Abstract
Day length is the primary cue used by many mammals to restrict reproduction to favourable spring and summer months, but it is unknown for any mammal whether the seasonal loss of fertility begins at the same time and occurs at the same rate in females and males; nor it established whether the termination of mating behaviour in males and females coincides with the loss of fertility. We speculated that females, owing to their greater energetic investment in reproduction, are the limiting sex in terminating offspring production in short days (SDs). Oestrous cycles and production of young were monitored in Syrian hamsters (Mesocricetus auratus) transferred from long days (LDs) to SDs. Females were mated to LD males after three to eight weeks of SD treatment; in a parallel experiment, males housed in SDs were mated to LD females. After five and eight weeks in SDs, at least twice as many males as females were fertile. Both males and females continued to copulate for several weeks after becoming infertile. The onset of seasonal infertility occurs earlier in females than males and the decline in fertility precedes the seasonal loss of mating behaviour in both sexes.
Keywords: photoperiod, sex differences, fertility, seasonality
1. Introduction
Many animal species restrict breeding to particular seasons and are reproductively quiescent the rest of the year. Because reproduction and offspring care are energetically costly, natural selection presumably favours production of young to coincide with abundant food availability and less challenging climatic conditions (Bronson & Heideman 1994). For small female mammals, high metabolic rates combined with the energetic demands of lactation often constitute an energy bottleneck (Bronson 1989; Hammond & Diamond 1992). In regions where an abundance of high quality food is available at certain times of the year, such species exhibit distinct breeding seasons, which are cued to variations in photoperiod, temperature and food availability (reviewed in Prendergast et al. 2002).
Photoperiodic mammals such as the Syrian hamster (Mesocricetus auratus) use day length as the primary exogenous cue to time the breeding season. In males, exposure to short photoperiods results in decreased secretion of pituitary gonadotrophins and testicular androgens, regression of the gonads and eventual loss of copulatory behaviour (Prendergast et al. 2002). Females also undergo gonadal changes after several weeks of short-day (SD) exposure, manifested by the transition from regular 4-day ovulatory oestrous cycles to anovulation (Seegal & Goldman 1975; Hauser & Benson 1986). After approximately 20 weeks of exposure to SD lengths, both male and female hamsters revert to the long-day (LD) phenotype; oestrous cycles resume and spermatogenesis is restored. At this time hamsters are considered to be refractory to SDs and their associated long-duration melatonin signals (Prendergast et al. 2002).
Physiological and behavioural changes associated with SD lengths have been documented in several hundred studies of rodents but to our knowledge, none has determined whether loss of fertility is initiated at the same time and occurs at the same rate in males and females. The relative importance of behavioural and physiological changes in limiting successful reproduction is also uncharted. Such information is for practical purposes unattainable in field studies of small nocturnal rodents, and, surprisingly, seems never to have been addressed in laboratory experiments.
Although structural and physiological changes in the gonads have been well characterized for some species, the relation between seasonal changes in hormone production and gonadal condition on the one hand, and fertility on the other, has not been explored. Testis involution is a heterogeneous process: at the same time that spermatocytes are degenerating, functional sperm can remain in the seminiferous tubules and epididymis (Jones 1999). Whether these sperm are viable, sufficiently motile and can be ejaculated in sufficient numbers to fertilize females are questions of physiological and adaptive significance. Offspring production will end if one sex becomes infertile before the other does, or if fertility wanes simultaneously in both sexes. Furthermore, infertility may result from deficits in mating behaviour instead of or in addition to impaired physiological processes.
We assessed the time course of changes in mating behaviour and fertility of females and males as a function of duration of exposure to SD lengths, and investigated how each of these factors contributes to termination of successful reproduction.
2. Material and methods
(a) Animals
Young adult female and male Syrian hamsters (M. auratus; Hsd:Han:AURA) were obtained from Harlan Sprague–Dawley (Haslett, MI, USA) and maintained in our laboratory in a 14L : 10D cycle (lights off at 14.00 PST). Animals were housed individually in polypropylene cages (48×25×15 cm) on Tek-Fresh bedding (Harlan Teklad, Madison, WI), and allowed to acclimate to laboratory conditions until 13 weeks of age, during which time female oestrous cyclicity was established. A subset of animals was then transferred to a short photoperiod (10L : 14D, lights off at 14.00) as described in experiments 1 and 2. Food (Prolab RMH 3000, Lab Diet, Richmond, IN) and water were available ad libitum. Ambient temperature was 22±2°C.
(b) Sexual experience and mating procedure
LD males were given sexual experience before they were used in the experiment. This procedure was adopted because to properly assess rates of decline of behaviour and fertility of LD females transferred to SDs, we needed to eliminate non-copulator males from the test pool. The stimulus females used as sexual partners for these males were bilaterally ovariectomized under isoflurane anaesthesia. Both ovaries were externalized through a single midline incision, the ovarian arteries were ligated and the ovaries excised. The wound was closed with sterile sutures. Several weeks later, these females were treated subcutaneously with 6 mm Silastic capsules (Dow Corning, Midland, MI; ID 1.98 mm, OD 3.18 mm) filled with crystalline oestradiol (Sigma Chemical, St Louis, MO). To induce behavioural oestrus, each female was injected with progesterone (0.5 mg in 0.2 ml peanut oil) 4 h before testing. Stimulus females were maintained in a 14L photoperiod. Only males that achieved six ejaculations with a stimulus female within 30 min on two separate tests were subsequently used in this study.
Matings to intact females were conducted under dim red light between 15.00 and 17.00 h in a 42×20×20 cm Plexiglass chamber. The chamber was situated over a tilted mirror that facilitated observation of copulatory behaviour. The occurrence and timing of mounts, intromissions and ejaculations were recorded. Ejaculations were characterized by the distinctive ejaculatory intromission (described by Bunnell et al. 1977), and the ensuing post-ejaculatory interval, during which males engaged in stereotypical behaviours that included anogenital grooming. In many instances, a copulatory plug indicative of sperm transfer was present in the female at the end of the ejaculatory intromission. Testing continued until the male achieved six ejaculations; mean latency to the sixth ejaculation was 8.0±0.2 min (mean±s.e.m.; range of 5.7–11.3 min, n=50, excluding a single outlier at 19.8 min). When both the male and female are in LDs, six ejaculations result in successful pregnancy in all females (Lanier et al. 1975; Huck et al. 1986). Stimulus males were drawn from a pool of 29 sexually experienced males who were mated up to three times at intervals of two weeks to SD or LD females. Copulatory capacity and sperm fertilizing capability of Syrian hamsters recover within 4–8 days after mating (Huck & Lisk 1985).
Prior to the beginning of the experiment, all females had manifested repeated 4-day oestrous cycles. They were not provided with sexual experience because 100% of virgin females tested at the appropriate stage of the oestrous cycle become pregnant and deliver litters during their initial mating test (Lanier et al. 1975; Huck et al. 1986). Prior sexual experience does not significantly affect subsequent sexual receptivity of female Syrian hamsters (Kohlert & Meisel 1999) which maintain a relatively immobile sexual posture (lordosis) for long intervals when first mounted and do not regulate the rate of male mounting attempts (Bradley et al. 2005).
(c) Female cycles and pregnancy
Oestrous cycles were monitored for each female by daily visual inspection of vaginal discharge. The presence of a post-oestrous discharge (PED) every fourth day (Orsini 1961) was the marker used to characterize the stage of the oestrous cycle. Monitoring of oestrous cycles was discontinued after animals were mated. A subset of 10 dams was monitored again beginning at the time the litters were weaned. Upon resumption of oestrous cycles after approximately 20 weeks of SD treatment, these females were mated for a second time to evaluate the fertility of photorefractory females maintained in SDs.
Females were mated 1–2 h after onset of darkness in Plexiglass chambers (described in §2b), and returned to a clean cage. Females were weighed at the time of mating and at regular intervals thereafter to judge whether they were pregnant. On the 16th day after mating, cages were inspected for presence of offspring four times at intervals of 3–4 h. An additional inspection was performed the next morning. Females were otherwise undisturbed beginning the day before and lasting until 8 days after parturition.
(d) Estimated testicular volume measurements
At the end of the 10th week of SD exposure, the length and width of the left testis was measured externally (±0.1 mm) under light anaesthesia induced with isoflurane vapours. The product of testis width squared times testis length (W2L) provides a measure of estimated testis volume (ETV) that is linearly and highly correlated with testis weight (Gorman & Zucker 1995).
(e) Statistics and data analysis
Mating and fertility comparisons between SD males or females and their respective LD controls, or between SD males and SD females, were performed using Fisher's exact test (FET) for analysis of small sample sizes of categorical data. In experiment 1, LD female control groups were tested concurrently with each SD group to control for possible effects of aging on fertility during the five weeks of mating tests. No ageing effect was found; the LD control response was uniform (100% mated and littered over the five weeks of testing) so the groups of LD females are treated as a single group of 24 females. For experiment 2, the LD control male matings were not age-matched, as we did not anticipate age-related decline in fertility; in fact, each of the 24 LD males mated to LD females sired young. Pooling of LD female controls to create a group with n=24 equal in size to the male LD control group allows for direct comparison of onset of fertility decline in males and females, with equal statistical power for males and females.
The relations between number of ejaculations and siring, between ETV and siring, and between litter size and day length were assessed using t-tests assuming unequal variances (JMP 5.1, SAS Institute, Inc.). In all cases, results were considered significant if the two-tailed p-value was less than 0.05.
(i) Experiment 1: decline of female fertility in short days
Four groups of females undergoing 4-day cycles were transferred from the 14L to the 10L photoperiod (n=9 per group); control groups of animals (n=6 per group) remained in LDs. At three, five, six and eight weeks after transfer to SDs, females were mated to rested, sexually experienced males. Age-matched control groups maintained in 14L were mated at each time point. Every control female at each time point delivered a litter. Female cyclicity, display of lordosis, post-mating weight gain, litter size at birth, and litter size, pup sex and weights at weaning (day 20) were recorded. Syrian hamster dams often cannibalize a portion of their litter (Schneider & Wade 1989). To minimize cannibalism, pups were not handled before day 8.
After extended periods in SDs, oestrous cycles of some females became irregular. In these instances, females were paired with males on the day consistent with their previous expected bout of sexual receptivity. Females that did not mate on this day were retested on each of the next three days. Pregnancies that resulted from pairings within this four-day interval were included in the data analysis. Matings were terminated if the female was aggressive towards the male.
(ii) Experiment 2: decline of male fertility in short days
Three groups of eight sexually experienced young males housed in SDs for five, eight or ten weeks were paired with LD receptive females and mated until they had achieved six ejaculations or for 30 min, whichever occurred first. Females were monitored for the presence of litters as above. ETVs were recorded at the end of week 10. A control group of 24 LD males was mated to LD females.
All procedures were approved by the Animal Care and Use Committee of the University of California at Berkeley.
3. Results
The results for experiments 1 and 2 are presented together to facilitate the comparison of the SD decline of fertility in males and females.
(a) Fertility decline of females
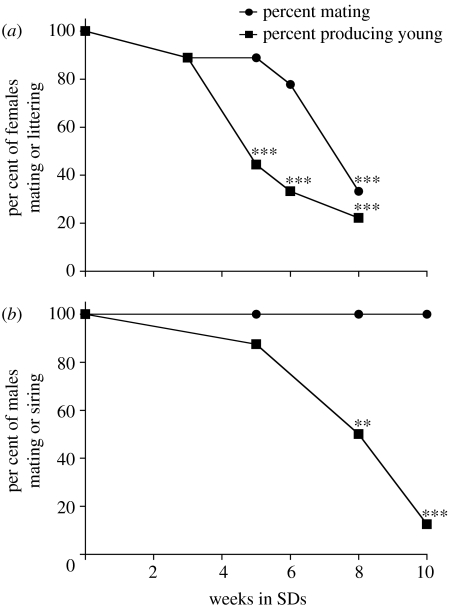
All LD females paired with LD males mated, became pregnant and littered (n=24). In contrast, the fertility of age-matched SD females mated to LD males declined significantly (figure 1a). After five weeks of exposure to SDs, only 4/9 females littered versus 100% of LD controls (p<0.001, FET); by week 8, only 2/9 SD females littered versus 100% of LD controls (p<0.001, FET). Among females that failed to deliver litters, several had received all six ejaculations from the male (4/5, 4/6 and 1/7 after five, six and eight weeks of SD treatment, respectively; figure 1a). Thus, reduced fertility in most females was not attributable to loss of sexual receptivity or failure of the male to achieve the requisite number of ejaculations.
Figure 1.
Fertility of SD Syrian hamsters mated to LD partners. (a) Female fertility: filled circles, per cent of females that mated during short day treatment. Filled squares, per cent of females that produced litters. (b) Male fertility: closed circles, per cent of males that mated in SD. Closed squares, per cent of males that sired offspring. LD controls are shown as 0 weeks in SD. Significance of difference between SD treatment and LD controls is indicated as **p<0.01, ***p<0.001.
(b) Fertility decline of males
All LD males paired with LD females mated and sired offspring (n=24). Unlike the majority of females, all SD males continued to mate until testing was terminated after 10 weeks of SD treatment (figure 1b). However, several aspects of mating behaviour (e.g. latency to first ejaculation) were eventually affected. By week 8, 16/16 SD males achieved six ejaculations, as did all LD controls. At week 10, all males ejaculated at least twice; male fertility nevertheless declined and several males that ejaculated six times did not sire young. Despite multiple ejaculations and sufficient stimulation to induce pseudopregnancy in their female partners (a condition resembling pregnancy, usually following infertile copulation, marked by persistence of the corpus luteum and other hormonal changes characteristic of the early and midstages of pregnancy), most males tested after 8 and 10 weeks of SD treatment failed to sire litters (3/7 and 1/8 males were successful compared with 100% of controls, (p<0.005 and <0.001, respectively, FET).
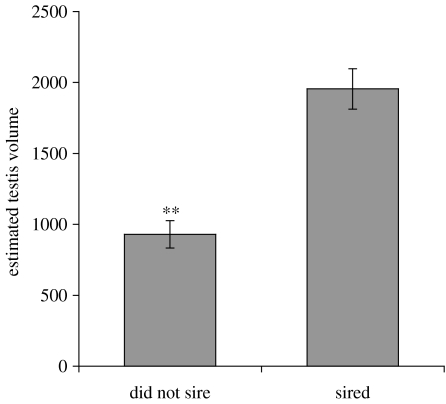
The ability of males to sire young in the week 10 matings was not related to the number of ejaculations (p>0.8, t-test): the single male that sired offspring ejaculated four times, whereas males that ejaculated six times failed to sire young. ETV at week 11 was predictive of fertility in week 8 and 10 tests (p<0.005, t-test, figure 2). No male with ETV<1500 (n=11) sired offspring, whereas every male with ETV>1500 (n=5) did so.
Figure 2.
Relation between siring at week 8 or 10 after transfer to SD and estimated testis volume. Males that did not sire litters had significantly smaller testes at the end of week 10 (**p<0.005). No male with ETV<1500 (n=11) sired young, and every male with ETV>1500 (n=5) sired offspring.
(c) Sex differences in mating behaviour and fertility
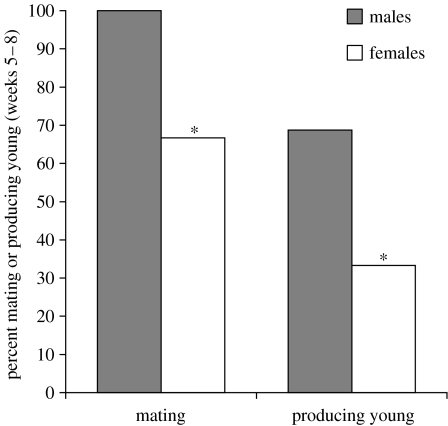
Mating behaviour ceased earlier in females than in males; after eight weeks of SD treatment, 8/8 males (100%) but only 3/9 females (33%) mated when paired with LD members of the opposite sex (3; p<0.01, FET). There was a parallel sex difference in fertility decline; SD females were less fertile than their LD counterparts by week 5, whereas males were first significantly less fertile than LD controls by week 8 (reported in §3b). Owing to the small intermediate numbers of hamsters that mated in the five and eight week time points, direct comparison of male and female fertility at each time point was of low statistical power: among animals that mated after five weeks of SD treatment 7/8 males (88%) and 4/9 females (44%) generated offspring (p<0.14). The comparable values for the week 8 tests were 4/8 males (50%) and 2/9 (22%) females producing offspring (p>0.3). Because of low power, fertility was assessed for data pooled for weeks five to eight for each sex. Male fertility in the five to eight week time period was significantly higher than female fertility: 11/16 males (69%) and 9/27 (33%) females that mated produced young (p<0.05) (figure 3).
Figure 3.
Fertility decline in males and females. At weeks 5–8, both mating and fertility were significantly lower in females than males (*: each p<0.05, FET).
(d) Female cyclicity
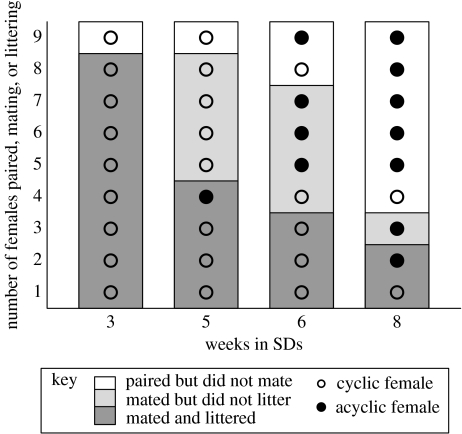
Females maintained regular 4-day oestrous cycles in LDs but became acyclic within approximately two through six weeks of transfer to SDs. Oestrous cycles, judged by the appearance of PED did not always correlate with sexual receptivity or fertility (figure 4). Some females mated while either acyclic or not manifesting normal 4-day cycles (n=6/36); two of these hamsters delivered litters. Conversely, some females that displayed regular cycles and mated did not deliver litters (n=5/36).
Figure 4.
Relation between mating, fertility, and cyclicity in females. The white portion of each bar represents hamsters that did not mate, the light grey portion those that mated but did not litter, and the dark grey portion those that mated and littered. Open circles denote individuals who had regular 4-day cycles; filled circles females who missed one or more cycles. Cyclic and acyclic females appear in each category.
LD females resumed visible cycles within 4–20 days of weaning their young. The PED always first appeared a multiple of 4 days from the date of weaning, irrespective of when females gave birth.
(e) Photorefractory females
Females housed continuously in SDs (n=10) resumed oestrous cycles between 17 and 23 weeks after their initial transfer to SDs (140±6 days). There was no relation between date of weaning and resumption of cyclicity.
In mating tests scheduled after 21–23 weeks of SD treatment, 100% of females mated to a criterion of six ejaculations and produced litters.
(f) Offspring characteristics
Maternal photoperiodic history affected litter size. Litter size at birth and weaning did not differ between age-matched LD and SD dams; however, primiparous SD photorefractory females had somewhat larger litters than did age-matched primiparous LD females at birth (10.0±0.6 for SD refractory versus 8.2±0.7 for LD controls, p=0.06), and significantly larger litters at weaning (8.6±0.5 for SD refractory versus 6.3±0.5 for LD controls, p<0.005, t-test).
4. Discussion
In field studies, it is difficult to assess whether curtailment of offspring production in waning day lengths reflects declining fertility in males or females. By mating SD animals with fertile LD partners, we demonstrate that in Syrian hamsters, female fertility declines in advance of male fertility; females, more than males, are responsible for terminating reproduction as day length decreases.
After eight weeks in SDs, male and female fertility were nearly equally low under conditions where each animal's sexual partner was a LD animal of high fecundity. In nature both males and females experience declining day lengths, as well as changes in other biotic and abiotic factors, hence it is plausible that production of young may cease after as few as four to five weeks of decreasing or SD lengths.
Unlike females, all males continued to engage in sexual behaviour throughout 10 weeks of SD treatment. This is congruent with well-established sex differences in post-castration persistence of sex behaviour of rodents; males copulate for several weeks after gonadal hormones are no longer detectable in the circulation (Meisel & Sachs 1994) whereas female receptivity declines within hours of ovariectomy (e.g. Moreines & Powers 1977). Since individual females remained fertile for several weeks after the majority had become infertile, males may benefit by retaining mating ability and fertility longer than most females; they can take advantage of the delayed onset of quiescence in the few remaining fertile females.
Since viable sperm can survive in the epididymis and testis after sperm production has ceased (Jones 1999), males that mate after androgen production has declined (Berndtson & Desjardins 1974) may still be able to inseminate females despite declining testicular dimensions.
Testis size, weight and histological characteristics, and presence of vaginal cycles have been used as proxies for fertility in SD lengths in numerous studies of rodents (Turek & Van Cauter 1994; Prendergast et al. 2002). Our study illustrates that manifestation of normal 4-day oestrous cycles, as assessed by PED, is an imperfect indicator of female fertility. Both cyclic and acyclic individuals refused to mate, mated but did not produce litters, or mated and produced litters (figure 4). ETV is an excellent indicator of male fertility; all males with ETV>1500 sired young whereas those with values below this threshold did not (figure 2). In vitro fertilizing (IVF) capacity of SD sperm was not measured in the present study, but our results suggest that it significantly underestimates in vivo male fertility. The proportion of SD males that sired offspring in the present study far exceeded that previously reported in IVF tests using sperm from SD males; under the latter conditions, fertilizing capacity of Syrian hamster sperm declined to 4% after six weeks in SDs, compared with 89% in LD controls (Holland et al. 1987). However, we found that 88 and 43% of SD males successfully sired young after five and eight weeks of SD treatment, respectively. This large discrepancy between in vivo and in vitro success may reflect changes in sperm capacitation attributable to mating.
Photorefractoriness in SD females, determined by the resumption of oestrous cycles, is accompanied by a return of fertility. Photorefractory primiparous females mated 20–25 weeks after transition to SDs had significantly larger litters than age and parity-matched LD hamsters. This is congruent with results from Siberian hamsters in which females raised for nine months in SDs before transfer to LDs remain fertile longer and show fewer signs of reproductive senescence than females raised from birth in LDs (Place et al. 2004).
We have demonstrated that on average, females are the limiting factor in offspring production after the onset of SD lengths and that male fertility declines soon thereafter. The sex difference in the seasonal decline in fertility documented here for Syrian hamsters probably applies to many other seasonally reproductive mammals, but the validity of this conjecture can only be decided by empirical test in other species. The females' greater energy investment in offspring, and the greater cost to females of failed reproduction, presumably account for the evolution of this sex difference.
Acknowledgments
We are grateful to Christiana Tuthill and the Office of Laboratory Animal Care for laboratory management, to the College of Natural Resources Statistics and Bioinformatics Consulting service, and to Matthew Paul and two anonymous reviewers for comments on an earlier version of the manuscript. This research was supported by grant HD 043098 from the National Institute of Child Health and Human Development.
References
- Berndtson W.E, Desjardins C. Circulating LH and FSH levels and testicular function in hamsters during light deprivation and subsequent photoperiodic stimulation. Endocrinology. 1974;95:195–205. doi: 10.1210/endo-95-1-195. [DOI] [PubMed] [Google Scholar]
- Bradley K.C, Haas A.R, Meisel R.L. 6-Hydroxydopamine lesions in female hamsters (Mesocricetus auratus) abolish the sensitized effects of sexual experience on copulatory interactions with males. Behav. Neurosci. 2005;119:224–232. doi: 10.1037/0735-7044.119.1.224. doi: 10.1037/0735-7044.119.1.224 [DOI] [PubMed] [Google Scholar]
- Bronson F.H. University of Chicago Press; Chicago, IL: 1989. Mammalian reproductive biology. [Google Scholar]
- Bronson F.H, Heideman P.D. Seasonal regulation of reproduction in mammals. In: Knobil E, Neill J.D, editors. Physiology of reproduction. Raven Press; New York, NY: 1994. pp. 541–583. [Google Scholar]
- Bunnell B.N, Boland B.D, Dewsbury D.A. Copulatory behavior of golden hamsters (Mesocricetus auratus) Behaviour. 1977;61:180–206. [Google Scholar]
- Gorman M.R, Zucker I. Seasonal adaptations of Siberian hamsters. II. Pattern of change in daylength controls annual testicular and body weight rhythms. Biol. Reprod. 1995;53:116–125. doi: 10.1095/biolreprod53.1.116. doi:10.1095/biolreprod53.1.116 [DOI] [PubMed] [Google Scholar]
- Hammond K.A, Diamond J. An experimental test for a ceiling on sustained metabolic rate in lactating mice. Physiol. Zool. 1992;65:952–977. [Google Scholar]
- Hauser U.E, Benson B. Rapid cessation of estrous cyclicity and depressed castration response in short photoperiod-treated, inbred LSH/SsLaK hamsters. Biol. Reprod. 1986;35:276–281. doi: 10.1095/biolreprod35.2.276. doi:10.1095/biolreprod35.2.276 [DOI] [PubMed] [Google Scholar]
- Holland M.K, Rogers B.J, Orgebin-Crist M.C, Danzo B.J. Effects of photoperiod on androgen-binding protein and sperm fertilizing ability in the hamster. J. Reprod. Fertil. 1987;81:99–112. doi: 10.1530/jrf.0.0810099. [DOI] [PubMed] [Google Scholar]
- Huck U.W, Lisk R.D. Determinants of mating success in the golden hamster (Mesocricetus auratus): I. Male capacity. J. Comp. Psychol. 1985;99:98–107. doi: 10.1037/0735-7036.99.1.98. doi:10.1037/0735-7036.99.1.98 [DOI] [PubMed] [Google Scholar]
- Huck U.W, Lisk R.D, Thierjung C. Stimulus requirements for pregnancy initiation in the golden hamster (Mesocricetus auratus) change with time of mating during the receptive period. J. Reprod. Fertil. 1986;76:449–458. doi: 10.1530/jrf.0.0760449. [DOI] [PubMed] [Google Scholar]
- Jones R.C. To store or mature spermatozoa? The primary role of the epididymis. Int. J. Androl. 1999;22:57–67. doi: 10.1046/j.1365-2605.1999.00151.x. doi:10.1046/j.1365-2605.1999.00151.x [DOI] [PubMed] [Google Scholar]
- Kohlert J.G, Meisel R.L. Sexual experience sensitizes mating-related nucleus accumbens dopamine responses of female Syrian hamsters. Behav. Brain Res. 1999;99:45–52. doi: 10.1016/s0166-4328(98)00068-0. doi:10.1016/S0166-4328(98)00068-0 [DOI] [PubMed] [Google Scholar]
- Lanier D.L, Estep D.Q, Dewsbury D.A. Copulatory behavior of golden hamsters: effects on pregnancy. Physiol. Behav. 1975;15:209–212. doi: 10.1016/0031-9384(75)90237-1. doi:10.1016/0031-9384(75)90237-1 [DOI] [PubMed] [Google Scholar]
- Meisel R.L, Sachs B.D. The physiology of male sex behavior. In: Knobil E, Neill J.D, editors. Physiology of reproduction. Raven Press; New York, NY: 1994. p. 3. [Google Scholar]
- Moreines J.K, Powers J.B. Effects of acute ovariectomy on the lordosis response of female rats. Physiol. Behav. 1977;19:277–283. doi: 10.1016/0031-9384(77)90339-0. doi:10.1016/0031-9384(77)90339-0 [DOI] [PubMed] [Google Scholar]
- Orsini M.W. The external vaginal phenomena characterizing the stages of the estrous cycle, pregnancy, pseudopregnancy, lactation and the anestrous hamster Mesocricetus auratus Water-house. Proc. Anim. Care Panel. 1961;11:193–206. [Google Scholar]
- Place N.J, Tuthill C.R, Schoomer E.E, Tramontin A.D, Zucker I. Short day lengths delay reproductive aging. Biol. Reprod. 2004;71:987–992. doi: 10.1095/biolreprod.104.029900. doi:10.1095/biolreprod.104.029900 [DOI] [PubMed] [Google Scholar]
- Prendergast B.J, Nelson R.J, Zucker I. Mammalian seasonal rhythms: behavior and neuroendocrine substrates. In: Pfaff D.W, Arnold A, Etgen A, Fahrbach S, Rubin R, editors. Hormones, brain and behavior. Academic Press; San Diego, CA: 2002. pp. 93–156. [Google Scholar]
- Schneider J.E, Wade G.N. Effects of maternal diet, body weight and body composition on infanticide in Syrian hamsters. Physiol. Behav. 1989;46:815–821. doi: 10.1016/0031-9384(89)90042-5. doi:10.1016/0031-9384(89)90042-5 [DOI] [PubMed] [Google Scholar]
- Seegal R.F, Goldman B.D. Effects of photoperiod on cyclicity and serum gonadotropins in the Syrian hamster. Biol. Reprod. 1975;12:223–231. doi: 10.1095/biolreprod12.2.223. doi:10.1095/biolreprod12.2.223 [DOI] [PubMed] [Google Scholar]
- Turek F.W, Van Cauter E. Rhythms in reproduction. In: Knobil E, Neill J, editors. The physiology of reproduction. 2nd edn. Raven Press; New York, NY: 1994. pp. 487–540. [Google Scholar]