Abstract
Extra-group paternity (EGP) can form an important part of the mating system in birds and mammals. However, our present understanding of its extent and ecology comes primarily from birds. Here, we use data from 26 species and phylogenetic comparative methods to explore interspecific variation in EGP in mammals and test prominent ecological hypotheses for this variation. We found extensive EGP (46% of species showed more than 20% EGP), indicating that EGP is likely to play an important role in the mating system and the dynamics of sexual selection in mammals. Variation in EGP was most closely correlated with the length of the mating season. As the length of the mating season increased, EGP declined, suggesting that it is increasingly difficult for males to monopolize their social mates when mating seasons are short and overlap among females in oestrus is likely to be high. EGP was secondarily correlated with the number of females in a breeding group, consistent with the idea that as female clustering increases, males are less able to monopolize individual females. Finally, EGP was not related to social mating system, suggesting that the opportunities for the extra-group fertilizations and the payoffs involved do not consistently vary with social mating system.
Keywords: extra-group paternity, mating systems, oestrous synchrony, mammals
1. Introduction
One of the most important recent advances in the study of mating systems has been the discovery that social bonds frequently do not reflect genetic mating systems because of extra-pair paternity (EPP; Birkhead & Møller 1992; Petrie & Kempenaers 1998; Griffith et al. 2002). Growing evidence that such EPP can be extensive and can form an important part of the sexual selection process within a population (e.g. Møller & Birkhead 1994; Møller & Ninni 1998; Sheldon & Ellegren 1999; Griffith et al. 2002) has led to an increasing interest in understanding variation in EPP among species. However, although recent evidence suggests that EPP is likely to be important in mammalian mating systems (e.g. Goossens et al. 1998; Fietz et al. 2000; Griffin et al. 2003; Ortega et al. 2003), our knowledge of the extent and ecology of EPP stems primarily from research on birds (reviewed in Griffith et al. 2002; Westneat & Stewart 2003). Patterns in mammals are likely to differ from those in birds, owing to the large differences between these taxa in parental care and social mating systems. Specifically, mammals show little paternal care and polygamy predominates, while birds typically show greater paternal care and are mostly monogamous (Clutton-Brock 1989; Davies 1991).
The growing number of genetic studies of paternity in mammals now allows us to examine interspecific variation in EPP in mammals. In this paper, we describe the extent of variation in EPP among mammals and test three prominent ecological hypotheses for this variation. We refer to extra-pair paternity as extra-group paternity (EGP); that is, the proportion of offspring fathered by males outside the social breeding group. We use the term EGP rather than EPP because mammals commonly live in groups that contain multiple breeding adult males and females. EGP is the same as EPP for species in which breeding groups consist of a single male in association with one or more females (monogamous and unimale polygynous systems).
The spatial and temporal clustering of oestrous females are two key factors thought to influence the ability of males to prevent their mates from engaging in extra-pair copulations (Emlen & Oring 1977; Birkhead & Møller 1992; Shuster & Wade 2003; van Noordwijk & van Schaik 2004). We used female group size (the number of adult females in a breeding group) to represent the spatial clustering of females and the length of the mating season as a measure of the temporal clustering of oestrous females. EGP is expected to be greater when females are in larger groups than in smaller groups, and when mating seasons are relatively short and overlap in oestrus among females is relatively high. Under these conditions, males are less likely to be able to monopolize individual females and prevent them from engaging in extra-group copulations. A third factor, the number of males in a social breeding group, may also influence EGP, because the level of EGP might be expected to decrease as the number of defending males increases (van Noordwijk & van Schaik 2004). Using phylogenetic comparative methods, we investigated the relative strengths of the relationships between EGP and these three ecological factors: female group size; mating season length; and the number of males in a breeding group. Finally, we also examined whether EGP varied among different types of social mating system and tested the suggestion that EGP is higher in polygynous systems than in monogamous ones, because males may be less effective at defending multiple mates (Arak 1984).
2. Material and methods
We searched the literature for estimates of EGP, the proportion of offspring fathered by males outside the social breeding group. To limit variation arising from large differences in resolution among genetic methods, we used only estimates that were based on DNA-based methods, such as microsatellite genotyping and multilocus minisatellite fingerprints, and did not use estimates based on other methods, such as allozyme variation (Griffith et al. 2002). Populations for which paternity data are available, but where males and females do not form social breeding groups and instead show other types of social mating system, are not included here because EGP is not defined in these cases (e.g. scramble competition in some ground squirrels and sheep and roving in some ungulates, both of which involve either one or both sexes searching for mates, associating very briefly while mating, and then moving on to search for more mates). We also compiled data on the average number of adult females and males in a breeding group, the length of the mating season and social mating system, wherever possible from the same population from which data on EGP were extracted. We used two measures for social mating system: one categorical and the other continuous. First, based on overt associations between adult males and females, we classified species as: monogamous (one male, one female); polygynous (one male, multiple females); and multi-male (multiple males, one or multiple females). Populations with both monogamous and polygynous males were classified as polygynous if more than 15% of males were polygynous (following Dunn et al. 2001). Second, we used a continuous measure of social mating system, namely breeding group sex ratio, which is the ratio of the mean number of females to that of males in a breeding group. This describes the mean number of females that a male associates with in monogamous and polygynous systems and the mean number of females per male for males in multi-male groups. Breeding group sex ratio, unlike categorical mating system, can thus integrate multiple types of mating associations into a single measure.
To take into account potential non-independence among species owing to common ancestry, we used phylogenetic generalized least-squares methods (Martins & Hansen 1997; Pagel 1999; Garland & Ives 2000; Freckleton et al. 2002). This technique addresses the concern that closely related species may be more similar to each other than to distantly related species by incorporating the degree of non-independence between species into the error structure of the statistical model. Unlike ordinary least-squares regression where data points are assumed to be independent, generalized least-squares (GLS) methods can be used to explicitly model how the covariance between species declines as their phylogenetic separation increases (Martins & Hansen 1997; Pagel 1999; Freckleton et al. 2002). We constructed a composite phylogenetic tree for the species in our study (electronic supplementary material 1). Phylogenetic relationships at the level of the family were based on Liu et al. (2001). Smaller-scale phylogenies were used for the relationships among genera and species within families (Purvis 1995; Bininda-Emonds et al. 1999; Michaux et al. 2001). As comparable branch lengths across the whole tree were not available, branch lengths were set to 1 in our analyses (Garland & Ives 2000; Freckleton et al. 2002). Several transformations of branch lengths were explored (Garland & Ives 2000), but these did not improve model fit, and had no qualitative effect on the results.
To test the relative abilities of ecological variables to explain variation in EGP, we ran a GLS regression with EGP as the response variable and the average number of females in a group, length of the mating season and the average number of males in a group as predictor variables. To explore the relationship between EGP and social mating system, we built separate GLS models, with categorical mating system and breeding group sex ratio as predictor variables. We also evaluated whether variation in data quality among studies might influence our findings by using weighted GLS models. These included the same explanatory and response variables as the unweighted ones, but, in addition, weighted the response variable by sample size (the number of offspring assigned paternity); that is, the sampling error variance in EGP was assumed to be inversely related to sample size (Griffith et al. 2002).
In all analyses, EGP was arcsine transformed and predictor variables were ln-transformed to meet assumptions of normality and linearity. We checked residuals for violations of model assumptions. For all analyses, the significance of fixed effects was assessed using conditional t- and F-tests (Pinheiro & Bates 2000). To assess the effect of phylogeny (incorporated into the error structure), models with phylogenetic structure (GLS models) were compared with models with the same fixed effects, but without phylogenetic information (ordinary least squares) using likelihood-ratio tests to see if including phylogeny improved model fit (Pagel 1997; Pinheiro & Bates 2000). Likelihood-ratio (LR) tests were also used to compare weighted and unweighted GLS models. All analyses were carried out in the statistical language R, v. 2.3.0 (R Development Core Team 2004). Models were fit using restricted maximum likelihood, GLS analyses were carried out using the function gls in the nlme package (Pinheiro et al. 2004), the distance matrix was derived by setting branch lengths to 1 and the expected correlation between species was assumed to decline exponentially with phylogenetic distance (Hansen & Martins 1996). All summary statistics for EGP shown in §3 are back-transformed from estimates obtained after arcsine transformation and standard errors are therefore asymmetrical. These are presented as mean (mean−1 s.e., mean+1 s.e.).
3. Results
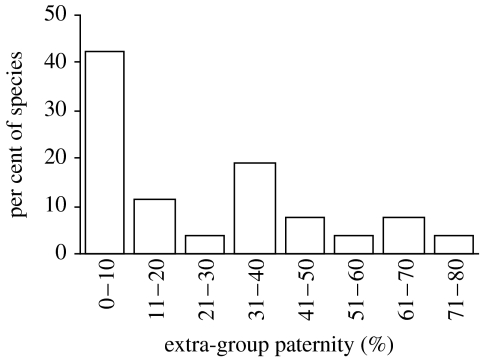
We obtained estimates of EGP from 26 species in 16 families and 6 orders. EGP ranged from zero in six species, including the California mouse Peromyscus californicus, the greater white-toothed shrew Crocidura russula and Kirk's dik-dik Madoqua kirkii, to 70% in the white-lined bat Saccopteryx bilineata and 80% in the red fox Vulpes vulpes (electronic supplementary material 2). Fifty-eight per cent of species showed frequencies of EGP greater than 10% and 46% of species frequencies greater than 20% (figure 1). Mean EGP across all species was 18.1%(13.3,23.4). Among species with EGP>0%, the mean value was 29.2%(23.9,34.8; N=20 species).
Figure 1.
Frequency distribution of extra-group paternity (EGP) in mammals. N=26 species.
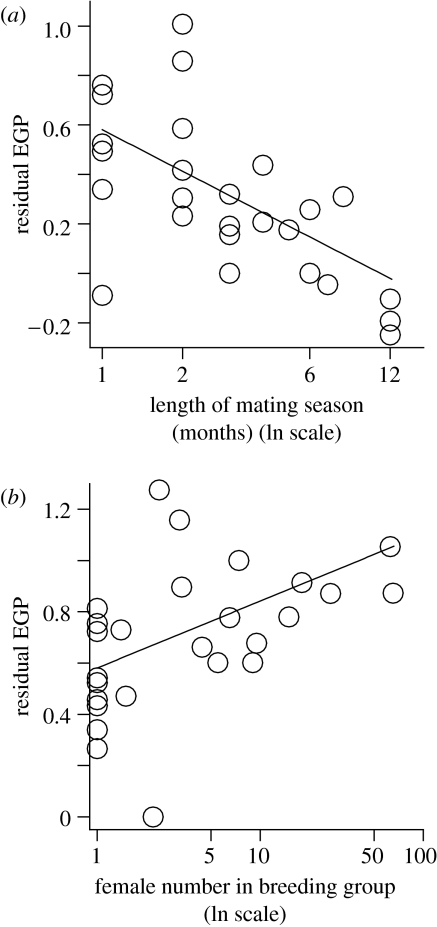
In the analysis of factors explaining variation in EGP, mating season length was the primary predictor and explained the most variation (table 1). EGP declined rapidly as mating season length increased (figure 2a). EGP was secondarily related to female numbers in a breeding group and showed a decelerating increase with female numbers (coefficient relating EGP to log(female numbers) was less than 1, table 1; figure 2b). EGP was not related to male numbers in a breeding group (table 1) and the effect of phylogeny was not significant (LR=0.004, N=26, d.f.=1, p=0.948). A weighted GLS model did not fit the data better than the unweighted one (LR=0.660, N=26, d.f.=1, p=0.417), suggesting that variation among studies in sample size did not influence our findings.
Table 1.
The effect of mating season length, female numbers in breeding group and male numbers in breeding group on EGP. (Results from both phylogenetic generalized least-squares and ordinary least-squares analyses are shown. EGP was arcsine transformed and all predictor variables were ln-transformed. N=26 species. Two-way interactions between predictor variables were tested, but none were significant (p>0.1 in all cases).)
| generalized least-squares model | ordinary least-squares model | ||||||
|---|---|---|---|---|---|---|---|
| coefficient (s.e.) | F | p | coefficient (s.e.) | F | p | change in R2 when removed from model (%) | |
| intercept | 0.581 | 0.581 | |||||
| (0.096) | (0.096) | ||||||
| mating season length | −0.242 | 13.809 | 0.0012 | −0.243 | 13.979 | 0.0011 | 33.8 |
| (0.065) | (0.065) | ||||||
| female numbers in breeding group | 0.113 | 6.285 | 0.0201 | 0.113 | 6.331 | 0.0197 | 15.3 |
| (0.045) | (0.045) | ||||||
| male numbers in breeding group | −0.045 | 0.374 | 0.5470 | −0.045 | 0.370 | 0.5494 | 0.9 |
| (0.074) | (0.074) | ||||||
Figure 2.
Extra-group paternity was (a) negatively related to the length of the mating season and (b) positively related to the number of females in the breeding group (N=26 species). Because EGP was significantly related to both variables, each panel displays partial residual plots of EGP. That is, residuals of EGP after subtracting the effect of the second significant predictor variable are plotted against the focal predictor variable (Neter et al. 1990). EGP was arcsine transformed for the analysis and residuals were calculated and best-fit lines drawn based on coefficients from the phylogenetic analysis (see table 1). Relationships between EGP and ecological variables were decelerating; hence, ecological variables were ln-transformed to meet linearity assumptions of statistical analyses.
EGP was not closely associated with social mating system. EGP varied widely within each category of mating system: monogamous—mean=8.0%(3.9,13.4), range=0–43.8%, N=8; multi-male—mean=18.7%(10.9,28.1), range=0–80%, N=11; polygynous—mean=31.9%(20.6,44.5), range=0–69.9%, N=7. EGP did not significantly differ among mating system categories (GLS: F2,23=1.875, N=26, p=0.176) nor was it significantly related to breeding group sex ratio (GLS: slope±1 s.e.=0.11±0.06, F1,24=3.368, N=26, p=0.079), although there was a tendency for EGP to increase with the degree of polygyny. In both these analyses, incorporating phylogenetic information did not significantly improve model fit (LR tests: p>0.05). As another way of evaluating an effect of phylogeny, we examined whether EGP differed among mammalian orders in our dataset that were represented by at least five species: carnivores (mean EGP=25.7%, range=0–80%, N=7); primates (mean=13.2%, range=0–43.8%, N=9); and rodents (mean=15.6%, range=0–61%, N=5). EGP did not vary significantly among these three orders (ANOVA: F2,18=0.483, p=0.625).
4. Discussion
(a) Variation in EGP
EGP varied widely among mammal species, from little or no EGP to over 60% EGP in several species, including the southern elephant seal Mirounga leonina, the white-lined bat S. bilineata and the red fox V. vulpes. EGP was not restricted to particular mammalian clades, instead it was extensively distributed across phylogenetic groups. In addition to being widely distributed, EGP levels were strikingly high; 46% of species in our dataset showed 20% or higher EGP.
The extensive distribution of EGP among mammals mirrors previous findings for birds (Griffith et al. 2002; Møller 2003). However, levels of EGP appear to be higher in mammals than in birds. For example, 18% of bird species (Griffith et al. 2002) as opposed to 46% of mammals (this study) show EGP levels greater than 20%. This difference may be related to the greater prevalence of paternal care in birds than in mammals. Several authors have suggested that when paternal care is important, females should be less likely to seek extra-pair copulations and thereby risk a reduction in paternal care from their social mate (Mulder et al. 1994; Birkhead & Møller 1996; Gowaty 1996; Bennett & Owens 2002).
(b) Ecological correlates of variation in EGP
Interspecific differences in EGP were most closely related to the length of the mating season, a measure of the degree of oestrous synchrony. EGP levels were the highest in species with short mating seasons (e.g. elephant seals Mirounga spp., fat-tailed dwarf lemur Cheirogaleus medius, Gunnison's prairie dog Cynomys gunnisoni) and declined as mating season length increased. This finding supports the idea that resident males may find it harder to prevent individual females from engaging in extra-group copulations, when mating seasons are short and, consequently, there is greater overlap among females in oestrus. Comparative studies of birds report some correlational evidence for a similar relationship between breeding synchrony and EGP (Stutchbury & Morton 1995; Møller & Ninni 1998; but see Weatherhead & Yezerinac 1998; Spottiswoode & Møller 2004).
EGP was secondarily related to the number of females in a breeding group, indicating that when females are in larger groups, they may be better able to engage in extra-pair copulations, perhaps because resident males then find it more difficult to closely monitor individual females. Finally, the lack of a relationship between EGP and the number of males in a breeding group suggests that the presence of a greater number of males in a group is not necessarily an effective defence against extra-group copulations.
To further investigate if the composition of breeding groups influences the frequency of extra-group fertilizations, we also examined the relationship between EGP and social mating system, which integrates both male and female numbers in breeding groups. EGP varied widely within each category of social mating system, did not significantly differ between types of mating system and was only weakly related to breeding group sex ratio. This suggests that the opportunities for EGP and/or its payoffs are not strongly influenced by social mating system and the degree of polygyny. In addition, these results indicate that polygyny may not impose a consistent cost on males in the form of a reduced paternity certainty (Arak 1984). Apart from the composition of breeding groups, the stability and cohesiveness of groups may also be important. For example, males may be less effective at guarding individual females and, consequently, EGP may be higher when groups are relatively unstable and there is movement into and out of groups (e.g. Cervus elaphus; Clutton-Brock et al. 1982) or when individuals within a group are dispersed while foraging, so that breeding females are frequently out of sight of resident males (e.g. Urocyon littoralis; Roemer et al. 2001; Clutton-Brock & Isvaran 2006).
Finally, in contrast to patterns in birds, we did not find a general phylogenetic signal in EGP, suggesting that the observed EGP levels reflect individuals responding to immediate ecological conditions. This result cannot be attributed to restricted sampling across clades, because, although our dataset is relatively small, the species we analysed are widely distributed across the mammalian phylogeny with representatives from 6 orders and 15 families. Furthermore, a simple comparison of EGP among three different orders also did not yield significant differences. In contrast, comparative studies of EGP in birds report a strong phylogenetic signal (Arnold & Owens 2002; Griffith et al. 2002). These studies propose that systematic differences among ancient lineages (families and orders) form an important source of variation among birds in EGP. This difference between mammals and birds is possibly related to differences in paternal care. The main hypotheses for the phylogenetic differences in EGP in birds involve variation among lineages in paternal care and the resultant variation in costs and benefits of EGP to females; when male care is essential, females should be less likely to engage in extra-pair copulations and risk a reduction in paternal care (e.g. Møller & Cuervo 2000; Arnold & Owens 2002). As paternal care is rare in mammals, it is unlikely to be an important selective factor leading to the kind of EGP variation among lineages that is seen in birds.
We have examined some of the ecological conditions thought to most strongly influence EGP. Other factors that are likely to be important and for which data are still lacking are variation among species in the costs and benefits to females and males from engaging in extra-pair copulations (Møller 2003; Westneat & Stewart 2003) and in counter tactics, such as mate-guarding (Clutton-Brock & Isvaran in press). An understanding of the nature and magnitude of these costs and benefits will allow an examination of how ecological conditions such as mating season length influence payoffs and thereby lead to variation among species in EGP.
Acknowledgments
We are grateful to D. Nussey, J. Pemberton, S. Quader, C. Spottiswoode, S. Dobson and an anonymous reviewer for their valuable comments on the manuscript, to J. Pemberton and D. Nussey for providing access to unpublished data, and to B. Bolker for his help with R code. K.I. was supported by the John Stanley Gardiner Fund and Magdalene College, Cambridge.
Supplementary Material
Electronic Supplementary Material includes (a) Appendix 1, which is a phylogenetic tree for the species used in our study and (b) Appendix 2, which is a table displaying estimates of extra-group paternity, mating season length, social mating system, breeding group sex ratio, and data sources for the species in our study
References
- Arak A. Sneaky breeders. In: Barnard C.J, editor. Producers and scroungers. Chapman and Hall; New York, NY: 1984. pp. 154–194. [Google Scholar]
- Arnold K.E, Owens I.P.F. Extra-pair paternity and egg dumping in birds: life history, parental care and the risk of retaliation. Proc. R. Soc. B. 2002;269:1263–1269. doi: 10.1098/rspb.2002.2013. doi:10.1098/rspb.2002.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett P.M, Owens I.P.F. Oxford University Press; Oxford, UK: 2002. Evolutionary ecology of birds: life history, mating systems and extinction. [Google Scholar]
- Bininda-Emonds O.R.P, Gittleman J.L, Purvis A. Building large trees by combining phylogenetic information: a complete phylogeny of the extant Carnivora (Mammalia) Biol. Rev. 1999;74:143–175. doi: 10.1017/s0006323199005307. doi:10.1017/S0006323199005307 [DOI] [PubMed] [Google Scholar]
- Birkhead T.R, Møller A.P. Academic Press; London, UK: 1992. Sperm competition in birds. [Google Scholar]
- Birkhead T.R, Møller A.P. Monogamy and sperm competition in birds. In: Black J.M, editor. Partnerships in birds. Oxford University Press; Oxford, UK: 1996. pp. 323–343. [Google Scholar]
- Clutton-Brock T.H. Mammalian mating systems. Proc. R. Soc. B. 1989;236:339–372. doi: 10.1098/rspb.1989.0027. [DOI] [PubMed] [Google Scholar]
- Clutton-Brock, T. H. & Isvaran, K. 2006 Paternity loss in contrasting mammalian societies. Biol. Lett (doi:10.1098/rsbl.2006.0531) [DOI] [PMC free article] [PubMed]
- Clutton-Brock T.H, Guinness F.E, Albon S.D. Edinburgh University Press; Edinburgh, UK: 1982. Red deer: behavior and ecology of two sexes. [Google Scholar]
- Davies N.B. Mating systems. In: Krebs J.R, Davies N.B, editors. Behavioural ecology: an evolutionary approach. Blackwell Scientific Publications; Oxford, UK: 1991. pp. 263–294. [Google Scholar]
- Dunn P.O, Whittingham L.A, Pitcher T.E. Mating systems, sperm competition, and the evolution of sexual dimorphism in birds. Evolution. 2001;55:161–175. doi: 10.1111/j.0014-3820.2001.tb01281.x. doi:10.1554/0014-3820(2001)055[0161:MSSCAT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Emlen S.T, Oring L.W. Ecology, sexual selection, and the evolution of mating systems. Science. 1977;197:215–223. doi: 10.1126/science.327542. [DOI] [PubMed] [Google Scholar]
- Fietz J, Zischler H, Schwiegk C, Tomiuk J, Dausmann K.H, Ganzhorn J.U. High rates of extra-pair young in the pair-living fat-tailed dwarf lemur, Cheirogaleus medius. Behav. Ecol. Sociobiol. 2000;49:8–17. doi:10.1007/s002650000269 [Google Scholar]
- Freckleton R.P, Harvey P.H, Pagel M. Phylogenetic analysis and comparative data: a test and review of evidence. Am. Nat. 2002;160:712–726. doi: 10.1086/343873. doi:10.1086/343873 [DOI] [PubMed] [Google Scholar]
- Garland T, Ives A.R. Using the past to predict the present: confidence intervals for regression equations in phylogenetic comparative methods. Am. Nat. 2000;155:346–364. doi: 10.1086/303327. doi:10.1086/303327 [DOI] [PubMed] [Google Scholar]
- Goossens B, Graziani L, Waits L.P, Farand E, Magnolon S, Coulon J, Bel M.-C, Taberlet P, Allainé D. Extra-pair paternity in the monogamous Alpine marmot revealed by nuclear DNA microsatellite analysis. Behav. Ecol. Sociobiol. 1998;43:281–288. doi:10.1007/s002650050492 [Google Scholar]
- Gowaty P.A. Battle of the sexes and origins of monogamy. In: Black J.M, editor. Partnerships in birds: the study of monogamy. Oxford University Press; Oxford, UK: 1996. pp. 21–52. [Google Scholar]
- Griffin A.S, Pemberton J.M, Brotherton P.N.M, McIlrath G, Gaynor D, Kansky R, O'Riain J, Clutton-Brock T.H. A genetic analysis of breeding success in the cooperative meerkat (Suricata suricatta) Behav. Ecol. 2003;14:472–480. doi:10.1093/beheco/arg040 [Google Scholar]
- Griffith S.C, Owens I.P.F, Thuman K.A. Extra pair paternity in birds: a review of interspecific variation and adaptive function. Mol. Ecol. 2002;11:2195–2212. doi: 10.1046/j.1365-294x.2002.01613.x. doi:10.1046/j.1365-294X.2002.01613.x [DOI] [PubMed] [Google Scholar]
- Hansen T.F, Martins E.P. Translating between microevolutionary process and macroevolutionary patterns: a general model of the correlation structure of interspecific data. Evolution. 1996;50:1404–1417. doi: 10.1111/j.1558-5646.1996.tb03914.x. doi:10.2307/2410878 [DOI] [PubMed] [Google Scholar]
- Liu F.R, Miyamoto M.M, Freire N.P, Ong P.Q, Tennant M.R, Young T.S, Gugel K.F. Molecular and morphological supertrees for eutherian (placental) mammals. Science. 2001;291:1786–1789. doi: 10.1126/science.1056346. doi:10.1126/science.1056346 [DOI] [PubMed] [Google Scholar]
- Martins E.P, Hansen T.F. Phylogenies and the comparative method: a general approach to incorporating phylogenetic information into the analysis of interspecific data. Am. Nat. 1997;149:646–667. doi:10.1086/286013 [Google Scholar]
- Michaux J, Reyes A, Catzeflis F. Evolutionary history of the most speciose mammals: molecular phylogeny of muroid rodents. Mol. Biol. Evol. 2001;18:2017–2031. doi: 10.1093/oxfordjournals.molbev.a003743. [DOI] [PubMed] [Google Scholar]
- Møller A.P. The evolution of monogamy: mating relationships, parental care and sexual selection. In: Reichard U.H, Boesch C, editors. Monogamy: mating strategies and partnerships in birds, humans and other mammals. Cambridge University Press; Cambridge, UK: 2003. pp. 29–41. [Google Scholar]
- Møller A.P, Birkhead T.R. The evolution of plumage brightness in birds is related to extrapair paternity. Evolution. 1994;48:1089–1100. doi: 10.1111/j.1558-5646.1994.tb05296.x. doi:10.2307/2410369 [DOI] [PubMed] [Google Scholar]
- Møller A.P, Cuervo J.J. The evolution of paternity and paternal care. Behav. Ecol. 2000;11:472–485. doi:10.1093/beheco/11.5.472 [Google Scholar]
- Møller A.P, Ninni P. Sperm competition and sexual selection: a meta-analysis of paternity studies of birds. Behav. Ecol. Sociobiol. 1998;43:345–358. doi:10.1007/s002650050501 [Google Scholar]
- Mulder R.A, Dunn P.O, Cockburn R.A, Lazenby-Cohen K.A, Howell M.J. Helpers liberate female fairy-wrens from constraints on extra-pair mate choice. Proc. R. Soc. B. 1994;255:223–229. [Google Scholar]
- Neter J, Wasserman W, Kutner M.H. Burr Ridge; Irwin, CA: 1990. Applied linear statistical models. [Google Scholar]
- van Noordwijk M.A, van Schaik C.P. Sexual selection and the careers of primate males: paternity concentration, dominance-acquisition tactics and transfer decisions. In: Kappeler P.M, van Schaik C.M, editors. Sexual selection in primates: new and comparative perspectives. Cambridge University Press; Cambridge, UK: 2004. pp. 208–221. [Google Scholar]
- Ortega J, Maldonado J.E, Wilkinson G.S, Arita H.T, Fleischer R.C. Male dominance, paternity, and relatedness in the Jamaican fruit-eating bat (Artibeus jamaicensis) Mol. Ecol. 2003;12:2409–2415. doi: 10.1046/j.1365-294x.2003.01924.x. doi:10.1046/j.1365-294X.2003.01924.x [DOI] [PubMed] [Google Scholar]
- Pagel M. Inferring evolutionary processes from phylogenies. Zoologica Scripta. 1997;26:331–348. doi:10.1111/j.1463-6409.1997.tb00423.x [Google Scholar]
- Pagel M. Inferring the historical patterns of biological evolution. Nature. 1999;401:877–884. doi: 10.1038/44766. doi:10.1038/44766 [DOI] [PubMed] [Google Scholar]
- Petrie M, Kempenaers B. Extra-pair paternity in birds: explaining variation between species and populations. Trends Ecol. Evol. 1998;13:52–58. doi: 10.1016/s0169-5347(97)01232-9. doi:10.1016/S0169-5347(97)01232-9 [DOI] [PubMed] [Google Scholar]
- Pinheiro J.C, Bates D.M. Springer; New York, NY: 2000. Mixed-effects models in S and S-PLUS. [Google Scholar]
- Pinheiro, J., Bates, D., DebRoy, S. & Sarkar, S. 2004 nlme: linear and nonlinear mixed effects models. R package version. 2006 3.1-71
- Purvis A. A composite estimate of primate phylogeny. Phil. Trans. R. Soc. B. 1995;348:405–421. doi: 10.1098/rstb.1995.0078. [DOI] [PubMed] [Google Scholar]
- R Development Core Team 2006. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org
- Roemer G.W, Smith D.A, Garcelon D.K, Wayne R.K. The behavioural ecology of the island fox (Urocyon littoralis) J. Zool. 2001;255:1–14. doi:10.1017/S0952836901001066 [Google Scholar]
- Sheldon B.C, Ellegren H. Sexual selection resulting from extra-pair paternity in collared flycatchers. Anim. Behav. 1999;57:285–298. doi: 10.1006/anbe.1998.0968. doi:10.1006/anbe.1998.0968 [DOI] [PubMed] [Google Scholar]
- Shuster S.M, Wade M.J. Princeton University Press; Princeton, NJ: 2003. Mating systems and strategies. [Google Scholar]
- Spottiswoode C, Møller A.P. Extrapair paternity, migration, and breeding synchrony in birds. Behav. Ecol. 2004;15:41–57. doi:10.1093/beheco/arg100 [Google Scholar]
- Stutchbury B.J.M, Morton E.S. The effect of breeding synchrony on extra-pair mating systems in songbirds. Behaviour. 1995;132:675–690. [Google Scholar]
- Weatherhead P.J, Yezerinac S.M. Breeding synchrony and extra-pair mating in birds. Behav. Ecol. Sociobiol. 1998;43:217–219. doi:10.1007/s002650050484 [Google Scholar]
- Westneat D.F, Stewart I.R.K. Extra-pair paternity in birds: causes, correlates, and conflict. Annu. Rev. Ecol. Syst. 2003;34:365–396. doi:10.1146/annurev.ecolsys.34.011802.132439 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Electronic Supplementary Material includes (a) Appendix 1, which is a phylogenetic tree for the species used in our study and (b) Appendix 2, which is a table displaying estimates of extra-group paternity, mating season length, social mating system, breeding group sex ratio, and data sources for the species in our study