Abstract
Males of many species adjust their reproductive behaviour according to the perceived risk of sperm competition. Although this phenomenon is widespread in insects and other animals, the mechanisms that allow mates to assess sperm competition levels remain largely unexplored. In this study, we analysed the mating behaviour of pairs of Tenebrio molitor beetles under three odour treatments representing increasing levels of sperm competition risk (SCR) and sperm competition intensity (SCI). Copula duration and male and female post-copulatory behaviour varied significantly with odour treatment. Both copula duration and post-copulatory associations (PCAs) increased significantly in odour treatments reflecting high male density. To our knowledge, this is the first study to report that insects may assess the actual density of potential competitors at the time of mating, a cue to SCR and SCI, on the basis of chemical cues. In T. molitor, males inhibit sperm release from the spermatophore of a rival male when remating takes place at short intervals. We show that, when sperm competition levels are high, PCAs increase female remating interval just above that necessary to prevent spermatophore inhibition by rival males. This finding strongly suggests that strategic male behaviour plays a ‘spermatophore guarding’ role in this species. Although common in insects with external spermatophore transfer, spermatophore guarding is not expected in species with rapid ejaculate transfer and internal spermatophore delivery. Our results reveal that spermatophore guarding may evolve, even under these circumstances, as an evolutionary response to short-term spermatophore inhibition or displacement mechanisms.
Keywords: sperm competition, mate guarding, chemical communication, Tenebrio molitor
1. Introduction
Parker's landmark paper (1970) on sperm competition introduced a powerful notion that delved deeply into Darwin's (1871) original view of sexual selection: sexual selection does not end with a male's access to a mating partner but is extended when the sperm from two or more males compete for the fertilization of a given set of ova (Parker 1998). Since then, sperm competition has been increasingly present in sexual selection studies and is presently recognized as one of its fundamental mechanisms (Thornhill & Alcock 1983; Smith 1984; Andersson 1994; Birkhead & Møller 1998; Simmons 2001). Its study has paved the way for understanding a host of male morphological, physiological and behavioural traits, and has shed light on some of the selection pressures that have shaped these adaptations (e.g. Birkhead & Møller 1998; Simmons 2001). Recent empirical and theoretical work has focused on the study of strategic reproductive behaviours that have evolved as a response to varying levels in sperm competition risk (SCR; the probability that a male's sperm will compete against the sperm of other males) and sperm competition intensity (SCI; average number of competing ejaculates; e.g. Parker et al. 1996, 1997; Parker 1998; Wedell et al. 2002; Engqvist & Reinhold 2005).
One of the main predictions arising from sperm competition theory is that changes in sperm competition levels (i.e. SCR and SCI) should favour strategic male allocation of sperm and post-copulatory mate guarding (Simmons 2001). This prediction rests on the assumption that males are somehow able to assess sperm competition levels, which may be accomplished in two ways. First, males may directly determine the risks from past matings by detecting whether a female has recently mated with other males, for example, by assessing the presence of semen in her reproductive tract (e.g. Cook & Gage 1995; Siva-Jothy & Stutt 2003). Second, males may assess the probability that a female will engage in future matings after mating with him. Several studies with insects have in fact shown that males achieve this by assessing either male density or the operational sex ratio at the time of mating (e.g. Gage 1991; Simmons 2001), but the stimuli involved remain unknown.
Tenebrio molitor is a highly polygynandrous beetle that has evolved several strategies in response to an evolutionary history of intense sperm competition (e.g. Happ 1969; Siva-Jothy et al. 1996; Drnevich et al. 2000; Griffith 2001; Drnevich 2003; Carazo et al. 2004). For example, males are capable of assessing the volume of sperm a female contains due to previous matings and adjust the volume of their ejaculate accordingly (i.e. sperm dilution; Drnevich et al. 2000; Drnevich 2003). As a consequence, T. molitor males have evolved the ability to respond to sperm dilution by increasing sperm transfer when mating in the presence of a rival male (Gage & Baker 1991). Similarly, when remating takes place at short intervals, T. molitor males are capable of preventing sperm release from the spermatophore of a rival male (i.e. spermatophore inhibition) and achieve near complete sperm precedence (Drnevich et al. 2000). In response to spermatophore inhibition, males have evolved a short-term anti-aphrodisiac that increases remating intervals by decreasing long-range female attractiveness (Happ 1969; Griffith 2001; Seybold & Vanderwel 2003). However, this anti-aphrodisiac does not prevent matings once a female encounters another male, and is probably only effective when male densities are low (Griffith 2001; Drnevich 2003). Thus, the risk of spermatophore inhibition is highest when male density is high (i.e. high SCI), precisely when theoretical models predict that sperm expenditure should be low (Parker et al. 1996; Parker 1998; Wedell et al. 2002; Engqvist & Reinhold 2005). Under these circumstances, male assessment of rival density at mating is crucial as it will not only reflect SCR and intensity levels, but also prevailing sperm competition sources that may require specific behavioural responses on the part of the male.
Out of several possible sources of information that T. molitor beetles may use to assess sperm competition levels, chemical cues seem the most obvious. Chemical cues play a major role in the reproductive behaviour of many insect species (Wyatt 2003), and can relay more subtle information than was hitherto recognized (e.g. Simmons 1989; Thornhill 1992; Moore et al. 1997; Bateman 1998). In particular, T. molitor relies heavily on chemical cues and exhibits an elaborate chemical communication system that does not merely function to attract mates (Tschinkel et al. 1967; Tanaka et al. 1986, 1989; Happ 1969; Happ & Wheeler 1969), but can convey complex information such as parasite load (Worden et al. 2000), condition (Rantala et al. 2003) and immunocompetence (Rantala et al. 2002). Furthermore, males of this species use chemical cues to assess SCR by detecting female reproductive status (Carazo et al. 2004). There is thus consistent theoretical and empirical evidence to suspect that male T. molitor may respond to increasing levels of sperm competition, and that this response may be mediated by chemical cues. We set to test these hypotheses by studying mating and post-copulatory behaviour in three different mating contexts: in the absence of odour cues from other males (low SCR), in the presence of odour cues from one male (high SCR but low SCI) and in the presence of odour cues from 10 males (certain SCR and high SCI).
2. Material and methods
(a) Beetle culture
All beetles used in this study originated from stock cultures maintained in our laboratory. These cultures have been running for more than 10 years, with regular contributions from other cultures and wild stock. All growth stages are kept together in plastic containers with a rearing medium consisting of white flour and wheat bran to which chunks of fruit, bread and various vegetables are added periodically. The surface of the culture is covered with filter paper, which is sprayed with water for moisture on a daily basis. All containers are stored in well-ventilated dark places, at ambient humidity and under temperature-controlled conditions.
Subjects used in our experiments were haphazardly collected from the stock cultures and sexed as pupae by examining the developing genitalia on the ventral side of the eighth abdominal segment (Bhattacharya et al. 1970). Individuals were examined under a dissecting microscope both as pupae and after eclosion, and beetles with obvious malformations were discarded. Sexed adults of the same age were kept separately in plastic containers measuring approximately 15 (height)×13×20 cm, until used in the experiments. Plastic containers were conditioned and maintained in the same way as stock cultures. All males and females used were at least 10 days post-eclosion at the beginning of the experiments. Before each trial, experimental males were given a 24 h mating period with a non-experimental female to ensure adequate motivation (Carazo et al. 2004) and to control male perception of mean SCR and intensity prior to testing (Engqvist & Reinhold 2005). Trials were conducted at a temperature of 22–25°C, at ambient humidity and under dim red light. All experimental females used were virgin, and all subjects took part in only one test.
(b) Experimental procedure
To determine whether T. molitor beetles use the odour cues of other males to assess sperm competition levels, we studied the reproductive behaviour of mating pairs that were randomly allocated to one of the three odour treatments reflecting increasing male density: (i) no odour cues, simulating low SCR (i.e. ‘no male’ treatment; n=22); (ii) odour cues left by one male, simulating high SCR but low SCI (i.e. ‘one male’ treatment; n=20); and (iii) odour cues left by 10 males, simulating certainty of SCR and high SCI (i.e. ‘10 males’ treatment; n=21). Experimental and donor beetles were haphazardly allocated to different treatments, and were thus of approximately the same age when tested (10–30 days old). All tests were conducted in a trial arena consisting of a Petri dish (radius 5.5 cm) inverted over a circular piece of filter paper bearing one of the three experimental treatments. Odours were collected by introducing male donors in a Petri dish lined with filter paper, and leaving them to mark for a 24 h period prior to the trial. The filter paper was then removed and placed, odour-side up, on a clean surface with a Petri dish inverted on top of it. An experimental male was placed in the centre of the arena for a 5 min habituation period during which males had access to the chemically laden substrate. At the end of the habituation period, an experimental female was placed in the arena and the trial began. If the experimental male failed to initiate courtship within 10 min, the trial was abandoned. We used a laptop computer equipped with event-recording software (JWatcher v. 0.9, Blumstein et al. 2000) to record the following behaviours:
Courtship. Begins with the male rapidly tapping the female with its antennae in a rhythmic way (i.e. tattoo). The male then climbs on top of the female making rapid forward–backward scraping movements with its prothoracic legs against the female's side and then proceeds to move its copulatory organ across the female's rear end until achieving intromission (end of courtship). Tattoo typically continues through courtship and only ends with the onset of copulation.
Copulation. The female lowers her last abdominal sternite and the male introduces the copulatory organ. The pair remain attached by the genitalia for a variable length of time.
Post-copulatory association (PCA). After withdrawing his copulatory organ, the male remains on top of the female and/or dismounts the female and stays immediately adjacent to (i.e. less than 1 cm apart) and usually in direct physical contact with her. PCA typically occurs in bouts that are interrupted by periods in which the members of the pair briefly lose contact with each other. Consequently, the duration of total PCA is difficult to measure and thus our operational measure of PCA was restricted to the first bout. Even though this measure is bound to underestimate actual PCA, it is an objective conservative measure that correlates strongly with overall PCA (E. Font, personal observation).
Tenebrio molitor females typically respond to PCA by remaining quiescent, but eventually begin to move after a variable period of time. It is not clear whether the end of PCA may be caused by female behaviour. Thus, we measured the time from the beginning of PCA (i.e. end of copula) to the occurrence of a female's first movement (FFM), both as an estimate of the degree of female acceptance towards PCA (e.g. Jablonski & Vepsäläinen 1995) and to control any possible effects of female behaviour over our estimate of PCA. FFM sample size was reduced owing to missing data (i.e. ‘no male’, n=19; ‘one male’, n=21; ‘10 males’, n=18). Trials were terminated at the end of PCA.
(c) The effect of post-copulatory association on female remating interval
Whether PCA affects female remating interval is an unresolved issue in T. molitor (e.g. Drnevich 2003). To function as a spermatophore guarding strategy, PCA should significantly increase female remating interval. As fitness returns of guarding are context dependent, the increase in female remating interval should be especially clear when sperm competition levels are high. We set to test this hypothesis by allowing virgin females to mate with a male, in the presence of odour cues from 10 rival males, and measuring subsequent female remating interval under three different post-mating contexts: (i) in the presence of the guarding male and one introduced male (i.e. ‘mate guarding’ treatment, n=10); (ii) no guarding male and one introduced male (i.e. ‘no guarding, one male’, n=10); and (iii) no guarding male and two introduced males (i.e. ‘no guarding, two males’, n=10). This last treatment was included to ensure that PCA effects over female remating time were not owing to the mere presence of two males that may compete with each other to achieve a mating and thus increase female remating time. We placed experimental females in a mating arena consisting of a Petri dish (radius 5.5 cm) inverted over a piece of filter paper bearing odour cues from 10 males. We made a hole in the inverted Petri dish (radius 3.5 cm) through which we could introduce a smaller Petri dish (radius 2.5 cm). This allowed us to cover the mating pair, once copulation had started, and to introduce one or two extra males in the intervening space delimited by the small and large Petri dishes. Immediately after the end of a copula, we removed the small Petri dish separating the mating pair from the introduced male(s) and either removed the mating male or left it to guard the female until the female remated with an introduced male.
(d) Data analysis
graphical exploration of our data variables indicated that we could not safely assume normality and homogeneity of variance owing to the presence of outliers. Thus, we rank transformed our data and performed a one-way robust ANOVA using SPSS v. 12.0. We graphically explored transformed data and conducted Levene's test to check for normality and homogeneity of variance after rank transformation (Quinn & Keough 2003). Wherever significant treatment effects were found, we conducted post hoc pairwise multiple comparison of group means using Tukey's HSD test. Significance level for rejection of the null hypothesis was set at α=0.05. All reported probabilities are two-tailed.
3. Results
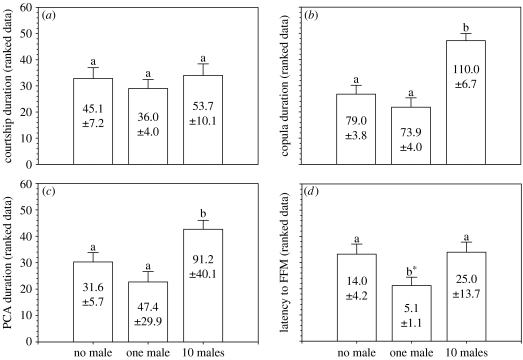
Levene's test confirmed that homoscedasticity could be safely assumed after rank transformation (courtship duration: F2,60=1.947, p=0.152; copula duration: F2,60=0.992, p=0.377; PCA: F2,60=0.208, p=0.813; and FFM: F2,57=0.869, p=0.425). Results of the robust ANOVA showed a highly significant treatment effect on copula duration (F2,60=17.085, p<0.001) and PCA duration (F2,60=7.522, p=0.001), but not on courtship duration (F2,60=0.406, p=0.668). Multiple pairwise comparisons (figure 1) revealed that there were no significant differences between the ‘no male’ and ‘one male’ treatment conditions for copula duration (Tukey's HSD, p=0.525) or PCA duration (Tukey's HSD, p=0.309), but comparisons between these two treatments and the ‘10 males’ condition were highly significant for both copula duration (Tukey's HSD, ‘no male’ versus ‘10 males’, p<0.001; ‘one male’ versus ‘10 males’, p<0.001) and PCA duration (Tukey's HSD, ‘no male’ versus ‘10 males’, p=0.047; ‘one male’ versus ‘10 males’, p=0.001). The latency to FFM during PCA was also significantly affected by treatment condition (F2,55=3.745, p=0.030). FFM was lower in the ‘one male’ than in the other treatments. Post hoc comparisons revealed that these differences were marginally non-significant (Tukey's HSD, ‘no male’ versus ‘one male’, p=0.058; ‘10 males’ versus ‘one male’, p=0.051), but a post hoc trend analysis (Quinn & Keough 2003) confirmed the existence of a significant quadratic trend across the three treatments (F2,57=6092, p=0.017; figure 1).
Figure 1.
(a) Courtship duration, (b) copula duration, (c) duration of PCA and (d) latency to a FFM in each of the three treatments: ‘no male’ (i.e. low SCR), ‘one male’ (i.e. high SCR) and ‘10 males’ (i.e. certain SCR and high SCI). Bar plots show data after rank transformation, as used in statistical analyses, but descriptive statistics of the raw data (, s) are provided within each bar. Bars with different letters have statistically significant differences (α<0.05) except in (d), where significant differences were detected by a post hoc trend analysis.
Homoscedasticity of data gathered in the female remating interval experiment was also confirmed by Levene's test (F2,30=0.845, p=0.441). The robust ANOVA revealed a highly significant treatment effect on female remating interval (F2,30=13.783, p<0.001), and multiple pairwise comparisons showed that average female remating time was significantly higher in the ‘mate guarding’ treatment than in either of the two treatments where male guarding was not allowed (Tukey's HSD, ‘male guarding’ versus ‘no guarding, one male’, p<0.001; ‘male guarding’ versus ‘no guarding, two males’, p<0.001, and ‘no guarding, one male’ versus ‘no guarding, two males’, p=0.999; figure 2).
Figure 2.
Female remating interval in each of the three post-mating treatments: ‘male guarding’, ‘no guarding, one male’ and ‘no guarding, two males’. Bar plots show data after rank transformation, as used in statistical analyses, but descriptive statistics of the raw data (, s) are provided within each bar. Bars with different letters have statistically significant differences (p<0.001).
4. Discussion
(a) Chemical assessment of sperm competition risk
Male T. molitor beetles exhibited significantly longer copulas and PCAs in the ‘10 males’ than in the ‘no male’ or ‘one male’ treatments, which shows that males are able to assess male density, a cue to future SCR and intensity, on the basis of odours left in the substrate by other males. In meadow voles, males increase their sperm investment when they mate in the presence of another male's odour (delBarco-Trillo & Ferkin 2004) and, in insects, Harris & Moore (2005) recently reported that the presence of conspecific odours during sexual development in Nauphoeta cinerea males triggers an ontogenetic adjustment of resources allocated to ejaculate components. Compared with the detection of conspecific presence, the chemosensory assessment of male density at mating is much more specific and potentially informative. It not only allows the transfer of information regarding the degree of SCR, but may also allow males to derive information about SCI, which requires the ability to assess specific male density. Furthermore, our results suggest that female latency to first movement (FFM) varied significantly across treatments, which offers the first evidence to suggest that females may also be able to chemically assess sperm competition levels. The fact that FFM and PCA treatment effects follow different trends (figure 1) rules out the possibility that PCA duration effects were owing to female rejective behaviour.
The actual chemical stimuli involved in the assessment of sperm competition levels remain unknown. Previous studies have revealed the existence of multiple pheromones in T. molitor (August 1971; Tanaka et al. 1986, 1989), including a male-produced sex pheromone recently identified as (Z)-3-dodecenyl acetate (Bryning et al. 2005), which appears to be a good candidate compound. While the function of this male-specific pheromone is to attract females (Bryning et al. 2005), it is widely available to other males that may acquire gross information about male density by detecting its relative concentration. Tenebrio molitor females are sensitive to increasing concentrations of the male pheromone (August 1971; Rantala et al. 2003), and it seems likely that males may also be capable of such discrimination. An alternative mechanism involving individually distinct signature odours (e.g. Moore et al. 1997) would allow males and females to identify individuals, and thus draw more accurate information regarding male density. Irrespective of the actual chemical stimuli involved, males are not likely to benefit by communicating their presence to other rivals. Thus, male assessment of sperm competition levels appears to be a case of intraspecific ‘interceptive eavesdropping’ (Peake 2005), whereby males would be using chemical compounds directed at other receivers, probably females, to detect male density.
(b) The evolution of strategic internal spermatophore guarding
In insect species with indirect sperm transfer, sperm is packed in a spermatophore that is either externally attached to the female's genital opening or introduced into her bursa copulatrix. Sperm transfer is not immediate in these species, and mate guarding has been suggested to function as a mechanism to guard sperm until it is released from the spermatophore into the female (i.e. spermatophore guarding). Spermatophore guarding is relatively common in insect species with external spermatophores (e.g. Orthoptera; Alcock 1994; Simmons 2001), but supposedly absent in species with internal spermatophores and rapid sperm release (Simmons 2001). In T. molitor, sperm transfer is an indirect two-step process. During copulation, males fill and transfer an invaginated spermatophore to the female's bursa copulatrix, where it will undergo eversion and finally burst to release sperm to the bursa 7–10 min after the end of copulation (Gadzama & Happ 1974). This 7–10 min period is crucial because rival males are able to inhibit sperm release from the spermatophore, thereby achieving near complete sperm precedence, when remating takes place before sperm release (Drnevich et al. 2000). Although this time window is very short, the probability of spermatophore inhibition increases with male density and its associated costs are very high. Thus, it seems likely that PCA and extended copulas may have evolved as a way to ensure complete sperm transfer when male density is high. Our results fit nicely with this hypothesis as: (i) both copula and PCA duration were significantly longer only when SCI was high, and (ii) the time devoted to extended copula and PCA suggests that guarding is aimed to avoid a short-term sperm competition mechanism. Furthermore, our results also show that, in T. molitor, PCA significantly increases female remating interval when SCR and intensity are high (i.e. ‘10 males’ treatment). Interestingly, mate guarding increased female remating interval just above 10 min (, 724.6±162.36 s), exactly what would be expected if the function of guarding was to allow complete sperm transfer to the bursa (i.e. 7–10 min; Drnevich et al. 2000). Altogether, our results paint a coherent picture that strongly suggests that PCA functions as an effective spermatophore guarding strategy in this species. We thus offer a plausible scenario for the evolution of internal spermatophore guarding in insects.
Alternative explanations, such as mate guarding, strategic sperm allocation or post-copulatory courtship mediating cryptic female choice (e.g. Alcock 1994; Birkhead & Møller 1998; Simmons & Siva-Jothy 1998; Simmons 2001), do not seem to explain our results. It is very unlikely that prolonged copulas and PCA could serve to guard females until oviposition because average second male sperm precedence in T. molitor is not strong (Drnevich et al. 2000) and the encounter rate with receptive females is very low in relation to the time from insemination to oviposition, which takes place deep in the grain pile hours after mating (Gerber & Sabourin 1984; Drnevich 2003). Furthermore, average PCA is far shorter than would be necessary for successfully guarding a female until oviposition (e.g. Font & Desfilis 2003; present study). Although prolonged copula duration, but not extended PCA, can be caused by an increase in the amount of sperm transfer (i.e. strategic sperm allocation), available theoretical and empirical evidence fails to support this possibility. Sperm competition theory predicts that sperm allocation should increase at high SCR levels until sperm competition is certain, but then drops consistently when a male has to face more than one competing ejaculate at high SCI levels (Parker et al. 1996; Parker 1998; Wedell et al. 2002; Engqvist & Reinhold 2005). Therefore, if extended copula duration was caused by an increase in the amount of sperm transferred to the female during mating, we would expect longer copula durations in the ‘one male’ treatment (Gage & Baker 1991), not in the ‘10 males’ treatment. Furthermore, Gage & Baker (1991) found no relationship between copula duration and the amount of sperm transferred in T. molitor, which seems to refute this hypothesis. Finally, prolonged copula duration and/or PCA could be interpreted as a form of copulatory courtship mediating cryptic female choice (Eberhard 1996), but this seems unlikely. All our experimental males were extracted from the same population, reared in the same conditions, and similar in size and age upon testing. We did not find differences in courtship duration across treatments even though courtship duration mediates cryptic female choice in Tribolium castaneum, a closely related species with a courtship behaviour which resembles that of T. molitor (Edvardsson & Arnqvist 2000). Additionally, our results suggest that females were more reluctant to accept male guarding behaviour in the ‘one male’ treatment than in the ‘no male’ and ‘10 males’ treatments, which does not seem to fit the cryptic female choice hypothesis (Eberhard 1996). Female T. molitor are highly promiscuous, gain multiple benefits from mating multiply with different males and, lacking a refractory period, can mate with several males in a row (Drnevich et al. 2001; Worden & Parker 2001; Drnevich 2003). Hence, one possible explanation for female behaviour is that, upon detecting the presence of another male and in a situation where the risk of spermatophore inhibition is low, a female may gain more by searching for future matings than by staying with a recent mate.
To sum up, we show that T. molitor beetles assess immediate SCR and intensity levels (i.e. male density) by inspecting chemical cues left in the substrate by other males. Our results suggest that T. molitor males not only exhibit strategic sperm competition avoidance behaviours (i.e. strategic sperm allocation and spermatophore guarding), but that their choice of behaviour may also vary according to prevailing sources of sperm competition (i.e. sperm dilution versus spermatophore inhibition). The existence of a trade-off between investment in sperm production and mate guarding, as suggested by recent empirical and theoretical studies (e.g. Warner et al. 1995; Alonzo & Warner 2000), would favour a shift from sperm allocation to spermatophore guarding strategies at high SCI levels. Future research should address this issue and explore the evolution of behavioural plasticity as a response to multiple sperm competition sources and avoidance mechanisms under different sociosexual contexts. We also show that extended copula and PCA duration seem to function as strategic spermatophore guarding strategies in T. molitor, a species with internal spermatophore delivery and rapid ejaculate transfer. We suggest that, in the face of short-term spermatophore inhibition or displacement mechanisms, spermatophore guarding may evolve even in species with internal spermatophore delivery and rapid ejaculate transfer, where it has traditionally not been expected.
Acknowledgments
We thank two anonymous referees, whose comments and criticisms were invaluable to improving our manuscript. We also wish to thank Carlos Sampedro for his help in the maintenance of insect cultures. P.C. was supported by a research grant (FPU) from the Ministerio de Educación y Ciencia of Spain.
References
- Alcock J. Postinsemination associations between males and females in insects: the mate-guarding hypothesis. Annu. Rev. Entomol. 1994;39:1–21. [Google Scholar]
- Alonzo S.H, Warner R.R. Allocation to mate guarding or increased sperm production in a Mediterranean wrasse. Am. Nat. 2000;56:266–275. doi: 10.1086/303391. doi:10.1086/303391 [DOI] [PubMed] [Google Scholar]
- Andersson M. Princeton MBE; Princeton, NJ: 1994. Sexual selection. [Google Scholar]
- August C.J. The role of male and female pheromones in the mating behaviour of Tenebrio molitor. J. Insect Physiol. 1971;17:739–751. doi:10.1016/0022-1910(71)90120-X [Google Scholar]
- Bateman P.W. Mate preference for novel partners in the cricket Gryllus bimaculatus. Ecol. Entomol. 1998;23:473–475. doi:10.1046/j.1365-2311.1998.00156.x [Google Scholar]
- Bhattacharya A.K, Ameel J.J, Waldbauer G.P. A method for sexing living pupal and adult yellow mealworms. Ann. Entomol. Soc. Am. 1970;63:1783. [Google Scholar]
- Birkhead T.R, Møller A.P. Academic Press; London, UK: 1998. Sperm competition and sexual selection. [Google Scholar]
- Blumstein, D. T., Evans, C. S. & Daniel, J. C. 2000 J Watcher 0.9: an introductory User's Guide. http://www.jwatcher.ucla.edu and http://galliform.psy.mq.edu.au/jwatcher
- Bryning G.P, Chambers J, Wakefield M.E. Identification of a sex pheromone from male yellow mealworm beetles, Tenebrio molitor. J. Chem. Ecol. 2005;31:2721–2730. doi: 10.1007/s10886-005-7622-x. doi:10.1007/s10886-005-7622-x [DOI] [PubMed] [Google Scholar]
- Carazo P, Sanchez E, Font E, Desfilis E. Chemosensory cues allow male Tenebrio molitor beetles to assess the reproductive status of potential mates. Anim. Behav. 2004;68:123–129. doi:10.1016/j.anbehav.2003.10.014 [Google Scholar]
- Cook P.A, Gage M.J.G. Effects of risk of sperm competition on the numbers of eupyrene and apyrene sperm ejaculated by the moth Plodia interpunctella (Lepidoptera: Pyralidae) Behav. Ecol. Sociobiol. 1995;36:261–268. [Google Scholar]
- Darwin C. D. Appleton and Company; New York, NY: 1871. The descent of man and selection in relation to sex. [Google Scholar]
- delBarco-Trillo J, Ferkin M.H. Male mammals respond to a risk of sperm competition conveyed by odours of conspecific males. Nature. 2004;431:446–449. doi: 10.1038/nature02845. doi:10.1038/nature02845 [DOI] [PubMed] [Google Scholar]
- Drnevich J.M. Number of mating males and mating interval affect last-male sperm precedence in Tenebrio molitor L. Anim. Behav. 2003;66:349–357. doi:10.1006/anbe.2003.2219 [Google Scholar]
- Drnevich J.M, Hayes E.F, Rutowski R.L. Sperm precedence, mating interval, and a novel mechanism of paternity bias in a beetle (Tenebrio molitor L.) Behav. Ecol. Sociobiol. 2000;48:447–451. doi:10.1007/s002650000257 [Google Scholar]
- Drnevich J.M, Papke R.S, Rauser C.L, Rutowski R.L. Material benefits from multiple mating in female mealworm beetles (Tenebrio molitor L.) J. Insect Behav. 2001;14:215–230. doi:10.1023/A:1007889712054 [Google Scholar]
- Eberhard W.G. Princeton University Press; Princeton, NJ: 1996. Female control: sexual selection by cryptic female choice. [Google Scholar]
- Edvardsson M, Arnqvist G. Copulatory courtship and cryptic female choice in red flour beetles Tribolium castaneum. Proc. R. Soc. B. 2000;267:559–563. doi: 10.1098/rspb.2000.1037. doi:10.1098/rspb.2000.1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engqvist L, Reinhold K. Pitfalls in experiments testing predictions from sperm competition theory. J. Evol. Biol. 2005;18:116–123. doi: 10.1111/j.1420-9101.2004.00792.x. doi:10.1111/j.1420-9101.2004.00792.x [DOI] [PubMed] [Google Scholar]
- Font E, Desfilis E. Courtship, mating, and sex pheromones in the mealworm beetle (Tenebrio molitor) In: Ploger B.J, Yasukawa K, editors. Exploring animal behaviour in laboratory and field. Academic Press; New York, NY: 2003. pp. 43–58. [Google Scholar]
- Gadzama N.M, Happ G.M. The structure and evacuation of the spermatophore of Tenebrio molitor L. (Coleoptera: Tenebrionidae) Tissue Cell. 1974;6:95–108. doi: 10.1016/0040-8166(74)90025-1. doi:10.1016/0040-8166(74)90025-1 [DOI] [PubMed] [Google Scholar]
- Gage M.J.G. Risk of sperm competition directly affects ejaculate size in the Mediterranean fruit fly. Anim. Behav. 1991;42:1036–1037. doi:10.1016/S0003-3472(05)80162-9 [Google Scholar]
- Gage M.J.G, Baker R.R. Ejaculate size varies with socio-sexual situation in an insect. Ecol. Entomol. 1991;16:331–337. [Google Scholar]
- Gerber G.H, Sabourin D.U. Oviposition site selection in Tenebrio molitor. Can. Ent. 1984;116:27–39. [Google Scholar]
- Griffith, O. L. 2001 The effect of mating on the pheromone system of the yellow mealworm beetle, Tenebrio molitor Honours Thesis, University of Winnipeg.
- Happ G.M. Multiple sex pheromones of the mealworm beetle, Tenebrio molitor L. Nature. 1969;222:180–181. doi: 10.1038/222180a0. doi:10.1038/222180a0 [DOI] [PubMed] [Google Scholar]
- Happ G.M, Wheeler J. Bioassay, preliminary purification, and effect of age, crowding, and mating in the release of sex pheromone by female Tenebrio molitor. Ann. Entomol. Soc. Am. 1969;62:846–851. [Google Scholar]
- Harris W.E, Moore P.J. Sperm competition and male ejaculate investment in Nauphoeta cinerea: effects of social environment during development. J. Evol. Biol. 2005;18:474–480. doi: 10.1111/j.1420-9101.2004.00816.x. doi:10.1111/j.1420-9101.2004.00816.x [DOI] [PubMed] [Google Scholar]
- Jablonski P, Vepsäläinen K. Conflict between sexes in the water strider, Gerris lacustris: a test of two hypotheses for male guarding behavior. Behav. Ecol. 1995;6:388–392. doi:10.1093/ceheco/6.4.388 [Google Scholar]
- Moore P.J, Reagan-Wallin N.L, Haynes K.F, Moore A.J. Odour conveys status on cockroaches. Nature. 1997;389:25. doi:10.1038/37888 [Google Scholar]
- Parker G.A. Sperm competition and its evolutionary consequences in the insects. Biol. Rev. 1970;45:525–567. [Google Scholar]
- Parker G.A. Sperm competition and the evolution of ejaculates: towards a theory base. In: Birkhead T.R, Møller A.P, editors. Sperm competition and sexual selection. Academic Press; San Diego, CA: 1998. pp. 3–54. [Google Scholar]
- Parker G.A, Ball M.A, Stockley P, Gage M.J.G. Sperm competition games: individual assessment of sperm competition intensity by group spawners. Proc. R. Soc. B. 1996;263:1291–1297. [Google Scholar]
- Parker G.A, Ball M.A, Stockley P, Gage M.J.G. Sperm competition games: a prospective analysis of risk assessment. Proc. R. Soc. B. 1997;264:1793–1802. doi: 10.1098/rspb.1997.0249. doi:10.1098/rspb.1997.0249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peake T.M. Eavesdropping in communication networks. In: Mcgregor P.K, editor. Animal communication networks. Cambridge University Press; Cambridge, UK: 2005. pp. 13–37. [Google Scholar]
- Quinn G.P, Keough M.J. 1st edn. Cambridge University Press; Cambridge, UK: 2003. Experimental design and data analysis for biologists. [Google Scholar]
- Rantala M.J, Jokinen I, Kortet R, Vainikka A, Suhonen J. Do pheromones reveal male immunocompetence? Proc. R. Soc. B. 2002;269:1681–1685. doi: 10.1098/rspb.2002.2056. doi:10.1098/rspb.2002.2056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rantala M.J, Kortet R, Kotiaho J.S, Vainikka A, Suhonen J. Condition dependence of pheromones and immune function in the grain beetle Tenebrio molitor. Funct. Ecol. 2003;17:534–540. doi:10.1046/j.1365-2435.2003.00764.x [Google Scholar]
- Seybold S.J, Vanderwel D. Biosynthesis and endocrine regulation of pheromone production in the Coleoptera. In: Blomquist G, Vogt R, editors. Insect pheromone biochemistry and molecular biology. Academic Press; San Diego, CA: 2003. pp. 137–200. [Google Scholar]
- Simmons L.W. Kin recognition and its influence on mating preferences of the field cricket, Gryllus bimaculatus (de Geer) Anim. Behav. 1989;38:68–77. doi:10.1016/S0003-3472(89)80066-1 [Google Scholar]
- Simmons L.W. Princeton University Press; Princeton, NJ: 2001. Sperm competition and its evolutionary consequences in the insects. [Google Scholar]
- Simmons L.W, Siva-Jothy M.T. Sperm competition in insects: mechanisms and the potential for selection. In: Birkhead T.R, Møller A.P, editors. Sperm competition and sexual selection. Academic Press; San Diego, CA: 1998. pp. 341–434. [Google Scholar]
- Siva-Jothy M.T, Stutt A.D. A matter of taste: direct detection of female mating status in the bedbug. Proc. R. Soc. B. 2003;270:649–652. doi: 10.1098/rspb.2002.2260. doi:10.1098/rspb.2002.2260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siva-Jothy M.T, Blake D.E, Thompson J, Ryder J.J. Short- and long-term sperm precedence in the beetle Tenebrio molitor: a test of the ’adaptive sperm removal’ hypothesis. Physiol. Psychol. 1996;21:313–316. [Google Scholar]
- Smith R.L, editor. Sperm competition and the evolution of animal mating systems. Academic Press; New York, NY: 1984. [Google Scholar]
- Tanaka Y, Honda H, Ohsawa K, Yamamoto I. A sex attractant of the yellow mealworm, Tenebrio molitor L., and its role in the mating behavior. J. Pesticide Sci. 1986;11:49–55. [Google Scholar]
- Tanaka Y, Honda H, Ohsawa K, Yamamoto I. Absolute configuration of 4-methyl-1-nonanol, a sex attractant of the yellow mealworm, Tenebrio molitor L. J. Pesticide Sci. 1989;14:197–202. [Google Scholar]
- Thornhill R. Female preferences for the pheromones of males with low fluctuating asymmetry in the Japanese scorpionfly (Panorpa japonica, Mercoptera) Behav. Ecol. 1992;3:277–283. [Google Scholar]
- Thornhill R, Alcock J. Harvard University; Cambridge MA: 1983. The evolution of insect mating systems. [Google Scholar]
- Tschinkel W, Willson C, Bern H.A. Sex pheromone of the mealworm beetle (Tenebrio molitor) J. Exp. Zool. 1967;164:81–86. doi: 10.1002/jez.1401640108. doi:10.1002/jez.1401640108 [DOI] [PubMed] [Google Scholar]
- Warner R.R, Shapiro D.Y, Marcanato A, Petersen C.W. Sexual conflict: males with highest mating success convey lowest fertilization benefits to females. Proc. R. Soc. B. 1995;262:135–139. doi: 10.1098/rspb.1995.0187. [DOI] [PubMed] [Google Scholar]
- Wedell N, Gage M.J.G, Parker G.A. Sperm competition, male prudence and sperm-limited females. Trends Ecol. Evol. 2002;17:313–320. doi:10.1016/S0169-5347(02)02533-8 [Google Scholar]
- Worden B.D, Parker P.G. Polyandry in grain beetles, Tenebrio molitor, leads to greater reproductive success: material or genetic benefits? Behav. Ecol. 2001;12:761–767. doi:10.1093/beheco/12.6.761 [Google Scholar]
- Worden B.D, Parker P.G, Pappas P.W. Parasites reduce attractiveness and reproductive success in male grain beetles. Anim. Behav. 2000;59:543–550. doi: 10.1006/anbe.1999.1368. doi:10.1006/anbe.1999.1368 [DOI] [PubMed] [Google Scholar]
- Wyatt T.D. Cambridge University Press; Cambridge, UK: 2003. Pheromones and animal behaviour. [Google Scholar]