Abstract
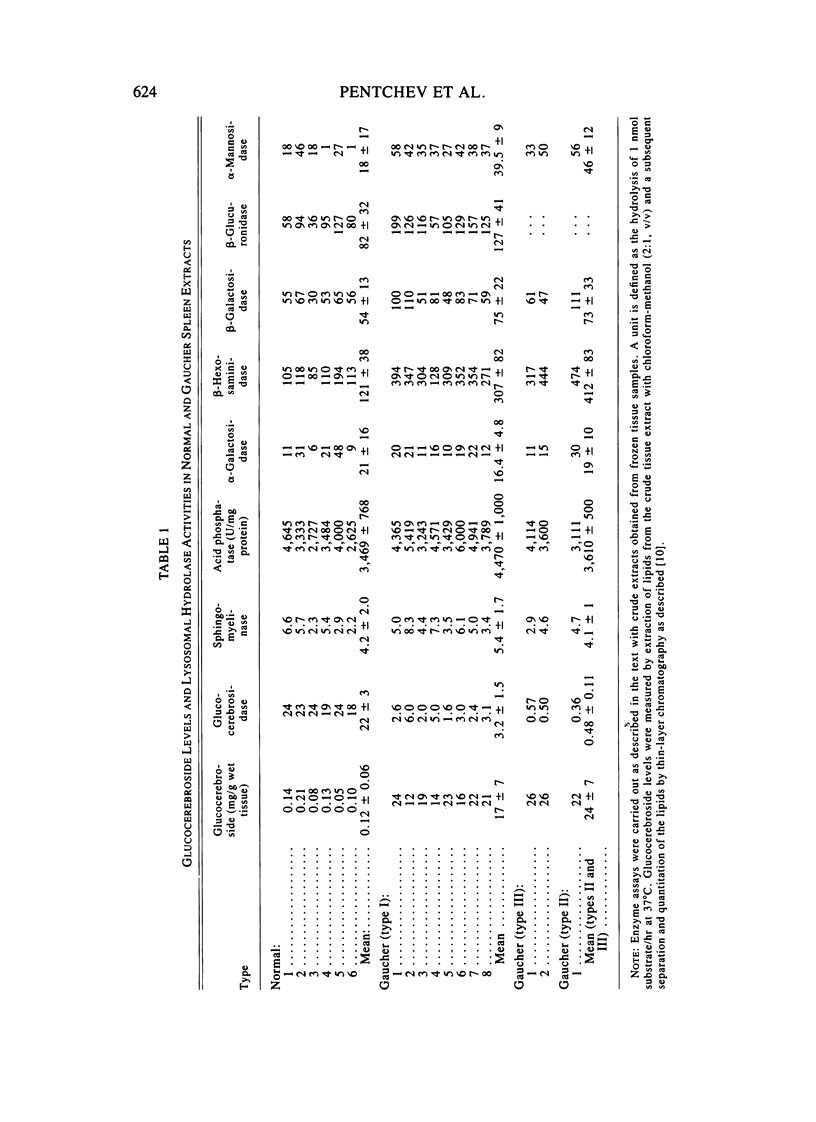
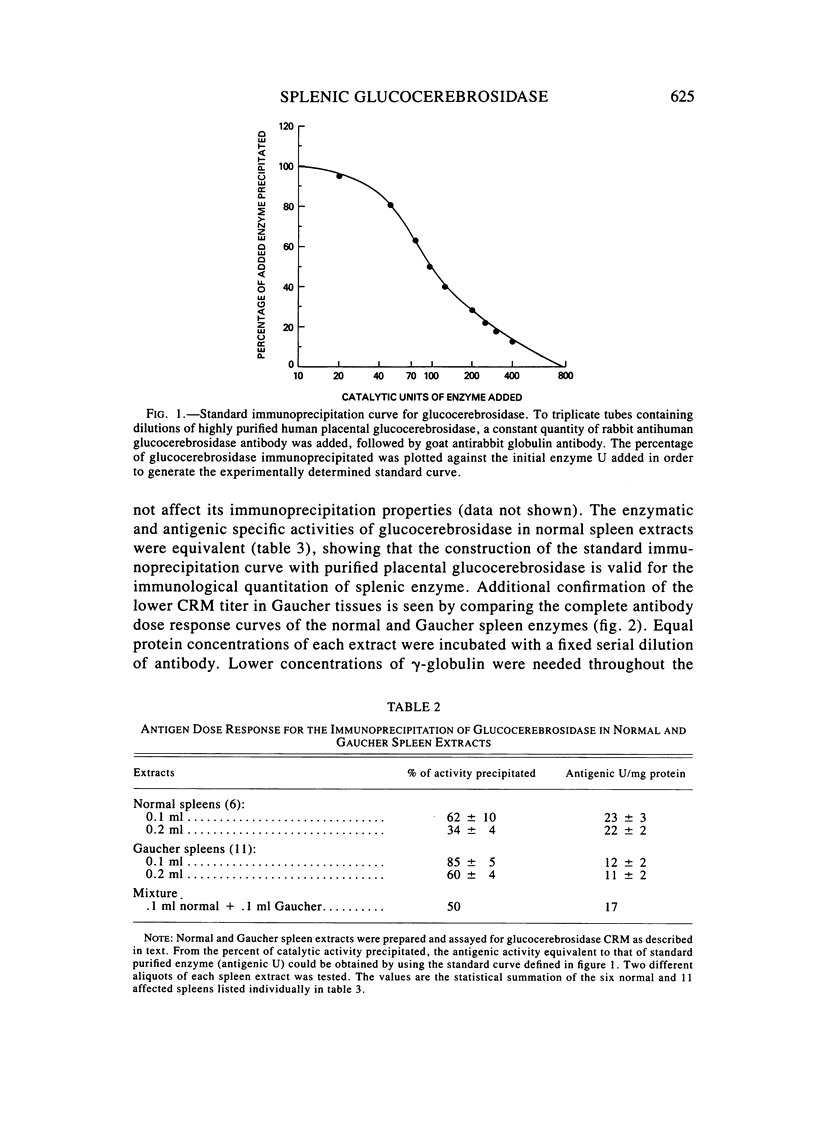
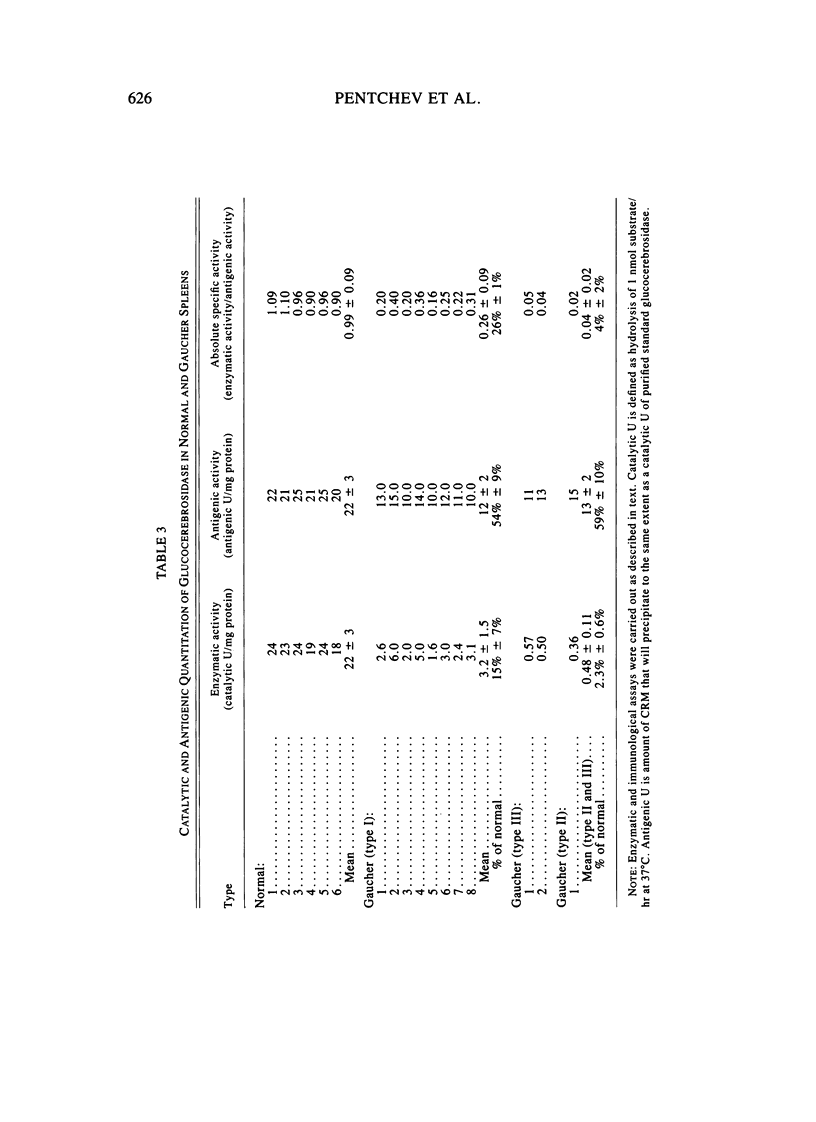
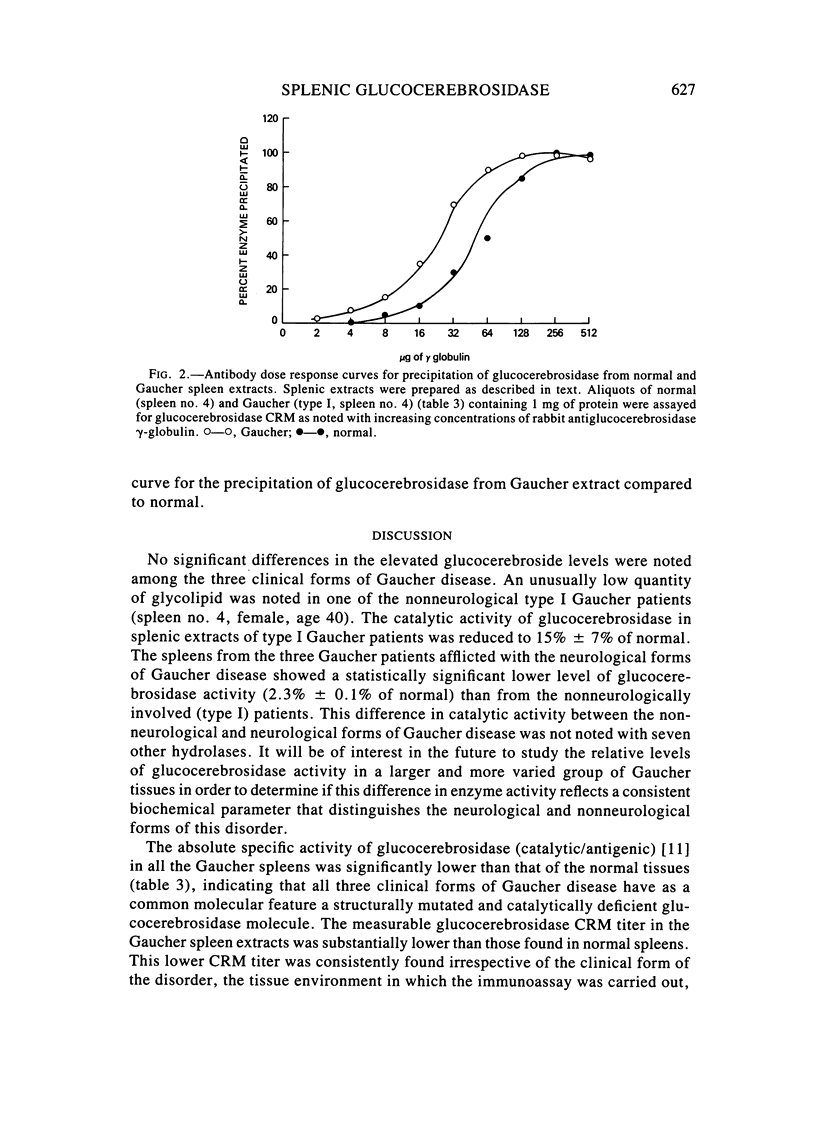
The enzymatic activity of glucocerebrosidase in splenic extracts of the adult nonneurological form of Gaucher disease (type I) was 15% +/- 7% of normal, and the titer of enzyme cross-reacting material (ECRM) in these spleens was 54% +/- 9% of normal. The titer of ECRM in splenic extracts of tissues obtained from patients with the neurological forms of Gaucher disease (types II and III) was essentially the same as in type I Gaucher spleens (59% +/- 10% of normal), but the measurable catalytic activity of glucocerebrosidase in these spleens was substantially lower than that found in type I Gaucher spleens (2.3% +/- 0.6% of normal). Thus, the attentuated glucocerebrosidase activity in spleens from all three forms of Gaucher disease appears to stem from a structurally mutated enzyme that is altered in its catalytic efficiency and possibly in its antigenic expression.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brady R. O., Johnson W. G., Uhlendorf B. W. Identification of heterozygous carriers of lipid storage diseases. Current status and clinical applications. Am J Med. 1971 Oct;51(4):423–431. doi: 10.1016/0002-9343(71)90249-x. [DOI] [PubMed] [Google Scholar]
- Carson D. A., Goldblum R., Seegmiller J. E. Quantitative immunoassay of adenosine deaminase in combined immunodeficiency disease. J Immunol. 1977 Jan;118(1):270–273. [PubMed] [Google Scholar]
- Daddona P. E., Frohman M. A., Kelley W. N. Radioimmunochemical quantitation of human adenosine deaminase. J Clin Invest. 1979 Sep;64(3):798–803. doi: 10.1172/JCI109526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furbish F. S., Blair H. E., Shiloach J., Pentchev P. G., Brady R. O. Glucocerebrosidase from human placenta. Methods Enzymol. 1978;50:529–532. doi: 10.1016/0076-6879(78)50058-x. [DOI] [PubMed] [Google Scholar]
- Pentchev P. G., Brady R. O., Blair H. E., Britton D. E., Sorrell S. H. Gaucher disease: isolation and comparison of normal and mutant glucocerebrosidase from human spleen tissue. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3970–3973. doi: 10.1073/pnas.75.8.3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentchev P. G., Brady R. O., Gal A. E., Hibbert S. R. The isolation and characterization of sphingomyelinase from human placental tissue. Biochim Biophys Acta. 1977 Aug 24;488(2):312–321. doi: 10.1016/0005-2760(77)90189-8. [DOI] [PubMed] [Google Scholar]
- Pentchev P. G., Brady R. O., Hibbert S. R., Gal A. E., Shapiro D. Isolation and characterization of glucocerebrosidase from human placental tissue. J Biol Chem. 1973 Aug 10;248(15):5256–5261. [PubMed] [Google Scholar]
- Pentchev P. G., Gal A. E., Booth A. D., Omodeo-Sale F., Fouks J., Neumeyer B. A., Quirk J. M., Dawson G., Brady R. O. A lysosomal storage disorder in mice characterized by a dual deficiency of sphingomyelinase and glucocerebrosidase. Biochim Biophys Acta. 1980 Sep 8;619(3):669–679. doi: 10.1016/0005-2760(80)90116-2. [DOI] [PubMed] [Google Scholar]


