Abstract
Amphibian species are declining at an alarming rate on a global scale in large part owing to an infectious disease caused by the chytridiomycete fungus, Batrachochytrium dendrobatidis. This disease of amphibians has recently emerged within Europe, but knowledge of its effects on amphibian assemblages remains poor. Importantly, little is known about the environmental envelope that is associated with chytridiomycosis in Europe and the potential for climate change to drive future disease dynamics. Here, we use long-term observations on amphibian population dynamics in the Peñalara Natural Park, Spain, to investigate the link between climate change and chytridiomycosis. Our analysis shows a significant association between change in local climatic variables and the occurrence of chytridiomycosis within this region. Specifically, we show that rising temperature is linked to the occurrence of chytrid-related disease, consistent with the chytrid-thermal-optimum hypothesis. We show that these local variables are driven by general circulation patterns, principally the North Atlantic Oscillation. Given that B. dendrobatidis is known to be broadly distributed across Europe, there is now an urgent need to assess the generality of our finding and determine whether climate-driven epidemics may be expected to impact on amphibian species across the wider region.
Keywords: climate-change, chytridiomycosis, Batrachochytrium dendrobatidis, amphibian declines, epidemiology
1. Introduction
Recent and predicted future patterns of global climate change are a major concern for many conservational ecologists. In many regions, climate change has resulted in warming over the past 30 years (Thomas et al. 2004). However, this effect is not uniform and the complexity of global climatic patterns means that temperatures in some areas to date have not changed measurably or have even cooled. Amphibians have been shown to be undergoing precipitous declines and species extinction on a global basis, and the recent Global Amphibian Assessment has shown that out of 5743 species, 1856 (32.5%) are globally threatened (Stuart et al. 2004). While a large percentage of these declines are attributable to direct anthropogenic effects, such as habitat loss, a substantial amount (48%) are classed as ‘enigmatic‘ declines with no identifiable cause (Stuart et al. 2004). The role of climate change in driving these declines has yet to be systematically addressed (Pounds 2001); however, several lines of evidence now suggest that climatic fluctuations may have a critical, and rapid, effect on amphibian populations by altering local densities (Carey & Alexander 2003) and species distributions (Araújo et al. 2006).
Recently, attention has been focused on the synergistic relationship between climate and infectious disease and their contribution to amphibian declines, known as the ‘climate-linked epidemic hypothesis’ (Harvell et al. 2002; Pounds et al. 2006). It has long been known that climate may play an indirect role in facilitating epidemics of amphibian infection disease (Kiesecker et al. 2001), and climatic fluctuations have been shown to encourage outbreaks of certain pathogens (Pounds & Crump 1994), such as the opportunistic fungus Saprolegnia ferax (Blaustein et al. 2003).
Chytridiomycosis is an emerging infectious disease of amphibians that is causing mass mortality and population declines worldwide (Berger et al. 1998; Daszak 2003). The causative agent, a non-hyphal zoosporic fungus, Batrachochytrium dendrobatidis, is a recently described species of the chytridiales (Longcore et al. 1999) which is known to infect over 93 species worldwide (www.jcu.edu.au/school/phtm/PHTM/frogs/chyglob.htm).
There are two major, non-mutually exclusive, hypotheses that attempt to explain the increasing impact of chytridiomycosis on global amphibian populations. The first hypothesis states that B. dendrobatidis is an introduced species whose spread can be best described by an epidemic ‘wave-like’ front (Lips et al. 2006). This hypothesis receives support from population genetic studies of the organism (Daszak et al. 2003; Morehouse et al. 2003) and observations that B. dendrobatidis is associated with asymptomatic carriage in anthropogenically widely dispersed species, for instance, the North American bullfrog (Rana catesbeiana) (Garner et al. 2006). On the other hand, it is also becoming clear that the colonized areas need to fit the ecological requirements for optimal growth, and successful infection, by B. dendrobatidis and this idea has led to a modification of the climate-linked epidemic hypothesis, known as the chytrid-thermal-optimum hypothesis (Pounds et al. 2006). Recent ecological niche models indicate that B. dendrobatidis presents a reasonably wide environmental tolerance and, consequently, is able to persist under a variety of temperature and precipitation regimens (Ron 2003). The optimal condition for growth of the chytrid in the laboratory is between 17 and 25°C, while at 28°C growth ceases and the organism dies after a week at above 29°C (Piotrowski et al. 2004). At lower temperatures, B. dendrobatidis is able to persist at 4°C, and can therefore overwinter in its hosts, even in temperate climates (Piotrowski et al. 2004). The chytrid-thermal-optimum hypothesis proposes an association between amphibian declines and increased cloud cover resulting from warming in the highlands of Central and South America, chiefly by the alteration of daytime radiant heating of microenvironments resulting in daytime cooling. This shift is hypothesized to bias the local environmental conditions towards the growth optimum of B. dendrobatidis, thus increasing the organism's pathogenicity on its hosts via unknown mechanisms. However, Pounds et al. (2006) did not focus on showing whether the pathogen was present, or causing disease, in the species studied, raising questions as to whether infection by B. dendrobatidis is actually involved in the observed species declines.
We have recently shown that B. dendrobatidis widely infects amphibian populations across Europe (Garner et al. 2005), suggesting that introductions of the pathogen are rather more ancient than were previously expected. However, mass mortalities are highly clustered within a relatively few high-altitude areas in Spain and France (S. Walker, M. C. Fisher & J. Bosch 2003–2006, unpublished data). These data are inconsistent with a wave-like front of introductions across Europe driving contemporary epidemics and suggest that environmental conditions influence host–pathogen dynamics in determining the outcome of infection within a site of introduction. Here, we develop the chytrid-thermal-optimum hypothesis by analysing a 28-year meteorological time-series within an alpine area located in a temperate zone before and after confirmed chytridiomycosis-related mass mortalities and species declines occurred. We show a significant association between the epidemic years and the specific climatic variables, chiefly temperature and humidity. Associations between climate and disease do not necessarily imply causation; however, by recognizing and describing a potentially potent ecological driver of these declines, we have created an important tool by which ecological modelling and laboratory studies can be used to dissect the complex interaction between amphibians, the chytrid, and their environment.
2. Material and methods
(a) Study area
The study area is included in the Peñalara Natural Park, a protected area (768 ha) in the Sierra de Guadarrama, Central Spain (40°50′ N, 3°57′ W). The area is located 1800–2200 m.a.s.l. and consists mainly of granitic outcrops, alpine meadows, heathlands dominated by Cytissus oromediterraneus and Juniperus communis nana, and the timberline of Pinus sylvestris pinewoods. Within the park, 242 ponds have been catalogued (Granados & Toro 2000), where 10 amphibian species presently breed. Populations of the midwife toad, Alytes obstetricans, have dramatically declined in the study area since 1997, owing to infection by the chytrid (Bosch et al. 2001). More recently, Salamandra salamandra has experienced a sharp decline in the park as a result of infection, and severe mass mortalities of Bufo bufo have also occurred for the same reason (Bosch & Martínez-Solano 2006; J. Bosch & S. Walker 2004–2006, unpublished observations).
(b) Data analyses
Climatic data were used from the neighbouring weather station of Puerto de Navacerrada, the nearest meteorological station located at a similar altitude (1890 m.a.s.l., 40°46′50″ N, 4°00′37″ W), 5.4 km away from the study area. Orographic characteristics of this locality and that of Peñalara Natural Park are very similar, and our experience supports the observation that data from Navacerrada can be generalized to the higher altitudes of the whole Guadarrama mountain range. Data for 20 meteorological variables were provided by the Spanish Instituto Nacional de Meteorología in the form of monthly values. Climatic information was gathered by this station for the period 1976–2003. This covers the time period for which there is good observational data of the amphibians’ biology within the study area, and therefore, the time span over which we can assert that chytridiomycosis has, or has not, occurred. Several meteorological variables were averages, while others were categorized as the number of days per month over which certain meteorological characteristics occurred (e.g. snow cover, mist, maximum temperature above 25°C; see table 1 for the variables considered).
Table 1.
Results of two-way ANOVAs analysing the climatic variation in the alpine area of the Sierra de Guadarrama. (Climatic variation has been partitioned into six factors according to the results of the PCA in the electronic supplementary material. The study period spans from 1976 to 2003. Sample units are monthly averages of each study year. Month, seasonal variation attributable to among-months differences; before–after chytridiomycosis (CHM), differences between two time periods according to chytridiomycosis infection (before, lacking the infection—1976 to 1996; after, with the chytridiomycosis outbreak—1997 to 2003; SS, sum of squares; %var., percentage of variance accounted for by each effect. Degrees of freedom are 11, 307 for the month term; 1, 107 for the before–after CHM term; and 11, 307 for the interaction in all ANOVAs.)
| SS | F | p | % var. | |
|---|---|---|---|---|
| PC1—thermal amplitude | ||||
| month | 207.6 | 150.6 | 0.000 | 62.9 |
| before–after CHM | 0.1 | 0.4 | 0.528 | 0.0 |
| interaction | 1.6 | 1.2 | 0.294 | 0.5 |
| PC2—number of hottest sunny days | ||||
| month | 105.6 | 16.5 | 0.000 | 32.0 |
| before–after CHM | 5.0 | 8.7 | 0.004 | 1.5 |
| interaction | 8.7 | 1.4 | 0.189 | 2.6 |
| PC3—precipitation gradient | ||||
| month | 48.8 | 5.3 | 0.000 | 14.8 |
| before–after CHM | 0.5 | 0.6 | 0.440 | 0.2 |
| interaction | 2.3 | 0.2 | 0.994 | 0.7 |
| PC4—storm frequency | ||||
| month | 67.0 | 7.6 | 0.000 | 20.3 |
| before–after CHM | 0.6 | 0.8 | 0.387 | 0.2 |
| interaction | 11.4 | 1.3 | 0.233 | 3.4 |
| PC5—increase in barometric pressure | ||||
| month | 34.3 | 3.6 | 0.000 | 10.4 |
| before–after CHM | 0.4 | 0.5 | 0.470 | 0.1 |
| interaction | 14.6 | 1.5 | 0.115 | 4.4 |
| PC6—mist frequency | ||||
| month | 21.3 | 3.4 | 0.000 | 6.5 |
| before–after CHM | 133.0 | 234.1 | 0.000 | 40.3 |
| interaction | 10.0 | 1.6 | 0.097 | 3.0 |
The annual land air and sea surface temperature anomalies from the Hadley Centre (Met Office, UK) based on the work of Jones et al. (2001), Folland et al. (1861) and Jones & Moberg (2003) are used here as comparisons alongside data collected from the Puerto de Navacerrada. The European Centre for Medium-Range Weather Forecasts (ECMWF) ERA40 reanalysis of the geopotential height of 850 hPa pressures is used for the period 1976–2002. This dataset has been used in order to determine the relationship between the global circulation patterns and the local climatology. General circulation patterns are principally determined by pressure fields, so any anomalous value for pressure will impact on the local climate, not only on the amplitude of the local variables, but also on the more general climate variability across the area of study.
A principal components analysis (PCA) was carried out with 20 meteorological variables describing 336 months (28 years×12 months per year) in order to obtain a reduced number of factors summarizing the climatic variation. The correlation matrix between the original meteorological variables was used, and varimax rotation was employed to synthesize and facilitate the comprehension of principal components (PCs). Six components with eigenvalues higher than 1 were then extracted.
Meteorological information was grouped into two time periods according to the lack (1976–1996) or presence (1997–2003) of observed chytrid-related mortalities. Comparisons between these two periods were carried out using two-way ANOVAs, including month as a second factor describing seasonal variation.
For the same two periods, data on the number of days with maximum temperatures equal or higher than values from 10 to 30°C in 1°C steps were computed for the interval from 15 July to 15 September (the time of the year when metamorphosis mainly takes place). The differences in number of days between the two periods were evaluated by the use of t-tests.
Finally, a set of composite geopotential height anomaly maps were calculated using the first three PCs (Von Storch & Zwiers 1999). First, anomaly height maps were calculated by subtracting monthly averages calculated from across the whole period 1976–2003. Subsequently, two average anomaly maps were determined: the first one being the average anomaly map when the PC is positive and higher than 0.5 (half of the standard deviation (s.d.)) and the second being the average anomaly map when the PC is negative and lower than −0.5 (half of the s.d.).
These average anomaly maps can be interpreted as the deviation from the medium-stage geopotential field that has led to a local extreme case of, for instance, the first PC. Mid-latitudinal weather events, such as Atlantic depressions, are the main driving force for local climatic conditions and we use these analyses to connect local conditions with the synoptic scale. For instance, our anomaly maps show whether positive cases of the first PC (local scale) are related to a reinforcement or weakness of the Azores anticyclone, or to an increase or decrease of the westerly flow over the Central Iberian Peninsula (synoptic scale).
3. Results
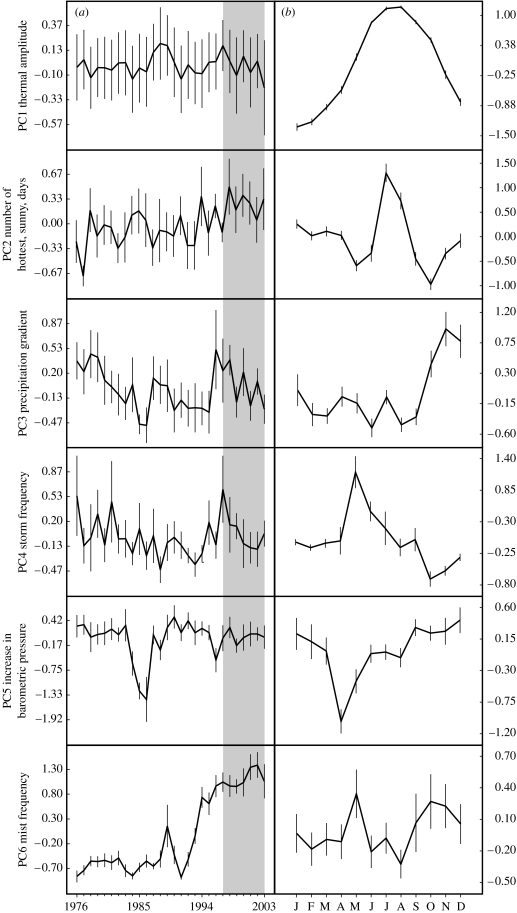
PCA was performed using variables defining the climatic characteristics of the study area. Six PCs accounted for 81% of the original among-years and within-year variations in the monthly averages of the 20 meteorological variables, shown in the electronic supplementary material. These PCs are analysed below considering seasonal (i.e. monthly) variations and differences between the periods before and after the occurrence of chytridiomycosis (see table 1 for ANOVA results and variance partitions, and figure 1 for monthly and yearly variations).
Figure 1.
(a) Yearly and (b) monthly variation in the climatic characteristics of an alpine area of the Sierra de Guadarrama. Sample size is 12 months for each year and 28 years for each month. Values represented are mean±1 s.e. Within grey areas are included the years of observed chytridiomycosis infection in the amphibian populations of Peñalara area. For significance of differences among years and months, see table 1.
The first climatic component (PC1, 36.4% of variance) specifies a thermal amplitude. PC1 is positively correlated with several measurements of environmental temperature and inversely with cloudiness, relative humidity, snowfall frequency/cover and the number of below-average cold days. Variations in this gradient are mainly linked with seasonal differences and are not related to the occurrence of chytridiomycosis (p=0.528, less than 0.1% of variance). The interaction between seasonal variation and chytridiomycosis was not significant, showing that the lack of climatic differences between the years before and after chytridiomycosis could not be accounted for by intra-annual variation (hereafter, this interaction term will only be commented if significant results are found).
The second component (PC2, 14.9% of variance) positively correlates with high levels of solar radiation and the number of days with very high maximum temperatures for this alpine area. PC2 inversely correlates with cloudiness and precipitation. There is a marked seasonal pattern in this climatic gradient, with marked peaks in summer months and minimum values in the cloudy months of spring and autumn. Controlling for seasonal patterns, there is a highly significant difference between years with and without chytridiomycosis infection (p=0.004), although this effect accounts for a very low proportion of climatic variance (1.5%). The number of summer hottest days, with high levels of solar radiation, was the largest in the epidemic period (post-1997).
The third component (PC3, 11.1% of variance) defines a precipitation gradient. PC3 is positively correlated to rainfall as well as cloudiness and inversely to solar radiation and barometric pressure. Periods before and after chytridiomycosis do not show a significant difference in this gradient (p=0.440, 0.2% of variance), although a significant difference among months was observed.
PC4 accounts for 6.8% of the variance and is directly related to the frequency of storms. This component is highly positively correlated with the number of days from which storms and hail were recorded. There is a significant seasonal variation in storm frequency, with maximum figures in late spring months (May and June), although differences between the periods when chytridiomycosis occurs are small and non-significant (p=0.387).
PC5 is associated with barometric pressure and correlates with the frequency of anticyclonic days (6.1% of variance). This component is significantly associated with monthly variation (the smallest values in this factor were measured in April and May), however, not with the occurrence of chytridiomycosis (p=0.470).
Finally, the sixth component (PC6, 5.7% of variance) is directly associated with the number of days that were observed to have high levels of mist and dew. There are significant monthly differences, although these account for a low proportion of the explained variance (the highest scores are in May and autumn months). The frequency of days with mist and dew began to increase from 1991 to 2003 and it significantly and consistently increased after 1997. The increase in the number of days with mist appears to be very abrupt and is not consistent with the pattern of the other climate variables. This component appears to be more associated with a change in the criteria of mist observations (which is often grouped together with low-level clouds in high-altitude areas) than that related to climate variability. For this reason, this component will not be further analysed.
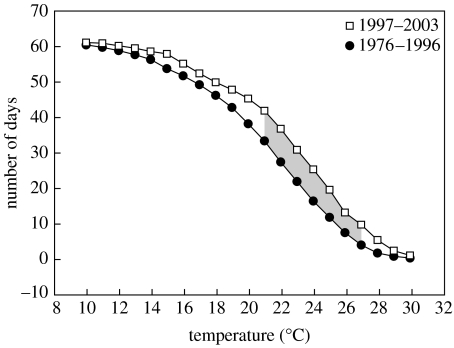
Figure 2 shows the variation in the number of days with maximum temperatures that are equal to, or higher than, values from 10 to 30°C in 1°C steps in the time-interval 15 July to 15 September. The number of days with maximum temperatures higher than 21–27°C was significantly lower in the period lacking chytridiomycosis (1976–1996) in relation to the period when amphibians suffered the disease (1997–2003).
Figure 2.
Variation in the number of days (ordinate) with maximum temperatures higher than several temperature values (abscissa) in the time-interval 15 July to 15 September. Two time periods are compared according to lack (1976–1996) or presence (1997–2003) of chytridiomycosis. The grey area denotes significant differences in the t-tests comparing these two time periods (p<0.05).
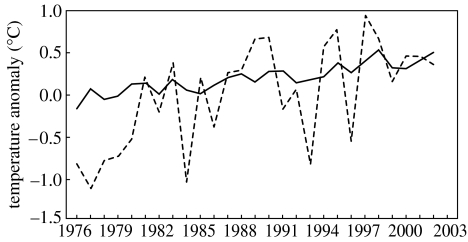
Figure 3 shows the changes in yearly mean surface temperature anomalies from 1976 to 2002 by comparing the climatological mean at the station alongside with the observed global data. A clear positive trend is observed for both the global and the local scale. However, the variability of the station anomalies is higher than that of the global ones, as expected and found by other authors for other alpine regions (Beniston et al. 1997). As illustrated, the higher anomaly values occur after 1995, reaching their maximum in 1997, with a value of 0.96°C at the beginning of the period where chytridiomycosis occurred (1997–2003).
Figure 3.
Yearly mean surface temperature anomalies for the station (dashed line) along with annual land air and sea surface temperature anomalies (solid line).
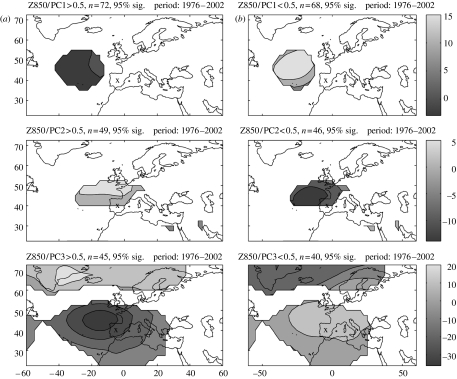
Figure 4a,b shows the composite maps of the 850 hPa geopotential height anomaly (850 GHA) for the first three PCs when their values are higher than half of the s.d. and lower than minus half of the s.d., respectively. Only anomalies where the correlation between the 850 GHA variation and the PC variation is statistically significant (using a t-test with a 95% significance level) are shown.
Figure 4.
Composite maps of the 850 GHA for the first three PCs when their value is (a) higher than half of the s.d. and (b) lower than minus half of the s.d. ‘X’ denotes the geographical location of the study area.
The composite maps for the first PC demonstrate a statistically significant association between positive pressure anomalies over the Atlantic, corresponding to the winter season for PC1 (figure 4a). Although the positive pressure anomalies are not centred over the station, they are likely to be affecting the advection of cold air over the Iberian Peninsula from northern Europe, which is the main source of cold events in this region. No inferences can be made for the anomaly map for the values of PC1>0.5, which corresponds to the summer season, since these values are close to 0.
The composite maps for PC2 show a significant relationship between the 850 GHA anomalies and the positive values of this component, which are related to high levels of insolation and days with very high maximum temperatures. On the contrary, negative values of the anomaly (figure 4b) are associated with cloudiness and precipitation.
Finally, the third set of composite maps shows high values on the left panel and low values on the right for PC3, which is mainly associated with precipitation. These anomaly patterns show clearly how the North Atlantic Oscillation, the main driver of climatic variability in this region, leads to precipitation. Here, positive values of PC3 over the study site lead to low levels of precipitation with the dipole behaviour of the anomalies being observed.
4. Discussion
The results we obtained are in accordance with the expected phenomena of global climate warming by showing that meteorological conditions have significantly changed in the studied area and over the period for which chytrid-related mass mortalities have occurred. In recent years, hotter and sunnier days appear to be more frequent in the area with temperature anomalies exhibiting a marked positive trend.
Importantly, a statistically significant relationship has been found between the general circulation patterns and the climatic variability at the study area. If changes in these general circulation patterns occur as a consequence of climate warming, then an increasing impact on the climate variability of the area of study is expected. Some of these changes have already been pointed out by other authors, such as those consisting of a north displacement of the North Atlantic Oscillation (Hurrell 1995) or that modelled under different greenhouse gas scenarios (Rodríguez-Fonseca et al. 2005). These patterns are expected to lead to significant changes in the climate variability of the region of study, consisting of higher numbers of sunnier and hotter days, and moderation of the lower temperatures as well as a shorter winter season.
Harvell et al. (2002) argued that since outbreaks of chytrid-related disease are particularly associated with cool, moist, high-altitude conditions, this disease may be one of a few for which climate warming could disrupt disease spread. However, the chytrid-thermal-optimum hypothesis (Pounds et al. 2006) argues that this statement may not apply to tropical areas of Central America and Northeast Australia where most chytrid-related declines occur, and here, we show that it is also not a valid statement for an alpine area in a temperate zone. In this region, global warming is moderating the naturally severe cool conditions, and among other consequences, as Harvell et al. (2002) pointed out, shorter, milder winters are expected to increase the incidence of disease.
As a general rule, temperature and water availability usually interact by determining fungal optimal conditions. The second climatic factor, PC2, for which we have demonstrated a significant effect, related to the occurrence of hot and sunny days, shows higher monthly values during July and August, these being the months when metamorphosis takes place. In general, most amphibian die-offs (or at least, the most recordable) occur in the period when animals complete metamorphosis and their skin becomes keratinized. In the days following metamorphosis, recent metamorphs remain close to the pond, searching for cool and damp places to hide. Our analysis shows that the number of days where maximum temperatures over the period of metamorphosis exceed 21–27°C has significantly increased in recent years. However, this recent warming has not increased the number of days where the maximum temperatures exceed 28°C, the critical threshold for successful persistence of B. dendrobatidis. In addition, even if this limit could be achieved in the area, survival and persistence of the pathogen may occur as a consequence of the active avoidance of high temperatures by the metamorphs preferential selection of damp and cool habitats within which to hide.
On the other hand, the sixth climatic factor, relating to the abundance of mist and dewfall, presents the highest values during May, when the breeding period of the amphibians takes place. Therefore, as may occur with the temperature scenario discussed earlier, a value of 100% relative humidity provided by overnight dewfall and mists during the breeding period could be a key determinant in facilitating the transmission of fungus among adults in terrestrial species. In fact, the most terrestrial species inhabiting the area (A. obstetricans and S. salamandra) are precisely the species most affected by chytridiomycosis, and they also exhibit nocturnal behaviour. This hypothesis is in accordance with the findings of Piotrowski et al. (2004) that zoospores of B. dendrobatidis swim less than 2 cm before they encyst therefore, the pathogen is probably spread from amphibian to amphibian by close or direct contact during mating when relative humidity is high.
A related factor is that transmission of B. dendrobatidis may have increased in the area as a result of shorter and milder winters. The two species that are most affected by chytridiomycosis (A. obstetricans and S. salamandra) share terrestrial habits and both the species breed in permanent ponds and have overwintering larvae. In the laboratory, the pathogen is able to persist and even grow slowly at low temperatures such as 4°C (Piotrowski et al. 2004). If in recent years, the water temperature has increased during the winter, the pathogen may now be growing faster on overwintering larvae, creating a greater infectious challenge upon metamorphosis.
The patterns and the associations that we have described here, while indicative of a link between the climate change and the onset of chytridiomycosis in our study populations, are unlikely to describe the whole story. To complete the epidemiological picture of the emergence of B. dendrobatidis and chytridiomycosis in Peñalara, it is necessary to also consider the hypothesis that the disease may have recently been introduced. Here also, it is possible that recent warming has contributed to the arrival of the pathogen. The most severe and the least predictable disease outbreaks may occur when climate change alters host or pathogen geographical ranges, causing formerly disjunct species and populations to converge (Davis & Shaw 2001). Amphibian species with a low susceptibility to B. dendrobatidis can act as reservoirs for the disease in the wild (Collins & Storfer 2003; Garner et al. 2005). The Iberian green frog, Rana perezi, is usually restricted to low elevations (Llorente & Arano 1997) and exhibits a very high plasticity, inhabiting virtually all regions of Spain. In the studied area, this species has expanded its range in the park (Martínez-Solano et al. 2003), doubling the number of ponds in which it was observed relative to 20 years ago. Rana perezi has been shown to carry the fungus (S. Walker & J. Bosch 2004–2005, unpublished data); however, the species susceptibility to the disease appears to be very low as no dead individuals or population declines for this species have been recorded. It may be that R. perezi or other thus far unidentified vectors are contributing to the spread of the pathogen.
Our data support the chytrid-thermal-optimum hypothesis and the climate change as a driver of chytridiomycosis in Peñalara, by defining an increase in a climatic envelope that is broadly similar to that described by Pounds et al. (2006) and showing that this climatic envelope is associated with observed increases in chytridiomycosis. However, we do not rule out the introduction of B. dendrobatidis in initiating the Peñalara epidemic. Chytrid-related declines are probably the result of a complex web of interaction, and the effects of climate will be conditional on other factors such as host density, amphibian community composition, microbial competitors and zooplankton predators, to name but a few. In order to disentangle this network into its key compartments, it is necessary to collect seasonal data on amphibian densities, contemporary and historical measurements of the prevalence and intensity of infection, seasonal mortalities, and fine-scale meteorological conditions from a range of sites that represent altitudinal clines. These data, in concert with molecular epidemiological analyses, will determine the relative roles of introduction and climate change in driving contemporary epidemics of this disease.
Recent surveys of amphibians across mainland Europe have shown that the chytrid is more broadly distributed, at least in Europe, than disease outbreaks predict (Garner et al. 2005). In the Iberian Peninsula, the pathogen occurs practically everywhere (J. Bosch, M. C. Fisher & S. Walker 2003–2006, unpublished data); however, relatively few sites of chytridiomycosis-driven mass mortalities have been recorded (see www.sosanfibios.org/spain.html). Therefore, it appears that, within mainland Europe at least, the dynamics of B. dendrobatidis and its hosts are more consistent with the climate-linked epidemic hypothesis rather than the occurrence of wave-like epidemic front of introductions such as is described by Lips et al. (2006), and that introductions of the pathogen are rather more ancient than might have been expected. As a consequence, our current efforts are focused on conducting European-wide screens for the disease, in concert with detailed ecological modelling and laboratory assessments of species susceptibility and disease transmission.
Acknowledgments
We thank the European Centre for Medium-Range Weather Forecasts (ECMWF) for providing the ERA40 reanalysis data. We thank all the people working at the Peñalara Natural Park for the facilities provided to complete this work. Funding for this study was provided from a project supported by the Fundación BBVA (PI: J.B.) and the Natural Environmental Research Council, NERC, UK (PI: M.F.).
Supplementary Material
Results of the principal components analysis summarizing the climatic variation throughout 12 months and 28 years (1991–2003) in the alpine area of the Sierra de Guadarrama
References
- Araújo M.B, Thuiller W, Pearson R.G. Climate warming and the decline of amphibians and reptiles in Europe. J. Biogeogr. 2006;33:1712–1728. doi:10.1111/j.1365-2699.2006.01482.x [Google Scholar]
- Beniston M, Diaz H.F, Bradley R.S. Climatic change at high elevation sites: an overview. Clim. Change. 1997;36:233–251. doi:10.1023/A:1005380714349 [Google Scholar]
- Berger L, et al. Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proc. Natl Acad. Sci. USA. 1998;95:9031–9036. doi: 10.1073/pnas.95.15.9031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein A.R, Romansic J.M, Kiesecker J.M, Hatch A.C. Ultraviolet radiation, toxic chemicals and amphibian population declines. Divers. Distrib. 2003;9:123–140. [Google Scholar]
- Bosch J, Martínez-Solano I. Chytrid fungus infection related to unusual mortalities of Salamandra salamandra and Bufo bufo in the Peñalara Natural Park (Central Spain) Oryx. 2006;40:84–89. doi:10.1017/S0030605306000093 [Google Scholar]
- Bosch J, Martínez-Solano I, García-París M. Evidence of a chytrid fungus infection involved in the decline of the common midwife toad (Alytes obstetricans) in protected areas of Central Spain. Biol. Conserv. 2001;97:331–337. doi:10.1016/S0006-3207(00)00132-4 [Google Scholar]
- Carey C, Alexander M. Climate change and amphibian declines: is there a link? Divers. Distrib. 2003;9:111–121. [Google Scholar]
- Collins J.P, Storfer A. Global amphibian declines: sorting the hypotheses. Divers. Distrib. 2003;9:89–98. [Google Scholar]
- Daszak P. Infectious disease and amphibian population declines. Divers. Distrib. 2003;9:141–150. doi:10.1046/j.1472-4642.2003.00016.x [Google Scholar]
- Daszak P, Cunningham A.A, Hyatt A.D. Infectious disease and amphibian population declines. Divers. Distrib. 2003;9:141–150. [Google Scholar]
- Davis M.B, Shaw R.G. Range shifts and adaptive responses to quaternary climate change. Science. 2001;292:673–679. doi: 10.1126/science.292.5517.673. doi:10.1126/science.292.5517.673 [DOI] [PubMed] [Google Scholar]
- Folland C.K, et al. Global temperature change and its uncertainties since 1861. Geophys. Res. Lett. 2001;28:2621–2624. [Google Scholar]
- Garner T.W.J, Walker S, Bosch J, Hyatt A.D, Cunningham A.A, Fisher M.C. Widespread European distribution of a global amphibian pathogen. Emerg. Infect. Dis. 2005;11:1639–1641. doi: 10.3201/eid1110.050109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner T.W.J, Perkins M.W, Govindarajulu P, Seglie D, Walker S, Cunningham A.A, Fisher M.C. Global distribution of Batrachochytrium dendrobatidis infection in introduced populations of the North American bullfrog, Rana catesbeiana. Biol. Lett. 2006;2:455–459. doi: 10.1098/rsbl.2006.0494. doi:10.1098/rsbl.2006.0494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granados I, Toro M. Limnología en el Parque Natural de Peñalara: nuevas aportaciones y perspectivas de futuro. In: Navalon L, Prieto D, editors. Segundas Jornadas Científicas del Parque Natural de Peñalara y del Valle de El Paular. Consejería de Medio Ambiente de la Comunidad de Madrid. Spain; Madrid: 2000. pp. 55–72. [Google Scholar]
- Harvell C.D, Mitchell C.E, Ward J.R, Altizer S, Dobson A.P, Ostfeld R.S, Samuel M.D. Climate warming and disease risks for terrestrial and marine biota. Science. 2002;296:2158–2162. doi: 10.1126/science.1063699. [DOI] [PubMed] [Google Scholar]
- Hurrell J. Decadal trends in the North Atlantic Oscillation: regional temperatures and precipitation. Nature. 1995;269:676–679. doi: 10.1126/science.269.5224.676. [DOI] [PubMed] [Google Scholar]
- Jones P.D, Moberg A. Hemispheric and large-scale surface air temperature variations: an extensive revision and an update to 2001. J. Clim. 2003;16:206–223. doi:10.1175/1520-0442(2003)016<0206:HALSSA>2.0.CO;2 [Google Scholar]
- Jones P.D, Osborn T.J, Briffa K.R, Folland C.K, Horton B, Alexander L.V, Parker D.E, Rayner N.A. Adjusting for sampling density in grid-box land and ocean surface temperature time series. J. Geophys. Res. 2001;106:3371–3338. [Google Scholar]
- Kiesecker J.M, Blaustein A.R, Belden L.K. Complex causes of amphibian population declines. Nature. 2001;410:681–683. doi: 10.1038/35070552. doi:10.1038/35070552 [DOI] [PubMed] [Google Scholar]
- Lips K.R, et al. Emerging infectious disease and the loss of biodiversity in a Neotropical amphibian community. Proc. Natl Acad. Sci. USA. 2006;103:3165–3170. doi: 10.1073/pnas.0506889103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorente G.A, Arano B. Rana perezi. In: Pleguezuelos J.M, editor. Distribución y biogeografía de los anfibios y reptiles en España y Portugal. Associatión Herpetológica Española-Universidad de Granada; Granada, Spain: 1997. pp. 164–166. [Google Scholar]
- Longcore J.E, Pessier A.P, Nichols D.K. Batrachochytrium dendrobatidis gen. et sp. nov, a chytrid pathogenic to amphibians. Mycologia. 1999;91:219–227. [Google Scholar]
- Martínez-Solano I, Bosch J, García-París M. Demographic trends and community stability in a montane amphibian assemblage. Conserv. Biol. 2003;17:238–244. doi:10.1046/j.1523-1739.2003.01096.x [Google Scholar]
- Morehouse E.A, James T.Y, Ganley A.R, Vilgalys R, Berger L, Murphy P.J, Longcove J.E. Multilocus sequence typing suggests the chytrid pathogen of amphibians is a recently emerged clone. Mol. Ecol. 2003;112:395–403. doi: 10.1046/j.1365-294x.2003.01732.x. [DOI] [PubMed] [Google Scholar]
- Piotrowski J.S, Annis S.L, Longcore J.E. Physiology of Batrachochytrium dendrobatidis, a chytrid pathogen of amphibians. Mycologia. 2004;96:9–15. [PubMed] [Google Scholar]
- Pounds J.A. Climate and amphibian declines. Nature. 2001;410:639–640. doi: 10.1038/35070683. doi:10.1038/35070683 [DOI] [PubMed] [Google Scholar]
- Pounds J.A, Crump M.L. Amphibian declines and climate disturbance: the case of the golden toad and the harlequin Frog. Conserv. Biol. 1994;8:72–85. doi:10.1046/j.1523-1739.1994.08010072.x [Google Scholar]
- Pounds J.A, et al. Widespread amphibian extinctions from epidemic disease driven by global warming. Nature. 2006;439:143–144. doi: 10.1038/nature04246. doi:10.1038/439143a [DOI] [PubMed] [Google Scholar]
- Rodríguez-Fonseca B, Sánchez E, Arribas A. Winter climate variability changes over Europe and the Mediterranean region under increased greenhouse conditions. Geophys. Res. Lett. 2005;32:L13702. doi:10.1029/2005GL022800 [Google Scholar]
- Ron S.R. Predicting the distribution of the amphibian pathogen Batrachochytrium dendrobatidis in the New World. Biotropica. 2003;37:209–221. doi:10.1111/j.1744-7429.2005.00028.x [Google Scholar]
- Stuart S.N, Chanson J.S, Cox N.A, Young B.B, Rodrigues A.S.L, Fischman D.L, Waller R.W. Status and treads of amphibian declines and extinctions worldwide. Science. 2004;306:1783–1786. doi: 10.1126/science.1103538. [DOI] [PubMed] [Google Scholar]
- Thomas C.D, et al. Extinction risk from climate change. Nature. 2004;427:145–148. doi: 10.1038/nature02121. doi:10.1038/nature02121 [DOI] [PubMed] [Google Scholar]
- Von Storch H, Zwiers F.W. Cambridge University Press; Cambridge, UK: 1999. Statistical analysis in climate research; p. 484. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Results of the principal components analysis summarizing the climatic variation throughout 12 months and 28 years (1991–2003) in the alpine area of the Sierra de Guadarrama