Abstract
The hypoactivity of dorsolateral prefrontal cortex in schizophrenics is well known. One cause of this hypoactivity may be defective corticocortical or thalamocortical connections. Recent imaging studies of the thalamus suggest reductions in volume of the whole thalamus and reduced activity in the medial group of thalamic nuclei, which may indicate loss of functional input to the cortex. Using stereological techniques in six pairs of individually matched brains from schizophrenics and controls, we measured the volumes and obtained estimates of the number of neurons in the three subnuclei (parvocellular, pc; densocellular, dc; magnocellular, mc) of the mediodorsal nucleus (MD) and from the ventral posterior medial nucleus. There was a significant reduction in total neuron number in MD as a whole but this neuron loss was largely restricted to MDpc and MDdc [−30.9 and −24.5%, respectively (P ≤ 0.01)]. MDmc and the control ventral posterior medial nucleus showed no significant changes in cell number. Because the subnuclei of MD have different connections and project to different areas of the frontal cortex, the specific loss of neurons in MDpc and MDdc has implications for the functional defects observed in schizophrenia.
Keywords: mediodorsal nucleus, prefrontal cortex, hypoactivity, stereology
The severe cognitive dysfunction of schizophrenia is associated with disturbances in activity of the dorsolateral prefrontal cortex (DLPFC) manifested by hypoactivity in imaging studies, deficiencies in tests of cognitive function, and putative, activity-dependent effects on gene expression for neurotransmitter- and receptor-related mRNAs. The causes of the functional disturbance frequently have been attributed to defective connectivity that could be both cortical and subcortical (1–4). The principal subcortical input to DLPFC arises in the mediodorsal nucleus (MD) of the thalamus, and recent imaging studies in schizophrenics have shown reductions in activity of the medial nuclei of the thalamus (5). Pakkenberg (6) had described earlier a reduction in the number of neurons in the MD of the thalamus in schizophrenics, but her figures for the control nuclei were only half of those reported for control nuclei in a later study (7), and Pakkenberg stated that her material was inadequate for accurately defining the borders of the large subnuclear divisions of the nucleus. Moreover, it was not stated whether neuronal loss was found in other thalamic nuclei as well.
MD is composed of three major subnuclei: the magnocellular, parvocellular, and densocellular nuclei. The nuclei are delineated by cytoarchitecture and distinguished by major differences in cortical and subcortical connections (8–15). The magnocellular subnucleus (MDmc) occupies the anteromedial aspect of MD and is composed of relatively large, deeply stained neurons. MDmc is dominated by inputs from the olfactory and entorhinal cortices but also receives input from the amygdala; it projects to ventromedial and orbital cortex in the frontal lobe. The parvocellular nucleus (MDpc) occupies most of the dorsolateral aspect of MD and possesses somewhat smaller, more densely packed neurons. MDpc receives input from the ventral pallium, substantia nigra, superior colliculus, and other midbrain structures and projects to dorsal and lateral areas of the prefrontal cortex, including the areas that appear to be compromised in schizophrenia. In monkeys, its dorsomedial part projects to dorsomedial cortical areas and its lateral part projects to dorsolateral areas. The densocellular nucleus (MDdc), in which we include the paralamellar part of MD, envelops MD laterally and posteriorly. Its neurons resemble those of the central lateral nucleus (CL). MDdc receives input from many of the same subcortical sites as CL and projects to the striatum and to premotor cortical areas.
The overall connectivity of MD makes it a likely candidate for pathological involvement in schizophrenia independent of the rest of the thalamus, but this needs to be determined. Because of the differences in connectivity of the three subnuclei of MD, specific loss in any one of them could have important implications for understanding the pathophysiology of this disorder. In the present study, the volumes of MD and its subnuclei and their content of neurons were measured by using stereological protocols in brains from individually matched schizophrenics and controls. As a control, comparative measurements were made on a sensory relay nucleus, the ventral posterior medial nucleus (VPM).
Methods
The thalami from brains of six schizophrenic and six control subjects from the Brain Tissue Repository of the Center for Neuroscience, University of California, Davis, were used. The schizophrenic patients were diagnosed by board-certified psychiatrists by using DSM-IV criteria (Diagnostic and Statistical Manual,4th Ed.), and brains were individually matched to the controls by age, sex, and autolysis time (time between death and freezing the brain). Control subjects had no clinical history of neurological or psychiatric disease and no history of substance abuse (Table 1). None of the schizophrenics were chronic alcoholics or suffered other potentially relevant conditions. After removal, the brain was cut into coronal slices ≈7.5 mm thick, frozen between super-cooled aluminum plates, and stored at −80°C. Blocks of the thalami were cut from the slices, raised to ≈4°C, and fixed in cold 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) overnight. Blocks then were infiltrated with 30% sucrose in 0.1 M phosphate buffer at 4°C, frozen in dry ice, and stored at −80°C. Thalami were serially sectioned in the frontal plane on a sliding microtome at 50 μm. Sections were organized in groups of 10; three were stained with thionin for cell counts and volume estimates, six were used for in situ hybridization histochemistry, and one was reacted for acetylcholine esterase activity (16). Sections from representative areas of the cortex revealed no evidence of major neurodegenerative pathology. All brains were examined by a board-certified neuropathologist and determined to be free of tumors, vascular disturbances, and degenerative pathology.
Table 1.
Case histories of matched pairs of brains
| Key | Pair | Sex | Age | Autolysis time, hr | Diagnosis | Age of onset | Medication | Cause of death |
|---|---|---|---|---|---|---|---|---|
| ● | 1 | M | 21 | 17 | Control | Overdose | ||
| 1 | M | 23 | 11 | Schizophrenic | 17 | Navane | Auto accident | |
| ■ | 2 | M | 51 | 21.5 | Control | Cardiac | ||
| 2 | M | 55 | 21.15 | Schizophrenic | 20 | Thorazine | Cancer | |
| ▴ | 3 | M | 72 | 12.5 | Control | Cardiac | ||
| 3 | M | 77 | 8 | Schizophrenic | 23 | Stelazine | Pneumonia | |
| ▾ | 4 | M | 78 | 11 | Control | Cardiac | ||
| 4 | M | 79 | 15 | Schizophrenic | 64* | Mellaril | Cardiac | |
| ♦ | 5 | M | 84 | 6 | Control | Cardiac | ||
| 5 | M | 83 | 7 | Schizophrenic | 20 | Mellaril | Cardiac | |
| 6 | F | 78 | 7.25 | Control | Cardiac | |||
| 6 | F | 79 | 7.3 | Schizophrenic | 20 | Mellaril/Prolixin | Cardiac |
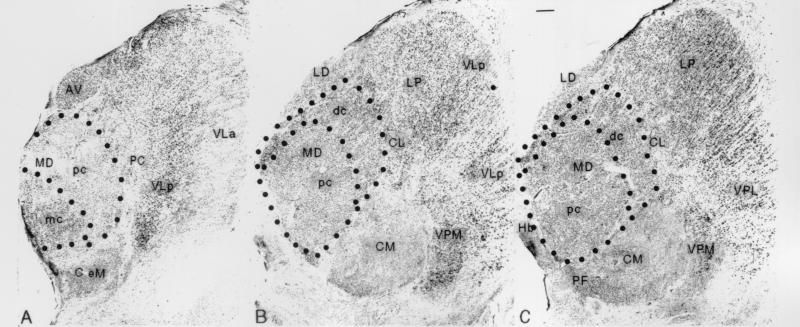
All quantitative studies were carried out on the left thalamus by observers blind to diagnosis. Every 20th Nissl-stained section was sampled in a systematic, random manner, resulting in at least 10 and as many as 19 sampled sections for each MD subnucleus and VPM. Volume estimates were determined from serial sections through the nuclei. MD, its subnuclei, and VPM were defined according to Hirai and Jones (17) (Fig. 1). Sections were projected onto the computer screen at ×2.5, and borders of the nuclei and subnuclei on the left side were drawn by an experienced observer based on cell size, packing density, and relation to the internal medullary lamina. The areas of MD, its subnuclei, and VPM were measured directly from the digitized outlines of each section, using a microscope stage equipped with stepping motors for x-, y-, and z-axis measurements (Ludl Electronics, Hawthorn, NY). Volume was calculated by multiplying the nuclear or subnuclear area by section thickness and the number of sections through each nucleus or subnucleus.
Figure 1.
Photomicrographs of Nissl-stained frontal sections through the anterior (A), middle (B), and posterior (C) levels of the mediodorsal nucleus, showing the MDmc, MDpc, and MDdc subnuclei outlined by interrupted lines. Other nuclei seen are the central lateral (CL), centre médian (CM), lateral dorsal (LD), lateral posterior (LP), ventral lateral anterior (VLa), ventral lateral posterior (VLp), ventral posterior lateral (VPL), and ventral posterior medial (VPM). (Bar = 1 mm.)
Cell counts were obtained with the aid of neurozoom fractionator software (Scripps Research Institute, La Jolla, CA, and Mount Sinai School of Medicine, New York). Absolute section thickness after histological processing was obtained from z-axis measurements. Using a counting frame measuring 80 μm × 100 μm, 1% of each subnucleus in a section was sampled with a ×60 high numerical aperture, oil-immersion objective. In each region, the area defined by the counting frame was optically dissected multiple times in 2-μm steps. After establishing upper and lower z-axis buffer zones, neuronal nucleoli coming into focus in each 2-μm step were counted. Nucleoli touching the left (y axis) and lower (x axis) borders of the counting box were excluded and those touching the opposite borders were included (17). Three hundred to 450 nucleoli were counted in each subnucleus. Using a coefficient of error (CE) formula (18), this excessive sampling regime resulted in maximum CE values for the subnuclei of 0.057–0.047, verifying adequacy of the sampling protocol.
Final neuron and volume estimates for MD as a whole, MDpc, MDdc, MDmc, and VPM were statistically analyzed by using a matched-pair Student's two-tailed t test. Covariates of age, sex, and autolysis time are accounted for in the matched-pair design of the study. Data were not pooled for further analysis because this diminishes the statistical power of the matched-pair design. To establish at what point any differences in MD, the three subnuclei, or VPM in each matched pair of brains acquired significance, the Bonferroni correction factor was applied. For this data set involving five comparisons in each pair, the corrected significant P value is ≤0.01. Estimates of percent differences between schizophrenics and controls as groups were obtained by averaging the percent difference between each matched pair of brains (Tables 2–5).
Table 2.
Estimates of average number of neurons in the three subnuclei and the entire MD in control and schizophrenic cases
| Subnuclei | No. of neurons ×
106
|
||
|---|---|---|---|
| Control | Schizophrenic | % Difference | |
| MDpc | 1.85 ± 0.25 | 1.26 ± 0.17 | −30.89* |
| MDdc | 1.16 ± 0.17 | 0.88 ± 0.23 | −24.45* |
| MDmc | 0.46 ± 0.07 | 0.39 ± 0.08 | −13.91 |
| Entire MD | 3.48 ± 0.40 | 2.54 ± 0.42 | −26.69* |
Number of neurons is average of all subjects in control or schizophrenic groups. Percent difference is the average of the percent differences between the matched pairs. P values are for individually matched pairs. *, P ≤ 0.01
Table 5.
Estimates of number of neurons, volume, and neuron density in VPM of control and schizophrenic cases
| VPM | Control | Schizophrenic | % Difference |
|---|---|---|---|
| Number of neurons | 137,376 ± 8,966.1 | 134,437 ± 12,402 | −3.78 |
| Nucleus volume, mm3 | 43.4800 ± 7.2747 | 45.39 ± 6.0583 | 6.99 |
| Neuron density, neurons/mm3 | 3,246.05 ± 682.78 | 4,370 ± 1,206 | −8.52 |
Number of neurons is average of all subjects in control or schizophrenic groups. Percent difference is the average of the percent differences between the matched pairs.
Results
Estimates of neuron number for the entire MD and its subnuclei from the six matched pairs of brains are shown in Table 2 and Fig. 2. In the schizophrenic cases, the number of neurons in the entire MD was reduced on average by 26.7% (P = 0.003). The estimated average number of neurons in MD of schizophrenics was 2.54 ± 0.42 million, and in MD of controls it was 3.48 ± 0.4 million.
Figure 2.
Estimates of the number of neurons in the MDpc, MDdc, and MDmc subnuclei and the entire MD nucleus in brains from the six matched pairs of schizophrenics and controls.
The reduction in the number of neurons was not homogeneous across MD (Table 2 and Fig. 2). In brains from schizophrenics, MDpc and MDdc demonstrated significant mean reductions in neuron number compared with controls, of 24.5% (P = 0.005) and 30.9% (P = 0.007), respectively. By contrast, the estimated number of neurons in MDmc of schizophrenics was only 13.9% less than in controls, and the difference was not statistically significant (P = 0.09). The percent decrease in neuron number in MDpc was significantly different from the decrease in neuron number in MDmc (P = 0.04), but the decrease in MDdc was not significantly different from the changes in MDpc and MDmc (P = 0.35 and P = 0.19, respectively). The decreases in neuron number showed no correlation with age, duration of illness, or autolysis time.
The volume of the entire MD in brains of schizophrenics showed a slight reduction of 16.8% in comparison with controls (Table 3 and Fig. 3). However, this was not statistically significant (P = 0.08). For individual subnuclei the data were more varied. An average reduction in volume of MDpc amounting to 16.8% was found in the brains of the schizophrenics in comparison with controls (P = 0.045). Taken alone, this would be considered significant. However, it is larger than the more restricted Bonferroni P value used in this study. Reductions in volume of MDmc and MDdc, amounting to 10% and 13.7%, respectively, failed to reach statistical significance (P = 0.32 and 0.25, respectively).
Table 3.
Estimates of average volumes of the three subnuclei and the entire MD in control and schizophrenic cases
| Subregion | Volume of nucleus,
mm3
|
||
|---|---|---|---|
| Control | Schizophrenic | % Difference | |
| MDpc | 488.8 ± 66.7 | 403.9 ± 63.6 | −16.81 |
| MDdc | 261.3 ± 57.2 | 209.9 ± 56.2 | −13.70 |
| MDmc | 112.4 ± 35.9 | 95.8 ± 31.8 | −10.01 |
| Entire MD | 862.6 ± 101.7 | 709.5 ± 138.0 | −16.80 |
Number of neurons is average of all subjects in control or schizophrenic groups. Percent difference is the average of the percent differences between the matched pairs.
Figure 3.
Estimates of the volumes of the MDpc, MDdc, and MDmc subnuclei and of the entire MD nucleus in brains from the six matched pairs of schizophrenics and controls.
The lack of significant decreases in nuclear volume affected estimates of neuron density, which ranged from 4,730 ± 1,206 to 3,171 ± 540 neurons/mm3 in the schizophrenics and from 4,576 ± 850 to 3,810 ± 384 neurons/mm3 in the controls. Neither MD as a whole nor any subnucleus showed significant changes in neuronal density (Table 4).
Table 4.
Estimates of average neuron density in the three subnuclei and the entire MD in control and schizophrenic cases
| Subregion | Neuron density,
neurons/mm3
|
||
|---|---|---|---|
| Control | Schizophrenic | % Difference | |
| MDpc | 3,810 ± 384 | 3,171 ± 540 | −15.51 |
| MDdc | 4,576 ± 850 | 4,314 ± 943 | −1.56 |
| MDmc | 4,379 ± 1,323 | 4,370 ± 1,206 | 8.91 |
| Entire MD | 4,054 ± 456 | 3,651 ± 714 | −8.07 |
Neuron density is average of all subjects in schizophrenic and control groups. Percent difference is the average of the percent differences between the matched pairs.
VPM showed no significant changes or trends in the number of neurons or in volume in brains of schizophrenics (Table 5).
No evidence of major gliosis or other pathological changes could be detected qualitatively in MD or VPM. All neurons counted appeared unshrunken in comparison with controls; this was confirmed in a preliminary nucleator analysis of the sizes of 150 neurons in each of two schizophrenics and their matched controls, which showed no pronounced differences in mean neuronal volume (5.8 × 103 ± 1.5 × 103 μm3, and 5.9 × 103 ± 1.7 × 103μm3, respectively).
Discussion
This study revealed that there are substantially fewer neurons in MD of schizophrenics than in MD of controls. The 27% reduction in total neuron number is less than the 40% reported by Pakkenberg (6). However, the numbers obtained for control cases are more than 100% greater than in the controls of Pakkenberg (6) and are similar to those reported for controls in other studies (7, 20, 21). The discrepancy between these results and those of Pakkenberg is likely to result from the stated difficulty that Pakkenberg had in delineating nuclear borders in her hematoxylin- and eosin-stained material. This discrepancy and the slight variations in the normal counts of other investigators also may be accounted for by variations in application of the optical fractionator. Despite the widespread theoretical acceptance and use of the optical fractionator to estimate particle number, there are several technical and theoretical concerns regarding its practical application (20, 22, 23). For this reason, sampling protocols used in this study were designed to result in exceptionally large sampling schemes.
Changes in total MD volume reported here indicate a trend toward decreased volume (−16.8%, P = 0.08) associated with schizophrenia. Previous studies in schizophrenia reported either no change in MD volume (24, 25) or a decrease in volume of the left MD (6). MRI studies of the thalamus in schizophrenics have reported both decreased (26–29) and unchanged (30–32) total thalamic volumes. A trend toward decreased volume is an expected accompaniment of neuron loss, and this would tend to offset any major alterations in neuronal packing density resulting from the loss of neurons.
A reduction in the overall neuronal population in MD of schizophrenics also has been reported in abstract form by Manaye et al. (33). We interpret it as loss of neurons. Potential causes of this loss are discussed below. The present study suggests that neuronal loss may be restricted to MD, although thalamic nuclei additional to VPM (which showed no change) will need to be studied to confirm this. Manaye et al. (33) also describe a reduction in volume of the anterodorsal nucleus in schizophrenia, as well as a reduction in neuron number in this nucleus. An important finding of the present investigation was the restriction of the neuron loss to the parvo- and densocellular subnuclei of MD, which project to DLPFC (11, 14, 15), and the lack of change in the magnocellular subnucleus, which projects mainly to the orbital and medial surfaces of the frontal lobe (10, 13, 14).
The substantial loss of neurons in MD subnuclei that project to DLPFC is significant in light of studies implicating DLPFC in the pathophysiology of schizophrenia. Imaging studies and tests of cognitive function indicate impaired activity of DLPFC in schizophrenics (34, 35), and although postmortem studies of the brains of schizophrenics sometimes have been inconsistent, there is increasing evidence for cellular pathology in this region. Increased (36–38), unchanged (39–41), and decreased (42, 43) neuron density have been reported in the DLPFC of schizophrenics, and there is evidence for activity-dependent changes in neuronal gene expression affecting the inhibitory, GABAergic (41, 44, 45), and excitatory, glutamatergic (46), neurotransmitter systems. These findings strengthen the belief that the defect of DLPFC function may stem from an underlying defect in connectivity. Recently reported alterations in the neuropil of the DLPFC in brains of schizophrenics (38, 47) further support this view. Reduction in the number of thalamic neurons projecting to DLPFC, as implied by the present results, is one obvious cause of diminished thalamocortical connections and reduced cortical neuropil, but reduction in corticocortical input also could be present and could be reflected in the consistent finding of enlargement of the lateral ventricles in schizophrenics (48, 49) because this implies a loss of subcortical white matter.
There is little functional evidence implicating the orbitomedial prefrontal cortex, to which MDmc projects, in schizophrenia, although some alterations in neuronal density, not based on stereological techniques, have been reported (36, 43, 50).
It remains uncertain whether the loss of neurons in two of the three subnuclei of MD is primary or secondary to pathology in DLPFC or in structures afferent to MDpc and MDdc. The histological appearances of the subnuclei in Nissl preparations did not provide evidence for any of the alternatives. It was significant, however, that there was no obvious gliosis and no overt neuronal pathology (see also ref. 51). Loss of neurons in the thalamus occurring as the result of acute, short-term cortical pathology is commonly accompanied by gliosis. Transneuronal degeneration occurring as the result of deafferentation, although leading in the long term to cell loss, usually is accompanied by shrinkage of cells, which was not evident in the present study. More extensive morphometric studies are needed to confirm the lack of gliosis and neuronal atrophy. Neuron loss during brain development is less likely to be accompanied by recognizable changes in surviving neurons or neuroglial cells. It could result from failure of neurons in the affected subnuclei of MD to establish synaptic connections in DLPFC or from a disproportionate degree of normally programmed cell death in the thalamus. It is also unknown yet whether the loss of neurons in MDpc and MDdc affects both relay neurons and interneurons or one of these populations specifically.
Note Added in Proof:
A publication reporting reduced numbers of neurons in the mediodorsal and anterior nuclei in brains of schizophrenics appeared while the present paper was under review (52).
Acknowledgments
We thank Phong Nguyen for help with preparation of the tissue; Dr. Christoph Schmitz for help with preparation of the manuscript; Dr. W. W. Tourtellotte, Ms. J. Sonnenshein, and Mr. J. Riehl at the National Neurological Research Specimen Bank, Los Angeles, for providing resources for storage and processing of brains; Mr. J. Beisner, Ms. J. Berndt, and the staff of the Orange County Sheriff/Coroner's office for neuropathological assessment; Dr. W. Lowell at the Ventura County Medical Examiner/Coroner's office; the staff of the Orange County Eye and Tissue Bank; Mr. P. Cartagena and the staff of the brain repository; and the families of the patients involved in this research. This work was supported by Grant MH54844 from the National Institutes of Health, United States Public Health Service.
Abbreviations
- DLPFC
dorsolateral prefrontal cortex
- MD
mediodorsal nucleus
- MDdc
MD densocellular division
- MDmc
MD magnocellular division
- MDpc
MD parvocellular division
- VPM
ventral posterior medial nucleus
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.150243397.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.150243397
References
- 1.Roberts G W, Bruton C J. Neuropathol Appl Neurobiol. 1990;16:3–16. doi: 10.1111/j.1365-2990.1990.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 2.Bogerts B. Schizophr Bull. 1993;19:431–445. doi: 10.1093/schbul/19.2.431. [DOI] [PubMed] [Google Scholar]
- 3.Jones E G. Schizophr Bull. 1997;23:483–501. doi: 10.1093/schbul/23.3.483. [DOI] [PubMed] [Google Scholar]
- 4.Harrison P J. Brain. 1999;122:593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- 5.Hazlett E A, Buchsbaum M S, Byne W, Wei T C, Spiegel-Cohen J, Geneve C, Kinderlehrer R, Haznedar M M, Shihabuddin L, Siever L J. Am J Psychiatry. 1999;156:1190–1199. doi: 10.1176/ajp.156.8.1190. [DOI] [PubMed] [Google Scholar]
- 6.Pakkenberg B. Arch Gen Psychiatry. 1990;47:1023–1028. doi: 10.1001/archpsyc.1990.01810230039007. [DOI] [PubMed] [Google Scholar]
- 7.Xuereb J H, Perry R H, Candy J M, Perry E K, Marshall E, Bonham J R. Brain. 1991;114:1363–1379. [PubMed] [Google Scholar]
- 8.Takagi S F. Trends Neurosci. 1980;13:313–315. [Google Scholar]
- 9.Yarita H, Iino M, Tanabe T, Kogure S, Takagi S F. J Neurophysiol. 1980;43:69–85. doi: 10.1152/jn.1980.43.1.69. [DOI] [PubMed] [Google Scholar]
- 10.Price J L, Amaral D G. J Neurosci. 1981;1:1242–1259. doi: 10.1523/JNEUROSCI.01-11-01242.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldman-Rakic P S, Porrino L J. J Comp Neurol. 1985;242:535–560. doi: 10.1002/cne.902420406. [DOI] [PubMed] [Google Scholar]
- 12.Jones E G. The Thalamus. New York: Plenum; 1985. [Google Scholar]
- 13.Barbas H, Henion T H, Dermon C R. J Comp Neurol. 1991;313:65–94. doi: 10.1002/cne.903130106. [DOI] [PubMed] [Google Scholar]
- 14.Siwek D F, Pandya D N. J Comp Neurol. 1991;312:509–524. doi: 10.1002/cne.903120403. [DOI] [PubMed] [Google Scholar]
- 15.Ray J P, Price J L. J Comp Neurol. 1993;337:1–31. doi: 10.1002/cne.903370102. [DOI] [PubMed] [Google Scholar]
- 16.Jones E G, Hendry S H, Liu X B, Hodgins S, Potkin S G, Tourtellotte W W. J Neurosci Methods. 1992;44:133–144. doi: 10.1016/0165-0270(92)90006-y. [DOI] [PubMed] [Google Scholar]
- 17.Hirai T, Jones E G. Brain Res Rev. 1989;14:1–34. doi: 10.1016/0165-0173(89)90007-6. [DOI] [PubMed] [Google Scholar]
- 18.Mayhew T M, Gundersen H J G. J Anat. 1996;188:1–15. [PMC free article] [PubMed] [Google Scholar]
- 19.Schmitz C. Anat Embryol. 1998;198:371–397. doi: 10.1007/s004290050191. [DOI] [PubMed] [Google Scholar]
- 20.Harding A J, Halliday G M, Cullen K. J Neurosci Methods. 1994;51:83–89. doi: 10.1016/0165-0270(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 21.Schmitz C, Rub U, Korr H, Heinsen H. Acta Neuropathol. 1999;97:623–628. doi: 10.1007/s004010051038. [DOI] [PubMed] [Google Scholar]
- 22.Guillery R W, Herrup K. J Comp Neurol. 1997;386:2–7. doi: 10.1002/(sici)1096-9861(19970915)386:1<2::aid-cne2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 23.Heinsen H, Rub U, Bauer M, Ulmar G, Bethke B, Schuler M, Bocker F, Eisenmenger W, Gotz M, Korr H, Schmitz C. Acta Neuropathol. 1999;97:613–622. doi: 10.1007/s004010051037. [DOI] [PubMed] [Google Scholar]
- 24.Rosenthal R, Bigelow L B. Brit J Psychiatry. 1972;121:259–264. doi: 10.1192/bjp.121.3.259. [DOI] [PubMed] [Google Scholar]
- 25.Lesch A, Bogerts B. Eur Arch Psychiatr Neurol Sci. 1984;234:212–219. doi: 10.1007/BF00381351. [DOI] [PubMed] [Google Scholar]
- 26.Andreasen N C, Swayze V W, II, Flaum M, Yates W R, Arndt S, McChesney C. Arch Gen Psychiatry. 1990;47:1008–1015. doi: 10.1001/archpsyc.1990.01810230024005. [DOI] [PubMed] [Google Scholar]
- 27.Andreasen N C, Arndt S, Swayze V, II, Cizadlo T, Flaum M, O'Leary D, Ehrhardt J C, Yuh W T. Science. 1994;266:294–298. doi: 10.1126/science.7939669. [DOI] [PubMed] [Google Scholar]
- 28.Flaum M, Swayze V W, II, O'Leary D S, Yuh W T, Ehrhardt J C, Arndt S V, Andreasen N C. Am J Psychiatry. 1995;152:704–714. doi: 10.1176/ajp.152.5.704. [DOI] [PubMed] [Google Scholar]
- 29.Buchsbaum M S, Someya T, Teng C Y, Abel L, Chin S, Najafi A, Haier R J, Wu J, Bunney W E., Jr Am J Psychiatry. 1996;153:191–199. doi: 10.1176/ajp.153.2.191. [DOI] [PubMed] [Google Scholar]
- 30.Corey-Bloom J, Jernigan T, Archibald S, Harris M J, Jeste D V. Am J Psychiatry. 1995;152:447–449. doi: 10.1176/ajp.152.3.447. [DOI] [PubMed] [Google Scholar]
- 31.Portas C M, Goldstein J M, Shenton M E, Hokama H H, Wible C G, Fischer I, Kikinis R, Donnino R, Jolesz F A, McCarley R W. Biol Psychiatry. 1998;43:649–659. doi: 10.1016/s0006-3223(97)00339-9. [DOI] [PubMed] [Google Scholar]
- 32.Arciniegas D, Rojas D C, Teale P, Sheeder J, Sandberg E, Reite M. Biol Psychiatry. 1999;45:1329–1335. doi: 10.1016/s0006-3223(97)00459-9. [DOI] [PubMed] [Google Scholar]
- 33.Manaye K F, Liang C L, Hicks P B, German D C, Young K A. Soc. Neurosci. Abstr. 1998. 484.3. [Google Scholar]
- 34.Weinberger D R, Berman K F, Zec R F. Arch Gen Psychiatry. 1986;43:114–124. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- 35.Maher B A, Manschreck T C, Woods B T, Yurgelun-Todd D A, Tsuang M T. Biol Psychiatry. 1995;37:144–150. doi: 10.1016/0006-3223(94)00203-F. [DOI] [PubMed] [Google Scholar]
- 36.Benes F M, McSparren J, Bird E D, SanGiovanni J P, Vincent S L. Arch Gen Psychiatry. 1991;48:996–1001. doi: 10.1001/archpsyc.1991.01810350036005. [DOI] [PubMed] [Google Scholar]
- 37.Selemon L D, Rajkowska G, Goldman-Rakic P S. Arch Gen Psychiatry. 1995;52:805–818. doi: 10.1001/archpsyc.1995.03950220015005. [DOI] [PubMed] [Google Scholar]
- 38.Selemon L D, Rajkowska G, Goldman-Rakic P S. J Comp Neurol. 1998;392:402–412. [PubMed] [Google Scholar]
- 39.Dunlap C B. Am J Psychiatry. 1924;3:403–421. [Google Scholar]
- 40.Ferrero C. Arch Neurol Psychiatry. 1947;59:41–70. [PubMed] [Google Scholar]
- 41.Akbarian S, Kim J J, Potkin S G, Hagman J O, Tafazzoli A, Bunney W E, Jr, Jones E G. Arch Gen Psychiatry. 1995;52:258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- 42.Colon E J. Acta Neuropathol. 1972;20:1–10. doi: 10.1007/BF00687897. [DOI] [PubMed] [Google Scholar]
- 43.Benes F M, Davidson J, Bird E D. Arch Gen Psychiatry. 1986;43:31–35. doi: 10.1001/archpsyc.1986.01800010033004. [DOI] [PubMed] [Google Scholar]
- 44.Akbarian S, Huntsman M M, Kim J J, Tafazzoli A, Potkin S G, Bunney W E, Jr, Jones E G. Cereb Cortex. 1995;5:550–560. doi: 10.1093/cercor/5.6.550. [DOI] [PubMed] [Google Scholar]
- 45.Volk D W, Austin M C, Pierri J N, Sampson A R, Lewis D A. Arch Gen Psychiatry. 2000;57:237–245. doi: 10.1001/archpsyc.57.3.237. [DOI] [PubMed] [Google Scholar]
- 46.Akbarian S, Sucher N J, Bradley D, Tafazzoli A, Trinh D, Hetrick W P, Potkin S G, Sandman C A, Bunney W E, Jr, Jones E G. J Neurosci. 1996;16:19–30. doi: 10.1523/JNEUROSCI.16-01-00019.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goldman-Rakic P S, Selemon L D. Schizophr Bull. 1997;23:437–458. doi: 10.1093/schbul/23.3.437. [DOI] [PubMed] [Google Scholar]
- 48.Pfefferbaum A, Zipursky R B. Schizophr Res. 1991;4:193–208. doi: 10.1016/0920-9964(91)90033-n. [DOI] [PubMed] [Google Scholar]
- 49.Lawrie S M, Abukmeil S S, Chiswick A, Egan V, Santosh C G, Best J J. Schizophr Res. 1997;25:155–166. doi: 10.1016/S0920-9964(97)00019-4. [DOI] [PubMed] [Google Scholar]
- 50.Kalus P, Senitz D, Beckmann H. J Neural Trans. 1997;104:549–559. doi: 10.1007/BF01277671. [DOI] [PubMed] [Google Scholar]
- 51.Richardson-Burns S M, Haroutunian V, Davis K L, Watson S J, Meador-Woodruff J H. Biol Psychiatry. 2000;47:22–28. doi: 10.1016/s0006-3223(99)00207-3. [DOI] [PubMed] [Google Scholar]
- 52.Young K A, Manaye K F, Liang C-L, Hicks P B, German D C. Biol Psychiatry. 2000;47:944–953. doi: 10.1016/s0006-3223(00)00826-x. [DOI] [PubMed] [Google Scholar]