Abstract
Perceptual biases can shape the evolution of signal form. Understanding the origin and direction of such biases is therefore crucial for understanding signal evolution. Many animals learn about species-specific signals. Discrimination learning using simple stimuli varying in one dimension (e.g. amplitude, wavelength) can result in perceptual biases with preferences for specific novel stimuli, depending on the stimulus dimensions. We examine how this translates to discrimination learning involving complex communication signals; birdsongs. Zebra finches (Taeniopygia guttata) were trained to discriminate between two artificial songs, using a Go/No-Go procedure. The training songs in experiment 1 differed in the number of repeats of a particular element. The songs in experiment 2 differed in the position of an odd element in a series of repeated elements. We examined generalization patterns by presenting novel songs with more or fewer repeated elements (experiment 1), or with the odd element earlier or later in the repeated element sequence (experiment 2). Control birds were trained with only one song. The generalization curves obtained from (i) control birds, (ii) experimental birds in experiment 1, and (iii) experimental birds in experiment 2 showed large and systematic differences from each other. Birds in experiment 1, but not 2, responded more strongly to specific novel songs than to training songs, showing ‘peak shift’. The outcome indicates that learning about communication signals may give rise to perceptual biases that may drive signal evolution.
Keywords: signal evolution, peak shift, birdsong, receiver psychology, sensory exploitation, generalization
1. Introduction
Understanding signal evolution requires an understanding of the adaptive significance of those signals, as well as of the proximate mechanisms involved in signal production and perception. A prime example is the evolution of signal form, which may be driven by sensory biases or biases resulting from the mechanisms involved in signal processing (e.g. Guilford & Dawkins 1991, 1993; Endler & Basolo 1998; Ryan 1998; Rowe & Skelhorn 2004). In many cases, signal processing is affected by learning. Predators, for instance, may learn to recognize aposematic prey (e.g. Edmunds 1974), while songbirds may learn to recognize the songs of neighbours (e.g. Brooks & Falls 1975). In such cases, individuals learn to discriminate particular signals from other similar ones. When confronted with a novel signal, the individual's response will be affected by what has been learned and, in particular, by the way in which knowledge about familiar signals is generalized to novel ones.
There is a large body of experiments that addresses the generalization patterns resulting from discrimination learning involving simple artificial stimuli such as lights of different wavelengths, tilted lines, tones of different amplitude or frequency, etc. (e.g. Purtle 1973; Ghirlanda & Enquist 2003). This has revealed that generalization can show remarkably different patterns and can even result in perceptual biases in which specific novel stimuli are preferred over familiar ones. In this paper, we examine the generalization patterns and biases that arise when the stimuli are complex and naturalistic animal communication signals; in this case birdsongs.
Stimulus generalization can be studied by training animals to discriminate between two stimuli that are differentially reinforced. One stimulus might indicate the presence of a reward (S+), the other (S−) might be neutral or linked to some punishment. If the training stimuli are relatively similar, the strongest response is often shifted away from the training stimuli towards more extreme stimuli, i.e. stimuli that increase the contrast between them. This ‘peak shift’ phenomenon has been known for a long time and has been studied extensively (e.g. Purtle 1973; Thomas et al. 1991; Mackintosh 1995; McLaren & Mackintosh 2002; Ghirlanda & Enquist 2003). In a review on stimulus generalization and peak shift, Ghirlanda & Enquist (2003) showed that generalization patterns differ strikingly according to whether the stimuli differ on so-called ‘intensity’ or ‘rearrangement’ dimensions. When learned differences concern the intensity of the stimuli, for instance tones of different amplitude, the generalization gradient is often monotonic. In this case, more extreme stimuli, like louder or softer tones than the training stimuli, may give rise to stronger responses than the training stimuli themselves, more or less in proportion to the increasing or decreasing intensity of the stimuli. On the other hand, when the stimuli are of equal intensity, but involve a rearrangement of stimulation over the receptors, for instance tones of the same amplitude but differing in frequency, the generalization usually peaks close to the familiar stimulus, i.e. may show a shift, but the response strength decreases rather than increasing with increasing difference from the familiar stimulus.
Applied to the context of signal evolution, the results of generalization studies using simple stimuli suggest that the response of an individual to novel signal variants, and hence the direction of selection, may be critically dependent on the contrast between the familiar signals.
Some recent studies have shown that skewed generalization patterns and even peak shifts may also result from learning about more complex or naturalistic stimuli, for instance in face perception in humans (Lewis & Johnston 1999; Spetch et al. 2004), the avoidance of aposematic prey (e.g. Gamberale & Tullberg 1996) and the development of sexual preferences by sexual imprinting (ten Cate et al. 2006). However, the presence of skewed generalization, as well as of different types of generalization patterns, in complex intraspecific communication signals still remains to be shown.
Birdsong is a complex vocal communication signal. Song functions in intra- and intersexual communication and song variation may be used to advertise species and individual identity, age, quality, motivation, etc. (e.g. Catchpole & Slater 1995). There is abundant evidence that both songbirds and non-songbirds learn to recognize different songs and other vocalizations. Most studies concern songbirds, where effects from prior experience on receiver response have been found in contexts like female song preferences at sexual maturity (e.g. Riebel 2003), discrimination between local and foreign song types (e.g. Nelson & Soha 2004) or between songs of mates and of other males (e.g. O'Loghlen & Beecher 1999). Furthermore, both songbirds (e.g. Weary 1990) and non-songbirds (e.g. Beckers et al. 2003) can be trained to discriminate between different vocalizations in skinner boxes. It is, however, largely unknown how discrimination learning involving songs differing in specific ways affects the responses to novel songs.
We examined song generalization patterns in the zebra finch (Taeniopygia guttata). The zebra finch is a prime model species for studying song learning, perception and preferences. Males acquire their song during an early sensitive phase (e.g. Slater et al. 1988). Both sexes recognize the tutor's song, which is preferred over unfamiliar song at adulthood (e.g. Adret 1993; Houx & ten Cate 1999; Riebel et al. 2002). Also, several studies have shown discrimination of songs learned at adulthood, for instance, in the context of recognizing mates (e.g. Miller 1979), colony members (Cynx & Nottebohm 1992), or familiar songs in general (Stripling et al. 2003).
Songs are complex multidimensional stimuli and two zebra finch songs may differ in many ways. We controlled this variability by constructing songs that varied systematically along two dimensions. In experiment 1, we manipulated the number of elements in the song (which might mimic an ‘intensity gradient’), and in experiment 2 the order of the elements (which might mimic a ‘rearrangement’ gradient). We show that the two experiments result in quite different generalization curves, both differing from those obtained after exposure to a single song, and comparable to the patterns described by Ghirlanda & Enquist (2003).
2. Material and methods
(a) Subjects and housing
We tested 23 male and 16 female adult zebra finches (T. guttata; age range: 0.5–3 years) originating from our breeding colony. The birds were naive to acoustical experiments. Each bird was used for one single experiment.
Prior to the experiments, the birds were housed in unisex groups, and kept on a 13.5 L : 10.5 D schedule at ±23°C. Drinking water, cuttlebone and a commercial seed mixture were available ad libitum.
The experiment took place in a skinner box (67(l)×26(d)×38(h) cm) consisting of wire mesh side and front walls and a wooden back wall, placed in a sound attenuated chamber. Two red pecking keys containing an LED and a small food hatch were located in the back wall. Songs were played through a Blaupunkt CB 4500 speaker behind an opening in the back wall. The overhead light source could be switched on and off during the experiment. The skinner box was placed in a sound attenuated chamber. The LEDs, the food hatch and the order in which the songs were played were controlled from outside this chamber, with a custom-made program that also recorded the key pecks.
(b) Discrimination learning
We used a ‘Go/No-Go’ procedure with a food award to train the birds to discriminate between two songs. A peck on key 1, which had the LED switched on, led to playback of the positive (‘Go’) stimulus song (S+). After the song finished, the LED in key 2 was switched on. Pecking this key opened the food hatch for a brief time period. When the bird pecked key 2 during at least 75% of the response intervals (i.e. used 75% of the opportunities to get food with a peck on key 2), the negative (‘No-Go’) stimulus song (S−) was introduced, and the bird had to learn not to peck after hearing this song. From then onwards, the bird heard either the S− or the S+ song after pecking key 1. In control experiments, the S− was not a song, but silence. If the bird now pecked on key 2 after hearing the S− song, it was punished with 15 s of darkness. During this training phase, the ratio S+ : S− was lowered, depending on the performance of the bird. When the bird had learned to discriminate between S+ and S− (reflected in a high ‘Go’ response for S+ and a low one for S−), the ratio between them was reset to 1 : 1. The criterion to proceed to the next phase, the generalization test, was that birds had to maintain a stable level of at least 75% correct responses for 3 days. During the 2 days after this we stopped reinforcing S+ and S− in 10% of the trials. This was to prevent extinction for the test song stimuli in the generalization test.
(c) Generalization test
During the generalization test, 90% of the playbacks of S+ and S− songs were played with reinforcement. In 10% of the cases, one of the test songs (see below), including the S+ and S−, was played randomly and non-reinforced. The fraction of times the bird pecked the second key was taken as response level and an indication of similarity with the S+ or S− as perceived by the bird. The test continued until each of the test songs had been played at least 100 times, which lasted on average three weeks. Not all songs were played the same number of times over this time period, owing to the randomization of stimulus presentation.
(d) Songs
Two types of song gradients were constructed from elements from ‘undirected’ songs of males recorded in a sound-attenuated chamber with a Sennheiser microphone and a Sony TC D5 tape recorder. The songs were digitized and edited with SIGNAL Sound Analysis System (Engineering Design, Belmont, MA). The parameter that varied in the two song gradients was element number of the song in experiment 1 and the element arrangement in experiment 2. To reduce the potential impact of using songs based on a single set of elements, we used two different element sets to construct two different song gradients for each experiment. In each case, they showed the same response patterns and hence we combined the data in the analyses.
(e) Experiment 1 (long/short)
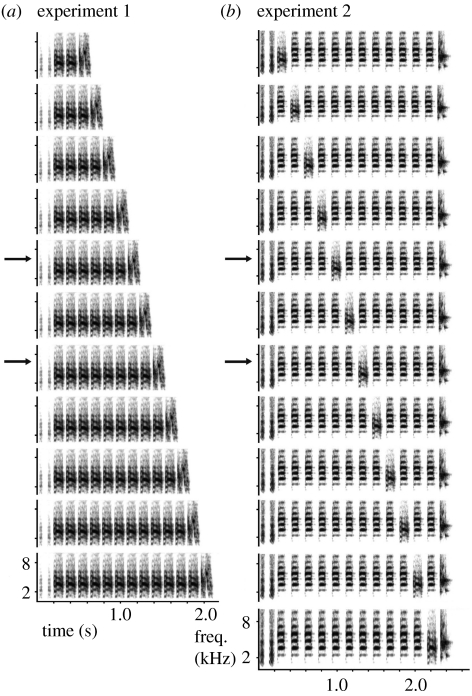
This song gradient consisted of songs with two ‘short slide’ or ‘introductory’ notes, a variable number of repetitions of a ‘combination note’ (description of note types follows Sturdy et al. 1999) and an ‘endnote’ (not classified by Sturdy et al.). We used 12 songs, in which the number of repetitions of the ‘combination note’ ranged from 2 to 11, to construct the gradient for the generalization test.
The S+ and S− songs had either six or eight repetitions (stimuli 5 and 7, see figure 1). We had two experimental groups. For the first one (the ‘S+ short’ group), seven birds were trained with stimulus 5 as S+ and stimulus 7 as S−. In the second set (the ‘S+ long’ group), six birds were trained on stimulus 7 as S+ and stimulus 5 as S−.
Figure 1.
Sonograms of one set of stimulus songs for (a) experiment 1 and (b) one for experiment 2. Stimuli for experiment 1 differ in the number of repeats of the central element; for experiment 2, stimuli differ in the position of an odd element in a series of repeated elements. Arrows indicate stimuli used as S+ and S− in each experiment.
Each group had a corresponding control experiment, where the S− song was replaced with silence. In the S+ short control group, four birds were trained, while in the S+ long control group three birds were trained.
(f) Experiment 2 (rearrangement)
The second gradient consisted of songs with two ‘short slide notes’, followed by 11 repetitions of a ‘flat note’ and one ‘combination element’. The latter was put in different positions in the flat note repetition row (see figure 1), keeping the number of flat note elements the same for each song. The song ends with an ‘endnote’. The S+ and S− were songs with the combination element at the 5th or 7th position after the short slide (figure 1b).
Again, we had two experimental groups. The first one consisted of seven birds trained on stimulus 5 as the S+, and stimulus 7 as S− (the ‘S+5’ group). In the second group, seven birds were trained on stimulus 7 as S+ and stimulus 5 as S− (the ‘S+7’ group). For each set of corresponding control experiments, three birds were trained on the S+, with the S− song replaced with silence.
(g) Analysis
Responses are measured as the fraction of the times that a particular bird pecked on the second key out of the first 100 times the bird heard the stimulus song. The response levels to S+ and S− were calculated from the response of the bird to the non-reinforced S+ and S−. Thus, from each bird we used the response fractions to each stimulus in our analysis, which provided us with six or seven data points per stimulus per experiment, or three data points per stimulus in the case of the corresponding control experiments. We used a cubic smoothing spline method to fit a smooth generalization curve through the thus calculated data points of the experiments (not the controls; Schluter 1988), and used bootstrapping with 1000 replicates to estimate a 95% confidence interval (CI) for this fit. The errors added to the randomly drawn data points were randomly drawn from a binomial distribution. Drawing was with replacement. Both spline fitting and bootstrapping were done with the program provided online by Schluter at http://www.zoology.ubc.ca/∼schluter/splines.html. The cubic spline method allows a description of a curve without a preset model, which makes it a non-parametric regression method. Our data fit the assumptions and distributions of this method: binary response variable and along a gradient. Our data samples are partly non-random, because each test subject evaluated all stimuli in a particular experiment. This spline method does not account for such data structure. The relative responses to the stimuli may be correlated within each test subject. However, our objective was to obtain a curve that described the average generalization of the test subjects, with some CIs for this description, to compare between experiments, not to compare the variances in responses between stimuli. Since the six or seven (depending on the group) replicates for each stimulus are independent data points, our data analysis does not over-parametrize our analysis.
The fitted spline can be set with a smoothing parameter, λ. A small value of λ makes a fitted spline very smooth, a large value very rough. We estimated the optimal λ using a cross-validation method provided by the program. To have a similar level of smoothness for the splines of both datasets within an experiment, we used the same λ to smoothen these curves, making sure that the mean response to S+ and S− fell within the CIs of the fit.
The curves from the control experiments were analysed differently. Since we had no clear prediction for the generalization gradient (excluding the response to the silence), we tested first for significant curvature. This was done by comparing the error sum of squares from a linear regression and the error sum of squares from an ANOVA, using the stimuli from the range as a categorical variable. The difference between the regression error and the ANOVA error was compared with an F-test. If this test was not significant, the linear regression was used to describe the control curve. If this test was significant, a nonlinear least-squares analysis was used to describe the control curve. Analysis of the control curves was done in R.
All analyses, both for the control and the S+/S− curves, were done assuming binomial distribution of the data, except for the nonlinear least-squares analysis. This was checked before fitting the models, and in each case no departure from this distribution was detected. The regression analysis was done with a GLM and a logit link function.
Additionally, we tested for peak shift with paired t-tests on the response ratio to S+ versus the stimulus closest to S+ on the side away from the S− (S+1). A similar analysis was done comparing S+ and the average of the S+ tail (all stimuli more extreme than S+, away from S−) to test for any cumulative effect at the more extreme stimuli. In the same way, we tested for peak shift towards more extreme values at the S− side. Corrections for multiple testing on the data for each experiment were done with a sequential Bonferroni test.
All analyses except the cubic spline and its bootstrapping were done in R (R Development Core Team 2005).
3. Results
(a) Discrimination learning
Birds differed in the time needed to reach the 75% correct responses for 3 days, the criterion to proceed to the generalization test. On average, 5011 (±819 s.e., n=28) playbacks of a reinforced S− were needed to reach the criterion to proceed to the generalization experiment. We fitted the number of times of exposure to S− in a GLM with Poisson distribution (corrected for overdispersion) with sex and experiment type. The minimal adequate model included only the experiment type (1 or 2), indicating that sex had no significant effect on our measure of discrimination learning. Birds that were trained with stimuli from song gradient 1 needed significantly more experience than birds trained on songs from gradient 2 (F1,26=5.05, p=0.033). Birds in control experiments needed significantly less experience with the negative stimulus (now a period of silence; F1,38=14.76, p<0.001). There was no difference in the number of playbacks of S− between control experiments 1 and 2. Although we had the impression that older birds took longer to train, we lacked the precise age of all birds and hence could not analyse this.
(b) Control curves
Only the control S+ short curve showed significant curvature and was fitted with a nonlinear least-squares analysis.
The other three control curves were fitted with a generalized linear regression. The logit link function yields estimates on that scale, so back transforming to the estimated y values follows the equation
(c) Experiment 1, long/short stimuli
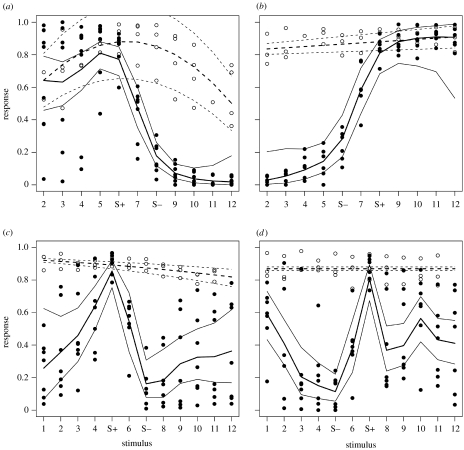
We set the smoothing parameter, λ, at −4. The curves and their CIs showed similar responses only to the ‘intermediate’ stimulus in between the S+ and S−. The S+ tail of the S+ long group does not overlap with the S− tail (more extreme stimuli at the S− side) of the S+ short group. In the same way, the S+ tail of the S+ short group does not overlap with the S− tail of the S+ long group. S+/S− location thus significantly alters the responses in the generalization tests. Furthermore, for both groups, the CIs of the S+ tails, but not of the S− tails, overlap with the estimates for the tail values of the control group (figure 2a,b).
Figure 2.
Data and generalization curves for experiments (a,b) 1 and (c,d) 2 and their controls. Open circles and dashed lines are for control experiments. Solid circles and solid lines are for S+/S− experiments. Thick lines (solid or dashed) are the curve estimates, thin lines are their corresponding 95% CIs. (a) Experiment 1, S+ short. (b) Experiment 1, S+ long. (c) Experiment 2, S+5. (d) Experiment 2, S+7.
For both groups, responses to S+ and S+1 were not significantly different. The average response over the S+ tail in the S+ long group was significantly higher than S+ (p=0.049, t5=2.49). However, for both groups, the responses to S−1 as well as to the average over the S− tail were significantly lower than to S− (S+ long group: p<0.01, t5=4.16 and p<0.01, t5=5.52, respectively; S+ short group: p<0.01, t6=4.17 and p<0.001, t6=4.36).
(d) Experiment 2, rearrangement stimuli
The smoothing parameter, λ, was set at −6. The S+ tails as well as the S− tails for both the S+7 and the S+5 group are intermediate to values of the S+ and S− stimuli. For both curves, the CI of the S+ includes the estimates for the control S+ values but, otherwise, the control curves and their CIs do not overlap with the curves of the experimental groups (figure 2c,d).
For both S+5 and S+7 groups, the responses to S+1 were significantly lower than to S+ (S+5: p<0.001, t6=−3.82; S+7: p<0.001, t6=−4.33). The average response in the S+ tail was lower than the S+ response in both groups (S+5: p<0.001, t6=−6.48; S+7: p<0.01, t6=−4.78). For both S+5 and S+7 groups, responses to S− did not differ significantly from S−1. However, the average response in the S− tail was significantly higher than S− in both groups (S+5: p<0.05, t6=−2.73; S+7: p<0.001, t6=−6.8).
(e) Comparing experiment 1 and 2
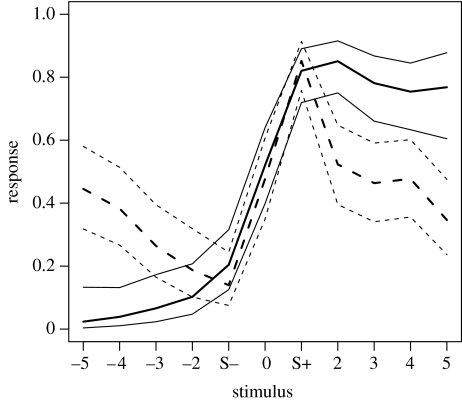
We compared the obtained generalization curves and their CIs of the S+ long and S+ short groups by redefining the stimuli, such that the stimulus intermediate to the S+ and S− was assigned the label 0, the S− the value −1 and the S+ the value +1. The other stimuli along the gradient were labelled according to their position relative to the S+ or S−: S+ tail all positive values and S− tail all negative values. This makes it possible to compare the shape of the generalization curves within and between the two experiments irrespective of which stimulus served as S+ and which as S−. The resulting curves of experiment 1 had overlapping CIs for all stimuli, except at stimulus +3 and +4, where there was a small difference. We then merged the datasets of both groups and fitted a common curve for experiment 1, using a λ of −6, and again 1000 bootstrap repeats (figure 3) on the 13 data points per stimulus.
Figure 3.
Generalization curves for the merged datasets of experiments 1 and 2. Solid thick line: curve for experiment 1. Solid thin lines: 95% CI for curve experiment 1. Dashed thick line: curve for experiment 2. Dashed thin lines: 95% CI for curve estimate of experiment 2.
Similarly, we compared the datasets of both groups in experiment 2. The CIs of the tails of the two groups now overlap, except at stimuli −5 and +2. We fitted a common curve for experiment 2 by merging the datasets, using a λ of −5, with 1000 bootstrap repeats (figure 3) on the 14 data points per stimulus.
The estimates of the curves for the combined datasets overlap for the S+, S−, the stimuli intermediate to these (stimulus 0) and the first two stimuli of the S− tail (stimuli −2 and −3). Otherwise, both S+ and S− tails are significantly different (figure 3).
4. Discussion
Our results show that the response to novel songs depends critically upon the song or songs about which the birds have learned before. With one exception, the flat generalization curves resulting from training on a single song indicate that control birds treat novel songs similar to familiar ones, without any skewed generalization. In contrast, if the birds are trained to discriminate between two different songs, there is a strong effect on the generalization curves. This effect differs strikingly between the two types of song contrasts.
When training songs differ in element number, most of the more extreme novel test songs (i.e. in the S− tails of both groups and the S+ tail in the S+ long group) give rise to stronger responses than familiar songs (i.e. S− or S+ songs). This is particularly striking for the responses to stimuli in the S− tail, for which the response does not change in the direction of the control curve, but continues to decline, thus demonstrating a peak shift. In spite of the higher complexity of the acoustic stimulus, this generalization pattern is very similar to that described by Ghirlanda & Enquist (2003) for stimuli differing in ‘intensity’, which give rise to more or less monotonic generalization patterns. In our experiment, ‘floor’ and ‘ceiling’ effects may have prevented the curves from being truly monotonic. This result suggests that element number is also perceived as an ‘intensity’ gradient. As higher sound intensities can give rise to monotonic curves (Pierrel & Sherman 1960; Thomas & Setzer 1972), more elements in a song might be perceived as a higher sound intensity, or as a song of longer duration.
The above pattern is quite different from the one obtained when the songs differ in element arrangement. Now, the training stimuli receive the strongest responses, with more extreme songs receiving significantly weaker responses. The overall pattern bears similarity to the generalization patterns that Ghirlanda & Enquist (2003) describe for rearrangement gradients. However, we did not obtain a shift towards more extreme stimuli. As such a shift depends on the magnitude of the contrast between the stimuli involved, and on the shape and slope of the generalization gradients (Purtle 1973; Ghirlanda & Enquist 2003), it might be that the contrast between our stimuli was too large to show peak shift.
From these results, we conclude that the generalization patterns of our training songs follow the same general rules as those of the more simple stimuli characteristic of most experiments on stimulus generalization. This includes the presence of skewed generalization in which particular novel songs get more extreme responses than familiar ones (S+ and S−). In contrast to a study by Cynx & Nottebohm (1992), in which males were better in performing acoustic discrimination than females, we obtained no sex differences.
How our findings translate to the generalizations of songs by zebra finches or any other birds under more natural conditions awaits further experiments. However, there is abundant evidence of learned song discrimination in birds. Discrimination learning occurs whenever there is a notable difference in reward associated with different stimuli. The way in which an individual is treated by its different neighbours or by individuals of its own and of another species might provide such different ‘rewards’ for song discrimination learning. Our results indicate that learning to discriminate different songs is likely to bias the responses to novel individuals singing novel songs. This bias may have evolutionary consequences, but will be a function of the differences between the songs encountered earlier, and not the result of selection for any adaptive benefit for a particular type of song. The song itself may be of neutral adaptive value, and whether the signal will be selected for or against will depend on the songs of other birds. If, for instance, familiar individuals vary in the number of elements in their songs in such a way that individuals having songs with a higher number of elements are preferred, longer songs may be treated as ‘super songs’. This may result in directional selection that may ultimately drive song evolution in the direction of longer songs. Hence, our results suggest that song evolution might be a profitable candidate area where signal evolution may be driven by ‘receiver psychology’ (Guilford & Dawkins 1991, 1993; Rowe & Skelhorn 2004).
Acknowledgments
We are very grateful to the biology workshop and, in particular, Rob van der Linden and Ab Gluvers for building and programming the skinner boxes and control units used in the experiments. We thank our colleagues and, in particular, Gabriel Beckers and Katharina Riebel for discussions and two anonymous referees for their constructive and helpful comments. The study was supported by grant 803-30-044 from NWO. The Leiden University Animal Experiments Committee approved the experimental protocols and animal care was according to institutional guidelines.
References
- Adret P. Operant conditioning, song learning and imprinting to taped song in the zebra finch. Anim. Behav. 1993;46:149–159. doi:10.1006/anbe.1993.1170 [Google Scholar]
- Beckers G.J.L, Goossens B.M.A, ten Cate C. Perceptual salience of acoustic differences between conspecifics and allospecific vocalizations in African collared-doves. Anim. Behav. 2003;65:605–614. doi:10.1006/anbe.2003.2080 [Google Scholar]
- Brooks R.J, Falls J.B. Individual song recognition in white-throated sparrows I. Discrimination of songs of neighbours and strangers. Can. J. Zool. 1975;53:879–888. [Google Scholar]
- Catchpole C.K, Slater P.J.B. Cambridge University Press; Cambridge, UK: 1995. Bird song: biological themes and variations. [Google Scholar]
- Cynx J, Nottebohm F. Role of gender, season and familiarity in discrimination of conspecific song by zebra finches (Taeniopygia guttata) Proc. Natl Acad. Sci. USA. 1992;89:1368–1371. doi: 10.1073/pnas.89.4.1368. doi:10.1073/pnas.89.4.1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmunds M. Longman; Harlow, UK: 1974. Defence in animals: a survey of anti-predator defences. [Google Scholar]
- Endler J.A, Basolo A.L. Sensory ecology, receiver biases and sexual selection. Trends Ecol. Evol. 1998;13:415–420. doi: 10.1016/s0169-5347(98)01471-2. doi:10.1016/S0169-5347(98)01471-2 [DOI] [PubMed] [Google Scholar]
- Gamberale G, Tullberg B.S. Evidence for a peak-shift in predator generalization among aposomatic prey. Proc. R. Soc. B. 1996;263:1329–1334. doi: 10.1098/rspb.1996.0195. [DOI] [PubMed] [Google Scholar]
- Ghirlanda S, Enquist M. A century of generalization. Anim. Behav. 2003;66:15–36. doi:10.1006/anbe.2003.2174 [Google Scholar]
- Guilford T, Dawkins M.S. Receiver psychology and the evolution of animal signals. Anim. Behav. 1991;42:1–14. doi:10.1016/S0003-3472(05)80600-1 [Google Scholar]
- Guilford T.C, Dawkins M.S. Receiver psychology and the design of animal signals. Trends Neurosci. 1993;16:430–436. doi: 10.1016/0166-2236(93)90068-w. doi:10.1016/0166-2236(93)90068-W [DOI] [PubMed] [Google Scholar]
- Houx B.B, ten Cate C. Song learning from playback in zebra finches: is there an effect of operant contingency? Anim. Behav. 1999;57:837–845. doi: 10.1006/anbe.1998.1046. doi:10.1006/anbe.1998.1046 [DOI] [PubMed] [Google Scholar]
- Lewis M.B, Johnston R.A. Are caricatures special? Evidence of peak shift in face recognition. Eur. J. Cogn. Psychol. 1999;11:105–117. [Google Scholar]
- Mackintosh N.J. Categorization by people and pigeons: the twenty-second Bartlett memorial lecture. Q. J. Exp. Psychol. B Comp. Physiol. Psychol. 1995;48:193–214. [Google Scholar]
- McLaren I.P.L, Mackintosh N.J. Associative learning and elemental stimulus representation: II. Generalization and discrimination. Anim. Learn. Behav. 2002;30:177–200. doi: 10.3758/bf03192828. [DOI] [PubMed] [Google Scholar]
- Miller D.B. Long-term recognition of father's song by female zebra finches. Nature. 1979;280:389–391. doi:10.1038/280389a0 [Google Scholar]
- Nelson D.A, Soha J.A. Perception of geographical variation in song by male Puget Sound white-crowned sparrows, Zonotrichia leucophrys pugetensis. Anim. Behav. 2004;68:395–405. doi:10.1016/j.anbehav.2003.08.027 [Google Scholar]
- O'Loghlen A.L, Beecher M.D. Mate, neighbour and stranger songs: a female song sparrow perspective. Anim. Behav. 1999;58:13–20. doi: 10.1006/anbe.1999.1125. doi:10.1006/anbe.1999.1125 [DOI] [PubMed] [Google Scholar]
- Pierrel R, Sherman J.G. Generalization of auditory intensity following discrimination training. J. Exp. Anal. Behav. 1960;3:313–322. doi: 10.1901/jeab.1960.3-313. doi:10.1901/jeab.1960.3-313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purtle R.B. Peak shift: a review. Psychol. Bull. 1973;80:408–421. doi:10.1037/h0035233 [Google Scholar]
- R Development Core Team, 2005 R foundation for statistical computing, Vienna, Austria.
- Riebel K. The “mute” sex revisited; vocal production and perception learning in female songbirds. Adv. Stud. Behav. 2003;33:49–86. [Google Scholar]
- Riebel K, Smallegange I.M, Terpstra N.J, Bolhuis J.J. Sexual equality in zebra finch song preference: evidence for a dissociation between song recognition and production learning. Proc. R. Soc. B. 2002;269:729–733. doi: 10.1098/rspb.2001.1930. doi:10.1098/rspb.2001.1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe C, Skelhorn J. Avian psychology and communication. Proc. R. Soc. B. 2004;271:1435–1442. doi: 10.1098/rspb.2004.2753. doi:10.1098/rspb.2003.2615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan M.J. Sexual selection, receiver biases, and the evolution of sex differences. Science. 1998;281:1999–2003. doi: 10.1126/science.281.5385.1999. doi:10.1126/science.281.5385.1999 [DOI] [PubMed] [Google Scholar]
- Schluter D. Estimating the form of natural selection on a quantitative trait. Evolution. 1988;42:849–861. doi: 10.1111/j.1558-5646.1988.tb02507.x. doi:10.2307/2408904 [DOI] [PubMed] [Google Scholar]
- Slater P.J.B, Eales L.A, Clayton N.S. Song learning in zebra finches: progress and prospects. Adv. Stud. Behav. 1988;18:1–34. [Google Scholar]
- Spetch M.L, Cheng K, Clifford C.W.G. Peak shift but not range effects in recognition of faces. Learn. Motiv. 2004;35:221–241. doi:10.1016/j.lmot.2003.11.001 [Google Scholar]
- Stripling R, Milewski L, Kruse A.A, Clayton D.F. Rapidly learned song-discrimination without behavioral reinforcement in adult male zebra finch (Taeniopygia guttata) Neurobiol. Learn. Mem. 2003;79:41–50. doi: 10.1016/s1074-7427(02)00005-9. doi:10.1016/S1074-7427(02)00005-9 [DOI] [PubMed] [Google Scholar]
- Sturdy C.B, Philmore L.S, Price J.L, Weisman R.G. Song-note discriminations in zebra finches (Taeniopygia guttata): categories and pseudocategories. J. Comp. Psychol. 1999;113:333–336. doi:10.1037/0735-7036.113.3.333 [Google Scholar]
- ten Cate C, Verzijden M.N, Etman E. Sexual imprinting can induce sexual preferences for exaggerated parental traits. Curr. Biol. 2006;16:1128–1132. doi: 10.1016/j.cub.2006.03.068. doi:10.1016/j.cub.2006.03.068 [DOI] [PubMed] [Google Scholar]
- Thomas D.R, Setzer J. Stimulus generalization gradients for auditory intensity in rats and guinea pigs. Psychon. Sci. 1972;28:22–24. [Google Scholar]
- Thomas D.R, Mood K, Morrison S, Wierletak E. Peak shift revisited: a test of alternative interpretations. J. Exp. Psychol. Anim. Behav. Process. 1991;17:130–140. doi: 10.1037//0097-7403.17.2.130. doi:10.1037/0097-7403.17.2.130 [DOI] [PubMed] [Google Scholar]
- Weary D.M. Categorization of song notes in great tits: which acoustic features are used and why? Anim. Behav. 1990;39:450–457. doi:10.1016/S0003-3472(05)80408-7 [Google Scholar]