Abstract
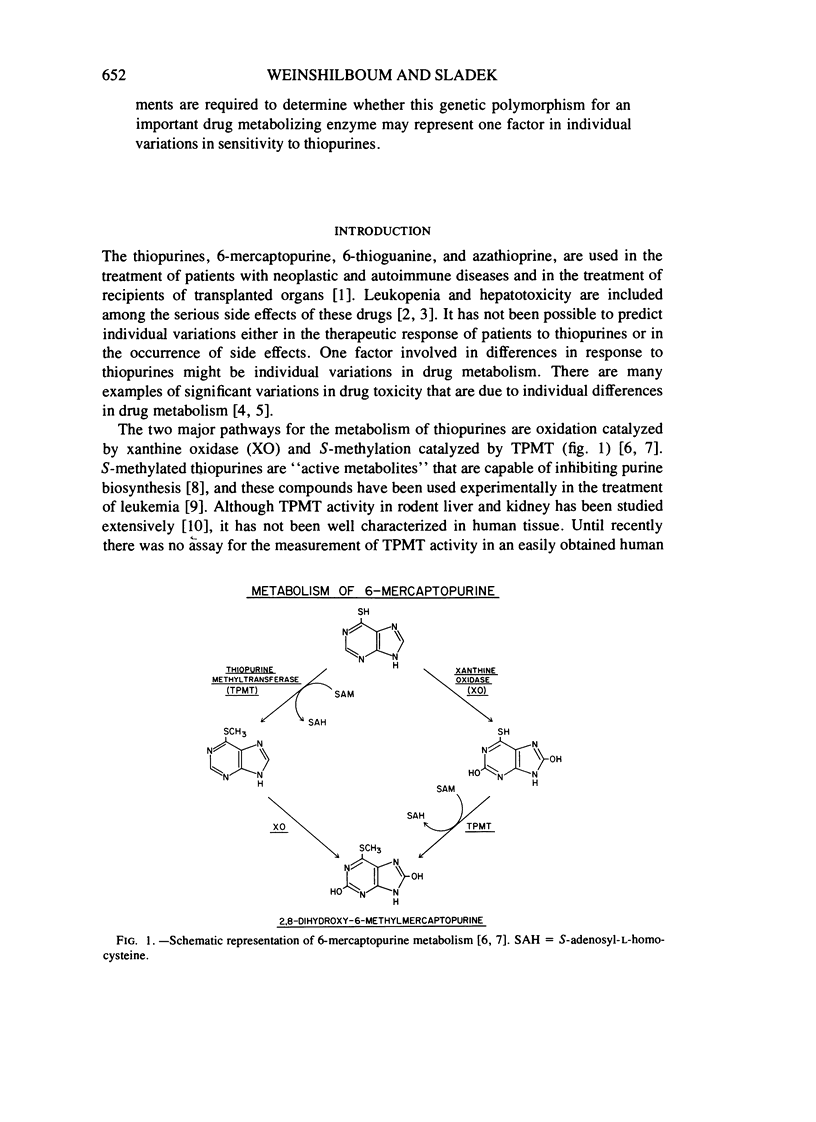
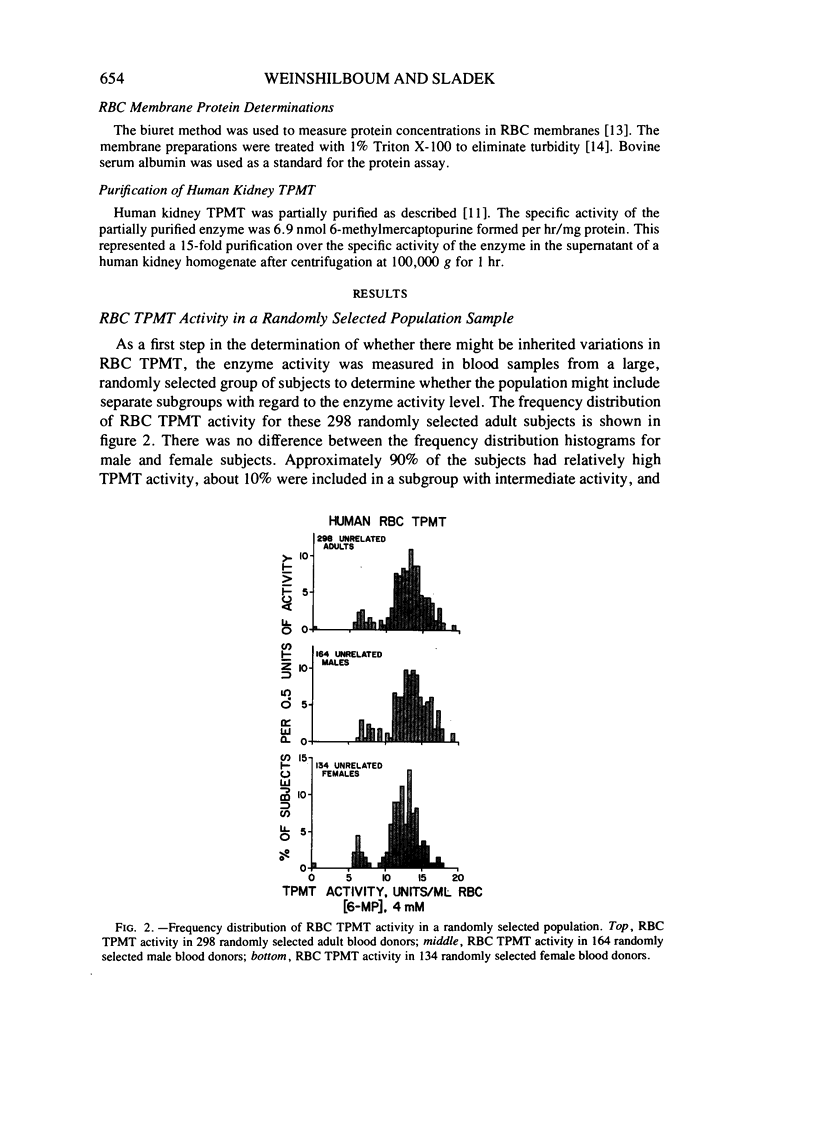
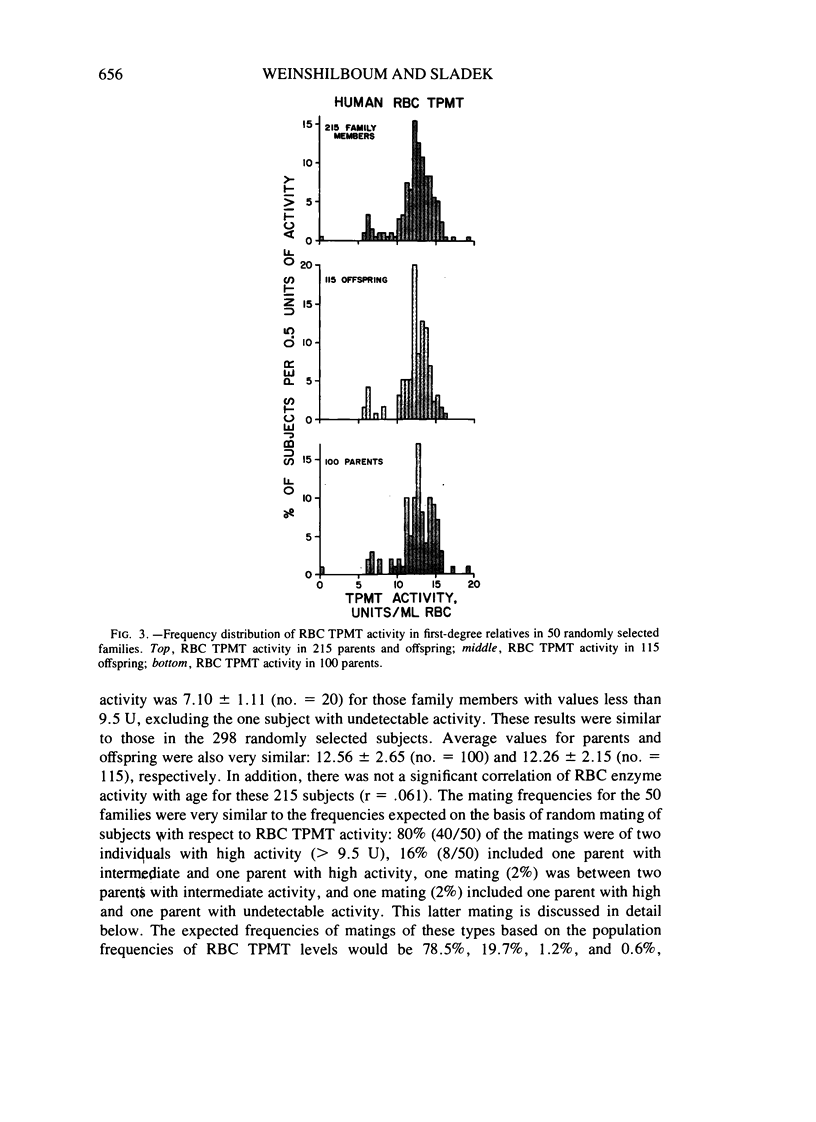
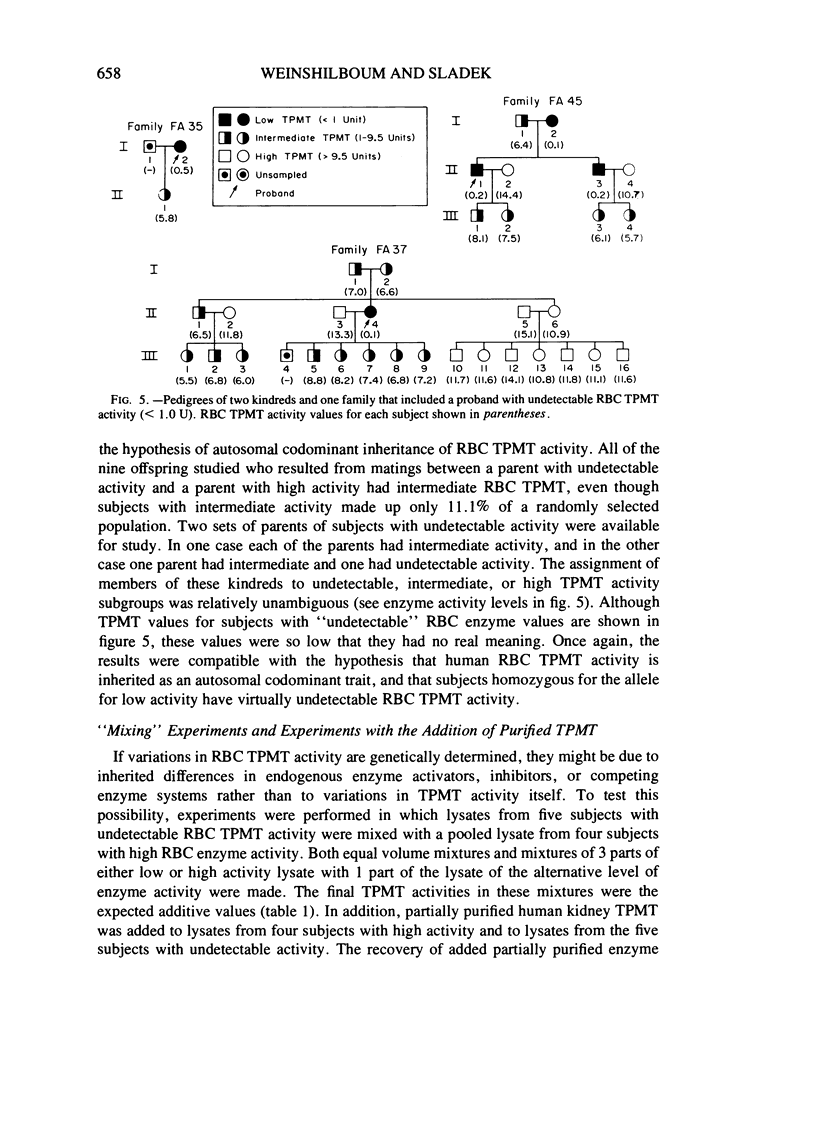
Thiopurine methyltransferase (TPMT) catalyzes thiopurine S-methylation, an important metabolic pathway for drugs such as 6-mercaptopurine. Erythrocyte (RBC) TPMT activity was measured in blood samples from 298 randomly selected subjects. Of the subjects, 88.6% were included in a subgroup with high enzyme activity (13.50 ± 1.86 U, mean ± SD), 11.1% were included in a subgroup with intermediate activity (7.20 ± 1.08 U), and 0.3% had undetectable activity. This distribution conforms to Hardy-Weinberg predictions for the autosomal codominant inheritance of a pair of alleles for low and high TPMT activity, TPMTL and TPMTH, with gene frequencies of .059 and .941, respectively. If RBC TPMT activity is inherited in an autosomal codominant fashion, then subjects homozygous for TPMTH would have high enzyme activity, subjects heterozygous for the two alleles would have intermediate activity, and subjects homozygous for TPMTL would have undetectable activity. The segregation of RBC TPMT activity among 215 first-degree relatives in 50 randomly selected families and among 35 members of two kindreds and one family selected because they included probands with undetectable RBC enzyme activity were also compatible with the autosomal codominant inheritance of RBC TPMT. For example, in eight matings between subjects with intermediate activity (presumed genotype TPMTL TPMTH) and subjects with high activity (presumed genotype TPMTH TPMTH), 47% (8/17) of the offspring had intermediate activity. This value is very similar to the 50% figure expected on the basis of autosomal codominant inheritance (χ2[1] = .059). Further experiments are required to determine whether this genetic polymorphism for an important drug metabolizing enzyme may represent one factor in individual variations in sensitivity to thiopurines.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Drayer D. E., Strong J. M., Jones B., Sandler A., Reidenberg M. M. In vitro acetylation of drugs by human blood cells. Drug Metab Dispos. 1974 Nov-Dec;2(6):499–505. [PubMed] [Google Scholar]
- Elion G. B. Symposium on immunosuppressive drugs. Biochemistry and pharmacology of purine analogues. Fed Proc. 1967 May-Jun;26(3):898–904. [PubMed] [Google Scholar]
- Freireich E. J., Bodey G. P., Harris J. E., Hart J. S. Therapy for acute granulocytic leukemia. Cancer Res. 1967 Dec;27(12):2573–2577. [PubMed] [Google Scholar]
- Lindsay R. H., Hulsey B. S., Aboul-Enein H. Y. Enzymatic S-methylation of 6-n-propyl-2-thiouracil and other antithyroid drugs. Biochem Pharmacol. 1975 Feb 15;24(4):463–468. doi: 10.1016/0006-2952(75)90129-x. [DOI] [PubMed] [Google Scholar]
- Mitchell J. R., Zimmerman H. J., Ishak K. G., Thorgeirsson U. P., Timbrell J. A., Snodgrass W. R., Nelson S. D. Isoniazid liver injury: clinical spectrum, pathology, and probable pathogenesis. Ann Intern Med. 1976 Feb;84(2):181–192. doi: 10.7326/0003-4819-84-2-181. [DOI] [PubMed] [Google Scholar]
- Pazmiño P. A., Weinshilboum R. M. Human erythrocyte phenol O-methyltransferase: radiochemical microassay and biochemical properties. Clin Chim Acta. 1978 Oct 16;89(2):317–329. doi: 10.1016/0009-8981(78)90331-5. [DOI] [PubMed] [Google Scholar]
- Perry H. M., Jr Late toxicity to hydralazine resembling systemic lupus erythematosus or rheumatoid arthritis. Am J Med. 1973 Jan;54(1):58–72. doi: 10.1016/0002-9343(73)90084-3. [DOI] [PubMed] [Google Scholar]
- REMY C. N. Metabolism of thiopyrimidines and thiopurines. S-Methylation with S-adenosylmethionine transmethylase and catabolism in mammalian tissues. J Biol Chem. 1963 Mar;238:1078–1084. [PubMed] [Google Scholar]
- Scanlon P. D., Raymond F. A., Weinshilboum R. M. Catechol-O-methyltransferase: thermolabile enzyme in erythrocytes of subjects homozygous for allele for low activity. Science. 1979 Jan 5;203(4375):63–65. doi: 10.1126/science.758679. [DOI] [PubMed] [Google Scholar]
- Tay B. S., Lilley R. M., Murray A. W., Atkinson M. R. Inhibition of phosphoribosyl pyrophosphate amidotransferase from Ehrlich ascites-tumour cells by thiopurine nucleotides. Biochem Pharmacol. 1969 Apr;18(4):936–938. doi: 10.1016/0006-2952(69)90069-0. [DOI] [PubMed] [Google Scholar]
- Weinshilboum R. M. Human erythrocyte catechol-O-methyltransferase: correlation with lung and kidney activity. Life Sci. 1978 Feb;22(7):625–630. doi: 10.1016/0024-3205(78)90343-0. [DOI] [PubMed] [Google Scholar]
- Weinshilboum R. M., Raymond F. A., Elveback L. R., Weidman W. H. Correlation of erythrocyte catechol-O-methyltransferase activity between siblings. Nature. 1974 Dec 6;252(5483):490–491. doi: 10.1038/252490a0. [DOI] [PubMed] [Google Scholar]
- Weinshilboum R. M., Raymond F. A. Inheritance of low erythrocyte catechol-o-methyltransferase activity in man. Am J Hum Genet. 1977 Mar;29(2):125–135. [PMC free article] [PubMed] [Google Scholar]
- Weinshilboum R. M., Raymond F. A., Pazmiño P. A. Human erythrocyte thiopurine methyltransferase: radiochemical microassay and biochemical properties. Clin Chim Acta. 1978 May 2;85(3):323–333. doi: 10.1016/0009-8981(78)90311-x. [DOI] [PubMed] [Google Scholar]
- Weinshilboum R. M., Sladek S., Klumpp S. Human erythrocyte thiol methyltransferase: radiochemical microassay and biochemical properties. Clin Chim Acta. 1979 Sep 15;97(1):59–71. doi: 10.1016/0009-8981(79)90025-1. [DOI] [PubMed] [Google Scholar]


