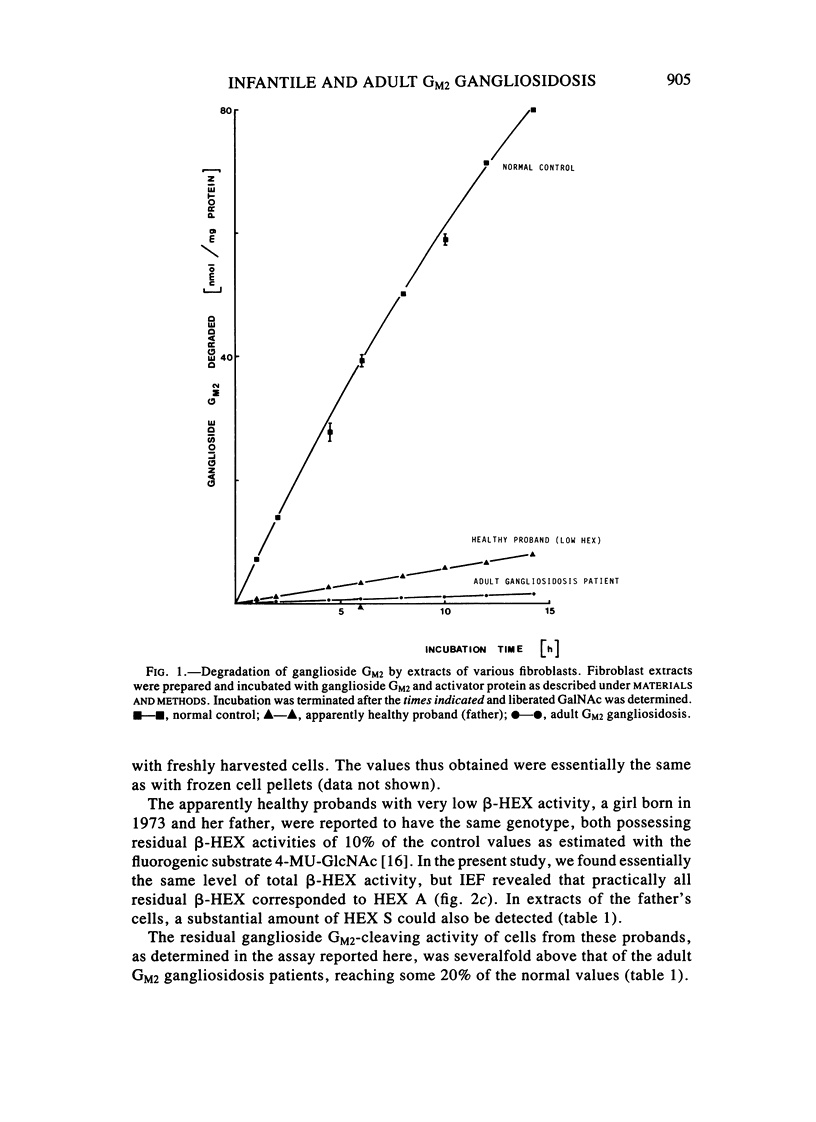
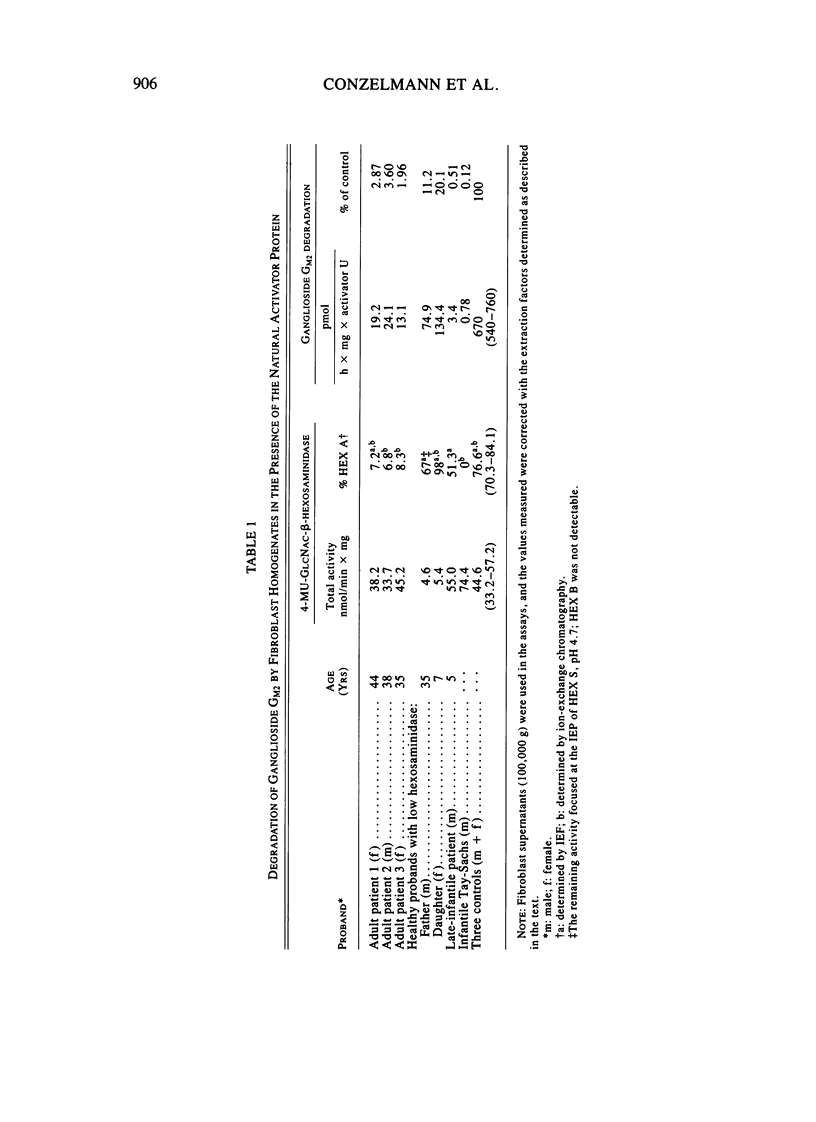
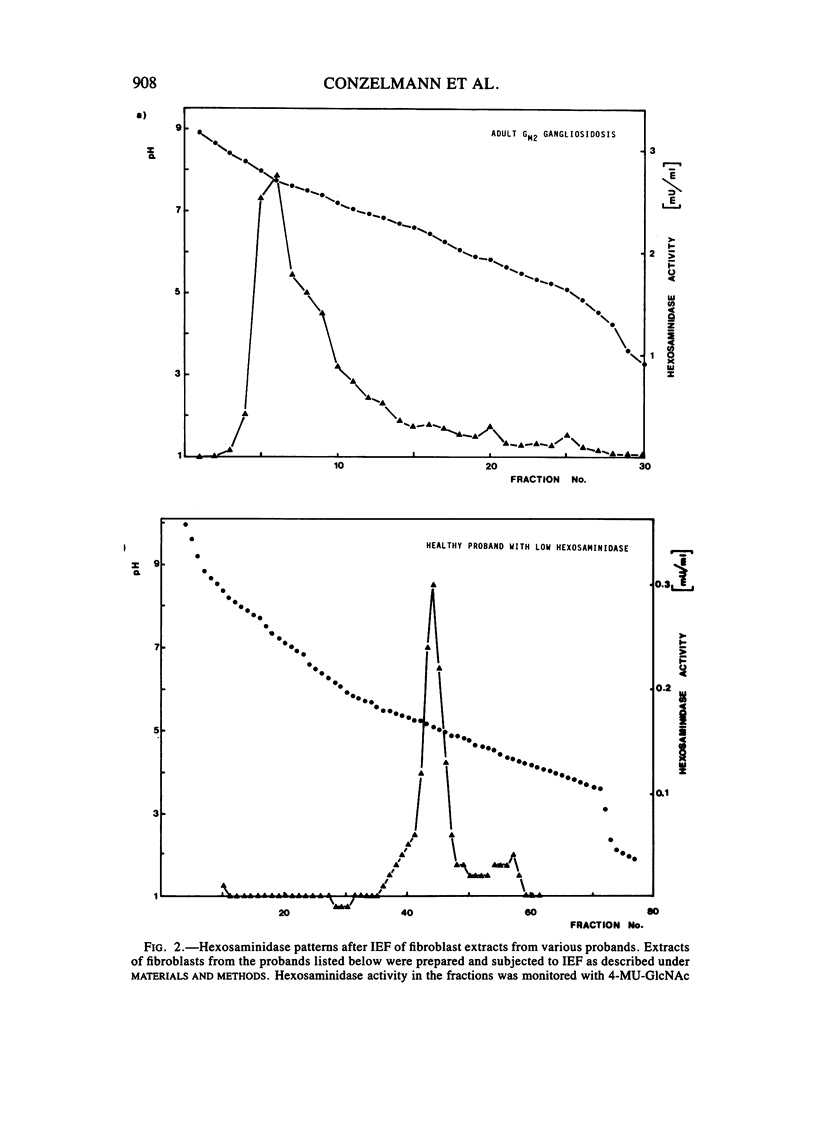
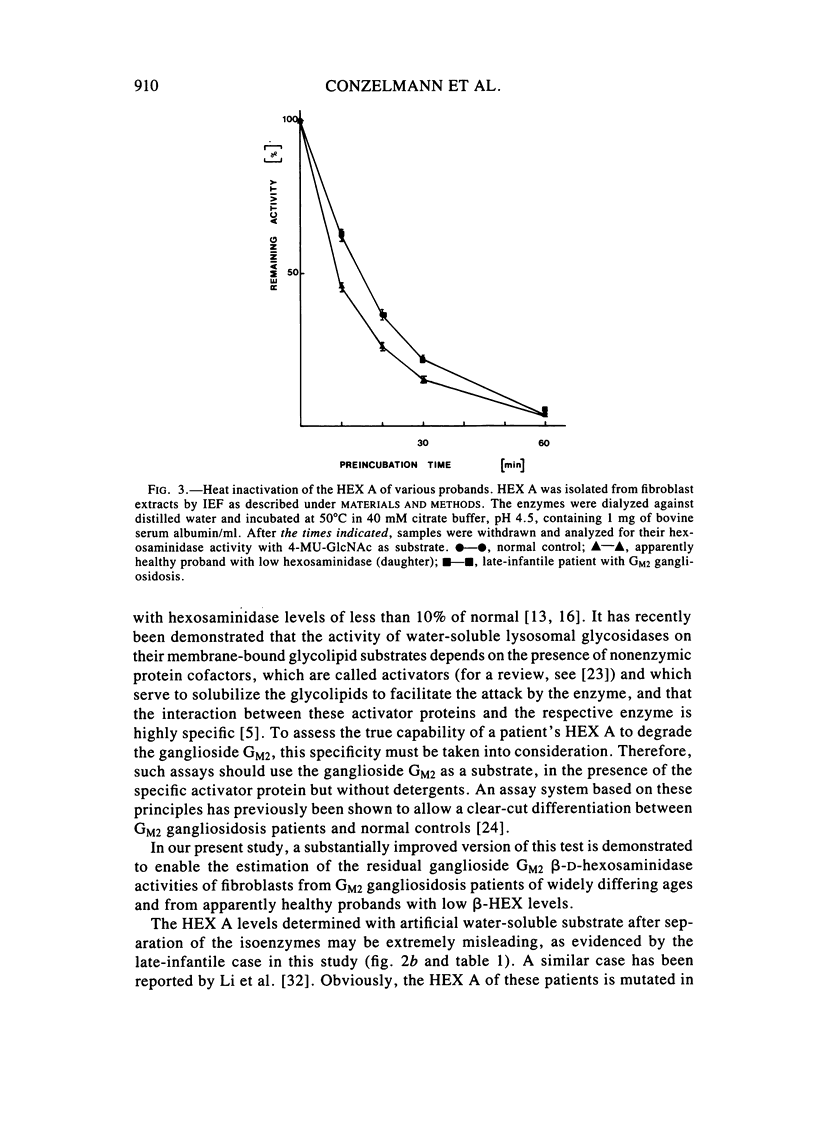
Abstract
A sensitive assay was developed to assess the ability of extracts from cultured fibroblasts to catabolize ganglioside GM2, in the presence of the natural activator protein but without detergents. This method, which permitted the reliable determination of residual activities as low as 0.1% of normal controls, was then used to measure ganglioside GM2 hydrolase activities in fibroblasts from several hexosaminidase variants. The residual activities thus determined correlated well with the clinical status of the respective proband: infantile Tay-Sachs (0.1% of normal controls), late-infantile (0.5%), and adult GM2 gangliosidoses (2%-4%) and healthy probands with "low hexosaminidase" (11% and 20%). In contrast, beta-hexosaminidase A levels as measured with the synthetic substrate 4-MU-GlcNAc could not be relied on for diagnostic purposes (the late-infantile patient studied retained 80% of the activity of controls).
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Conzelmann E., Burg J., Stephan G., Sandhoff K. Complexing of glycolipids and their transfer between membranes by the activator protein for degradation of lysosomal ganglioside GM2. Eur J Biochem. 1982 Apr 1;123(2):455–464. doi: 10.1111/j.1432-1033.1982.tb19789.x. [DOI] [PubMed] [Google Scholar]
- Conzelmann E., Sandhoff K. AB variant of infantile GM2 gangliosidosis: deficiency of a factor necessary for stimulation of hexosaminidase A-catalyzed degradation of ganglioside GM2 and glycolipid GA2. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3979–3983. doi: 10.1073/pnas.75.8.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conzelmann E., Sandhoff K. Purification and characterization of an activator protein for the degradation of glycolipids GM2 and GA2 by hexosaminidase A. Hoppe Seylers Z Physiol Chem. 1979 Dec;360(12):1837–1849. doi: 10.1515/bchm2.1979.360.2.1837. [DOI] [PubMed] [Google Scholar]
- Dreyfus J. C., Poenaru L., Svennerholm L. Absence of hexosaminidase A and B in a normal adult. N Engl J Med. 1975 Jan 9;292(2):61–63. doi: 10.1056/NEJM197501092920201. [DOI] [PubMed] [Google Scholar]
- Dreyfus J. C., Poenaru L., Vibert M., Ravise N., Boue J. Characterization of a variant of beta-hexosaminidase: "hexosaminidase Paris". Am J Hum Genet. 1977 May;29(3):287–293. [PMC free article] [PubMed] [Google Scholar]
- Erzberger A., Conzelmann E., Sandhoff K. Assay of ganglioside GM2-N-acetyl-beta-D-galactosaminidase activity in human fibroblasts employing the natural activator protein--diagnosis of variant forms of GM2 gangliosidosis. Clin Chim Acta. 1980 Dec 22;108(3):361–368. doi: 10.1016/0009-8981(80)90342-3. [DOI] [PubMed] [Google Scholar]
- Fischer G., Jatzkewitz H. The activator of cerebroside sulphatase. Purification from human liver and identification as a protein. Hoppe Seylers Z Physiol Chem. 1975 May;356(5):605–613. doi: 10.1515/bchm2.1975.356.1.605. [DOI] [PubMed] [Google Scholar]
- Geiger B., Arnon R. Chemical characterization and subunit structure of human N-acetylhexosaminidases A and B. Biochemistry. 1976 Aug 10;15(16):3484–3493. doi: 10.1021/bi00661a014. [DOI] [PubMed] [Google Scholar]
- Kelly T. E., Reynolds L. W., O'Brien J. S. Segregation within a family of two mutant alleles for hexosaminidase A. Clin Genet. 1976 May;9(5):540–543. doi: 10.1111/j.1399-0004.1976.tb01609.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee J. E., Yoshida A. Purification and chemical characterization of human hexosaminidases A and B. Biochem J. 1976 Dec 1;159(3):535–539. doi: 10.1042/bj1590535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S. C., Hirabayashi Y., Li Y. T. A new variant of type-AB GM2-gangliosidosis. Biochem Biophys Res Commun. 1981 Jul 30;101(2):479–485. doi: 10.1016/0006-291x(81)91285-7. [DOI] [PubMed] [Google Scholar]
- Li S. C., Li Y. T. An activator stimulating the enzymic hydrolysis of sphingoglycolipids. J Biol Chem. 1976 Feb 25;251(4):1159–1163. [PubMed] [Google Scholar]
- Navon R., Argov Z., Brand N., Sandbank U. Adult GM2 gangliosidosis in association with Tay-Sachs disease: a new phenotype. Neurology. 1981 Nov;31(11):1397–1401. doi: 10.1212/wnl.31.11.1397. [DOI] [PubMed] [Google Scholar]
- Navon R., Geiger B., Yoseph Y. B., Rattazzi M. C. Low levels of beta hexosaminidase A in healthy individuals with apparent deficiency of this enzyme. Am J Hum Genet. 1976 Jul;28(4):339–349. [PMC free article] [PubMed] [Google Scholar]
- Navon R., Padeh B., Adam A. Apparent deficiency of hexosaminidase A in healthy members of a family with Tay-Sachs disease. Am J Hum Genet. 1973 May;25(3):287–293. [PMC free article] [PubMed] [Google Scholar]
- O'Brien J. S., Tennant L., Veath M. L., Scott C. R., Bucknall W. E. Characterization of unusual hexosaminidase A (HEX A) deficient human mutants. Am J Hum Genet. 1978 Nov;30(6):602–608. [PMC free article] [PubMed] [Google Scholar]
- Okada S., O'Brien J. S. Tay-Sachs disease: generalized absence of a beta-D-N-acetylhexosaminidase component. Science. 1969 Aug 15;165(3894):698–700. doi: 10.1126/science.165.3894.698. [DOI] [PubMed] [Google Scholar]
- SVENNERHOLM L. Quantitative estimation of sialic acids. II. A colorimetric resorcinol-hydrochloric acid method. Biochim Biophys Acta. 1957 Jun;24(3):604–611. doi: 10.1016/0006-3002(57)90254-8. [DOI] [PubMed] [Google Scholar]
- Sandhoff K., Andreae U., Jatzkewitz H. Deficient hexosaminidase activity in an exceptional case of Tay-Sachs disease with additional storage of kidney globoside in visceral organs. Pathol Eur. 1968;3(2):278–285. [PubMed] [Google Scholar]
- Sandhoff K., Christomanou H. Biochemistry and genetics of gangliosidoses. Hum Genet. 1979;50(2):107–143. doi: 10.1007/BF00390234. [DOI] [PubMed] [Google Scholar]
- Sandhoff K., Conzelmann E., Nehrkorn H. Specificity of human liver hexosaminidases A and B against glycosphingolipids GM2 and GA2. Purification of the enzymes by affinity chromatography employing specific elution. Hoppe Seylers Z Physiol Chem. 1977 Jul;358(7):779–787. doi: 10.1515/bchm2.1977.358.2.779. [DOI] [PubMed] [Google Scholar]
- Sandhoff K., Harzer K., Wässle W., Jatzkewitz H. Enzyme alterations and lipid storage in three variants of Tay-Sachs disease. J Neurochem. 1971 Dec;18(12):2469–2489. doi: 10.1111/j.1471-4159.1971.tb00204.x. [DOI] [PubMed] [Google Scholar]
- Sandhoff K. The biochemistry of sphingolipid storage diseases. Angew Chem Int Ed Engl. 1977 May;16(5):273–285. doi: 10.1002/anie.197702733. [DOI] [PubMed] [Google Scholar]
- Sandhoff K. Variation of beta-N-acetylhexosaminidase-pattern in Tay-Sachs disease. FEBS Lett. 1969 Aug;4(4):351–354. doi: 10.1016/0014-5793(69)80274-7. [DOI] [PubMed] [Google Scholar]
- Srivastava S. K., Beutler E. Hexosaminidase-A and hexosaminidase-B: studies in Tay-Sachs' and Sandhoff's disease. Nature. 1973 Feb 16;241(5390):463–463. doi: 10.1038/241463a0. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Suzuki K. Specific radioactive labeling of terminal n-acetylgalactosamine of glycosphingolipids by the galactose oxidase-sodium borohydride method. J Lipid Res. 1972 Sep;13(5):687–690. [PubMed] [Google Scholar]
- Tallman J. F., Brady R. O., Navon R., Padeh B. Ganglioside catabolism in hexosaminidase A-deficient adults. Nature. 1974 Nov 15;252(5480):254–255. doi: 10.1038/252254a0. [DOI] [PubMed] [Google Scholar]
- Vidgoff J., Buist N. R., O'Brien J. S. Absence of -N-acetyl-D-hexosaminidase A activity in a healthy woman. Am J Hum Genet. 1973 Jul;25(4):372–381. [PMC free article] [PubMed] [Google Scholar]
- Zerfowski J., Sandhoff K. Juvenile GM2-gangliosidose mit veränderter Substratspezifität der Hexosaminidase A. Acta Neuropathol. 1974 Mar 26;27(3):225–232. doi: 10.1007/BF00687632. [DOI] [PubMed] [Google Scholar]


