Abstract
N- and P/Q-type Ca2+ channels regulate a number of critical physiological processes including synaptic transmission and hormone secretion. These Ca2+ channels are multisubunit proteins, consisting of a pore-forming α1, and accessory β and α2δ subunits each encoded by multiple genes and splice variants. β subunits alter current amplitude and kinetics. The β2a subunit is associated with slowed inactivation, an effect that requires the palmitoylation of two N-terminal cysteine residues in β2a. In the current manuscript, we studied steady state inactivation properties of native N- and P/Q-type Ca2+ channels and recombinant N-type Ca2+ channels. When bovine α1B and β2a and human α2δ were coexpressed in tsA 201 cells, we observed significant variations in inactivation; some cells exhibited virtually no inactivation as the holding potential was altered whereas others exhibited significant inactivation. A similar variability in inactivation was observed in native channels from bovine chromaffin cells. In individual chromaffin cells, the amount of inactivation exhibited by N-type channels was correlated with the inactivation of P/Q-type channels, suggesting a shared mechanism. Our results with recombinant channels with known β subunit composition indicated that inactivation could be dynamically regulated, possibly by alterations in β subunit palmitoylation. Tunicamycin, which inhibits palmitoylation, increased steady-state inactivation of Ca2+ channels in chromaffin cells. Cerulenin, another drug that inhibits palmitoylation, also increased inactivation. Tunicamycin produced a similar effect on recombinant N-type Ca2+ channels containing β2a but not β2b or β2a subunits mutated to be palmitoylation deficient. Our results suggest that Ca2+ channels containing β2a subunits may be regulated by dynamic palmitoylation.
Voltage-dependent Ca2+ channels are multimeric membrane proteins critical for a wide variety of cellular functions, including synaptic transmission, cellular growth, differentiation, and migration. Ca2+ channels are classified based on their biophysical, pharmacological, and, more recently, molecular properties. Functionally, Ca2+ channels have been classified as L-, N-, P/Q-, R- and T-type; ten different α1 genes, with multiple splice variants, have been identified and called α1A-α1I and α1S (1, 2). Both N- and P/Q-type Ca2+ channels have been directly linked to neurotransmitter release (3–6) and are associated with synaptic release proteins (7–11). In chromaffin cells, N-type Ca2+ channels, when activated by themselves, trigger catecholamine release (12). L- and P/Q-type channels can also initiate catecholamine release from chromaffin cells. Many chromaffin cells express an N-type current that exhibits little inactivation during long depolarizations and does not show reduced channel availability at depolarized holding potentials (13). A similar noninactivating N-type current has been described in chick ciliary ganglion (14). However, most neuronal N-type channels show robust inactivation (3), which suggests that inactivation may be regulated in a cell-specific manner. Changes in the inactivation properties of Ca2+ channels may have significant effects on neurotransmitter release, thus providing another means of controlling neuronal activity.
Both N- and P/Q-type Ca2+ channels are multisubunit proteins, consisting of at least three subunits. The α1 subunit is the pore-forming subunit, which is composed of four homologous domains each containing six transmembrane-spanning domains. The β subunit is an intracellular protein, which interacts with the I–II loop of the α1 subunit and probably other sites as well and changes the voltage-dependent properties of the channel (2). In most cases, coexpression of the β subunit increases the surface expression of the channel complex. The other auxiliary subunit, α2δ, is largely extracellular, with a single transmembrane-spanning domain, and has similar but smaller effects on voltage-dependent properties and channel expression. The potential for diversity within these multiprotein complexes is large because each subunit is coded for by multiple genes and splice variants. For instance, the β subunit has four known genes (β1-β4) and multiple splice variants. Different β subunits have opposing effects on voltage-dependent inactivation with the β2a isoform, slowing voltage-dependent inactivation, whereas the other β subunits accelerate inactivation (15). The β2 gene codes for four known splice variants, including the β2a and β2b variants, which differ only at their N terminals (16).
Palmitoylation is a reversible lipid modification seen in a large number of proteins, but its function is not well understood. The enzymes that transfer and remove the long chain fatty acid to proteins usually via thioester bonds to a cysteine have only recently been described (for review see ref. 17). Palmitoylation targets certain proteins to the membrane and alters protein–protein associations in others (17, 18). The β2a subunit contains two cysteines in the N terminus that are known to be palmitoylated (19, 20). The β2b and other β subunits do not have any cysteines in their N termini (16, 20). Mutagenesis studies have demonstrated that these palmitoylated cysteines are important for the reduced inactivation as well as other unique properties of the β2a subunit (21). The palmitoylation levels of certain proteins are dynamically regulated and correlated with the protein's function (22, 23), which raises the possibility of a similar form of regulation in Ca2+ channels. In this report, we describe the effect of inhibiting dynamic palmitoylation on Ca2+ channel function in both native Ca2+ channels found in bovine adrenal chromaffin cells and recombinant channels expressed in tsA cells. Tunicamycin, which inhibits dynamic palmitoylation, increased steady-state inactivation of Ca2+ channels in chromaffin cells, without affecting their peak current size or current–voltage relationship. Tunicamycin also produced a similar effect on recombinant N-type Ca2+ channels containing β2a subunits but not β2b or palmitoylation-deficient β2a(Cys3,4Ser) subunits (i.e., subunits in which Cys3 and Cys4 were altered to Ser). N-type Ca2+ current, made by coexpressing bovine α1B and β2a and human α2δ in tsA 201 cells, exhibited a dramatic variation in inactivation between cells; this result suggests that inactivation may be dynamically regulated, possibly by alterations in β subunit palmitoylation.
Methods
Bovine Chromaffin Cell Ca2 + Channel cDNAs.
Cloning of the bovine chromaffin cell α1B and β2a (GenBank no. AF174417) and β2b (GenBank no. AF174418) subunit cDNAs from a bovine chromaffin cell library was previously described (24). β2a(Cys3,4Ser) subunit cDNA was a kind gift of Marlene Hosey (Northwestern University, Chicago, IL). The human α2δ subunit cDNA was kindly provided by R. J. Miller (University of Chicago, Chicago, IL). Each subunit was subcloned into pcDNA3.1(+) (Invitrogen) for transfection. CD8, a T lymphocyte cell surface protein, was cotransfected to allow identification of transiently transfected cells.
Cell Culture and Transfection.
Bovine chromaffin cells were prepared and cultured as previously described (13). Briefly, bovine adrenal glands were digested with collagenase, and the dissociated cells were purified by density gradient centrifugation. Cells were plated on collagen coated glass coverslips (0.15 × 106 cells/cm2) and maintained at 37°C in 92.5% air/7.5% CO2 and 90% humidity. Half of the media was replaced daily and recordings were done 2–5 days after plating. The cultures are somewhat enriched for epinephrine- over norepinephrine-containing cells.
tsA 201 cells were used for transient transfection and were grown in MEM containing 10% FCS/2 mM glutamine/100 units/ml penicillin/100 μg/ml streptomycin. Cells were maintained at 37°C in 95% air/5% CO2 and 90% humidity. Cells were transfected with Qiagen (Chatsworth, CA)-purified plasmids in a 15:10:5:3 ratio of α1B, either β2a or β2b or β2a(Cys3,4Ser), α2δ, and CD8 DNAs by using Lipofectamine Plus (GIBCO/BRL). Cells were replated for electrophysiology on poly-d-lysine-coated glass coverslips the day before recording. Recordings were done 50–72 h after transfection. Transfected cells were visually detected after incubation with anti-CD8 attached microbeads (Dynal, Great Neck, NY).
Electrophysiology.
Cells were voltage-clamped in the whole-cell configuration of the patch clamp mode with List (List Electronics, Darmstadt, Germany) EPC-7 or AxoPatch 1C (Axon Instruments, Foster City, CA) patch clamps. Gigaohm seals were obtained in an extracellular Tyrode's solution (130 mM NaCl/20 mM glucose/10 mM Hepes/1 mM MgCl2/2 mM KCl/10 mM CaCl2, pH adjusted to 7.3 with NaOH). ICa was measured in an extracellular tetraethylammonium (TEA)/Ba2+ solution (140 mM TEACl/10 mM glucose/10 mM Hepes/10 mM BaCl2 for transfected cells or 2 mM BaCl2 for chromaffin cells, pH adjusted to 7.3 with TEAOH). For chromaffin cell recordings, 1 μM nitrendipine and 100 nM tetrodotoxin were added to the extracellular TEA/Ba2+ solution to block L-type Ca2+ channels and sodium channels, respectively. Electrodes were pulled from glass capillary tubes (Drummond Scientific, Broomall, PA), coated with Sylgard (Dow-Corning) and fire polished to a final resistance of approximately 2 MΩ when filled with a CsCl-based internal solution (110 mM CsCl/10 mM EGTA/20 mM Hepes/4 mM MgCl2/0.3 mM GTP/6 mM ATP/14 mM creatine phosphate, pH adjusted to 7.3 with CsOH). For chromaffin cell recording, the cells were continuously perfused with recording solution at a rate of 2–3 ml/min. In some bovine chromaffin cell recordings, ω-conotoxin GVIA (ω-Cgtx; Alomone Labs, Jerusalem, Israel) was used to separate the total ICa into ω-Cgtx-insensitive (P/Q-type current) and ω-Cgtx-sensitive (N-type current). ω-Cgtx was added to the bath at a final concentration of 1 μM.
Some cells were treated with tunicamycin (Sigma) or cerulenin (Biomol) before recording. Tunicamycin was dissolved in DMSO at 10 mg/ml and stored at 4°C. Cells were placed in Opti-MEM media (GIBCO/BRL) containing freshly diluted tunicamycin (5.95 μM) or 0.05% DMSO for 1 h at 37°C. Using a short incubation time minimizes tunicamycin's effects on glycosylation. Cerulenin was dissolved in 95% ethanol and diluted in Opti-MEM to a final concentration of 98.5 μM, and cells were treated for 2 h at 37°C. Control experiments were completed in the presence of the appropriate carrier, i.e., tunicamycin experiments were compared with DMSO controls, and cerulenin experiments were compared with ethanol controls.
Steady-state inactivation curves were generated by 25-ms step depolarizations, delivered either every 30 or 60 s to a test pulse of 0 mV or +10 mV for bovine chromaffin cells or transfected tsA cells, respectively. Cells were held at various holding potentials for 60 s to attain steady-state inactivation before the test pulse. For Fig. 2, steady-state inactivation curves from each cell were obtained and then ω-Cgtx was added to the bath to block N-type current before repeating the inactivation protocol. The ω-Cgtx-insensitive current (or P/Q-type current), which remained after the addition of ω-Cgtx, was subtracted from the total current to obtain the ω-Cgtx-sensitive current (N-type current). Current–voltage (I–V) relationships were obtained by 25-ms step depolarizations to various test potentials every 30 s. All data presented here are leak- and capacitance-subtracted. Series resistance was partially compensated (>60%) by using the series resistance compensation circuit of the patch clamp.
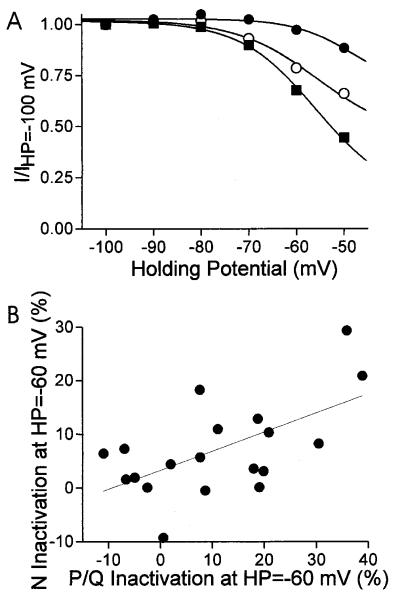
Figure 2.
Variability of steady state inactivation properties in individual bovine chromaffin cells. Steady-state inactivation was recorded after 60 s at each holding potential and plotted as normalized Ca2+ current versus holding potential. (A) Steady-state inactivation of total Ca2+ current in three representative cells. Ca2+ current was elicited by a step depolarization to 0 mV. (B) The amount of inactivation observed at −60 mV, as compared with −100 mV, was correlated between N- and P/Q-type Ca2+ currents. Figure plots the percentage of inactivation at HP = −60 mV of ω-Cgtx-sensitive (N-type) versus ω-Cgtx-insensitive (P/Q-type) current. Each point represents a single cell. ω-Cgtx was used to isolate the N- and P/Q-type currents. The solid line is a linear regression fit to the data. The correlation coefficient for the fit is 0.595 whereas P (the probability that R is zero) is 0.007.
Data Analysis.
For steady-state inactivation curves, ICa from each cell at each holding potential were normalized to the peak current at the most hyperpolarized holding potential and displayed as a function of holding potential. The normalized data were averaged across cells. The peak current size as plotted in the bar graphs is defined as the peak ICa elicited from the most hyperpolarized holding potential in the inactivation protocol. The Student t test was used to compare treatment groups.
Results
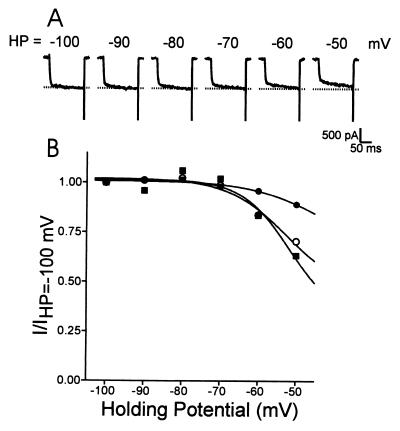
We have previously described a noninactivating N-type Ca2+ channel in bovine chromaffin cells (13). Like other N-type channels, the chromaffin cell channel is sensitive to ω-Cgtx. But, unlike most other N-type channels, it exhibits little voltage-dependent inactivation as the holding potential was changed in the range of −100 mV to −50 mV; most neuronal N-type channels exhibit robust inactivation in this voltage range (25). We concluded that the noninactivating N-type current comprised bovine α1B, β2a, and α2δ because coexpression of these bovine cDNAs, in either Xenopus oocytes or HEK 293 cells, produced a noninactivating N-type Ca2+ current that mimicked the endogenous chromaffin cell currents (24). Surprisingly, however, not every cell exhibited noninactivating N-type currents, even though every transfection included the same Ca2+ channel subunits. Fig. 1A shows Ca2+ currents from a noninactivating tsA cell, transfected with bovine α1B and β2a and human α2δ, elicited by a 160-ms depolarization to 10 mV from a variety of holding potentials. Each holding potential was maintained for 60 s before depolarizing the cell. Fig. 1B plots channel availability as a function of holding potential for three different cells treated in an identical manner, including the one shown in Fig. 1A. For this figure, the peak current recorded at each holding potential was normalized by the peak current obtained at HP = −100 mV. Note that one cell shows little change in channel availability as the holding potential was changed, but that the other two show large changes. It is unlikely that these results are due to complementation by an endogenous β subunit because there was no measurable current observed in experiments where β2a was not coexpressed with α1B and α2δ, suggesting that no endogenous β subunit was present (not shown). In heterologous expression systems, the β2a subunit exhibits the least voltage-dependent inactivation regardless of the α1 subunit (2, 21). Mutagenesis studies have mapped this property of the β2a subunit to the N terminus at the site of two cysteines that are palmitoylated (21). The variability in channel availability shown in Fig. 1 may involve β subunit palmitoylation. [Channel availability was not tested at very depolarized potentials (>−40 mV) because these potentials activate Ca2+ currents. N-type Ca2+ channel inactivation appears to involve some current dependence (see ref. 26), which would complicate studies of voltage-dependent inactivation.]
Figure 1.
Variability of steady-state inactivation properties of heterologous N-type channels expressed in tsA 201 cells. The cells were transiently transfected with bovine α1B, bovine β2a, and α2δ subunits. (A) Currents elicited by test depolarizations to 10 mV. The holding potential was varied, as indicated, in the range from −100 mV to −50 mV. The dashed line corresponds to the peak current value recorded at −100 mV. (B) Normalized current amplitude obtained by dividing peak current at each holding potential by the peak current obtained at −100 mV, for three different cells. Each holding potential was maintained for 60 s, before a test pulse to +10 mV was applied to determine channel availability. There was no evidence of complementation by an endogenous β subunit.
During the course of our earlier studies we observed that the inactivation properties of endogenous Ca2+ currents in bovine chromaffin cells also varied considerably (Fig. 2). In these experiments, L-type Ca2+ currents were blocked with nitrendipine, and >95% of the remaining current could be blocked with a combination of ω-Cgtx and ω-agatoxin IVA, suggesting that only N- and P/Q-type Ca2+ channels are present on these cells. Steady-state inactivation curves from three representative cells, for both N- and P/Q-type Ca2+ current, are plotted in Fig. 2. The inactivation of total ICa at a holding potential of −60 mV (HP-60 mV) ranges from 3% to 32%, and this variability is seen even between experiments performed on the same day. Interestingly, when the experiments were done under conditions that allowed separation of the two current types, we observed a significant correlation between the inactivation of the N- and P/Q-type currents at HP = −60 mV (Fig. 2B). This observation suggested to us that inactivation of the two current types may be controlled by a shared mechanism. The amount of inactivation exhibited at HP = −60 mV was not correlated with peak current size or the fraction of total current that is N-type current (data not shown). Previously, we had interpreted data of the type shown in Fig. 2 to mean that the cells were expressing different β subunits, which can give rise to changes in steady state inactivation. In light of our results shown in Fig. 1, where steady state inactivation varied even in cells that presumably expressed Ca2+ channels with a constant subunit composition, we wanted to determine whether some of the differences observed in chromaffin cells were due to the palmitoylation state of β2a.
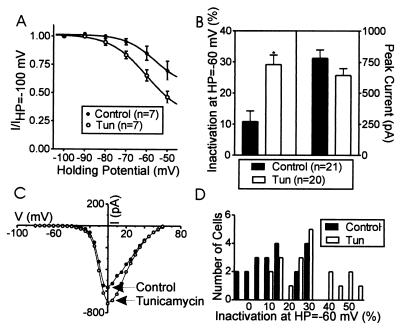
In the experiments shown in Fig. 3, tunicamycin (5.95 μM) was used to alter cellular palmitoylation. Cells were exposed to tunicamycin for 1 h before the experiment. Fig. 3 shows averaged data from chromaffin cells in the presence and absence of tunicamycin. The steady state inactivation curve was shifted in the hyperpolarizing direction by tunicamycin (Fig. 3A). When the amount of inactivation observed at HP = −60 mV, as compared with HP = −100 mV, is plotted, it is clear that tunicamycin induced a significant increase in inactivation (10.8 ± 3.42%, control, 29.2 ± 3.02%, tunicamycin, Fig. 3B). Tunicamycin did not affect the kinetics of activation or inactivation within the 25-ms test pulse (not shown). Nor was the current amplitude significantly altered by tunicamycin or its voltage dependence of activation (Fig. 3 B and C). Fig. 3D plots the distribution of inactivation for all of the individual cells observed at HP = −60 mV (compared with −100 mV) in both control and tunicamycin-treated cells. Although there is overlap in the two populations, they are clearly shifted, one relative to the other.
Figure 3.
Tunicamycin, a compound that blocks palmitoylation, shifted the steady state inactivation properties of chromaffin cells in the hyperpolarizing direction. (A) Steady-state inactivation properties for a group of control cells and cells treated with tunicamycin. (B) Comparison of inactivation at HP = −60 mV compared with HP = −100 mV and peak current size with and without tunicamycin treatment. The * indicates a statistical difference between control and treatment group (P < 0.05). (C) Plot of current–voltage (I–V) relationship in two representative cells with and without tunicamycin treatment. No significant shift in activation parameters was apparent. (D) Distribution of inactivation observed in different cells at HP = −60 mV. Cells treated with tunicamycin are to the right of the distribution, i.e., show large inactivation, whereas control cells are to the left, i.e., show more modest inactivation.
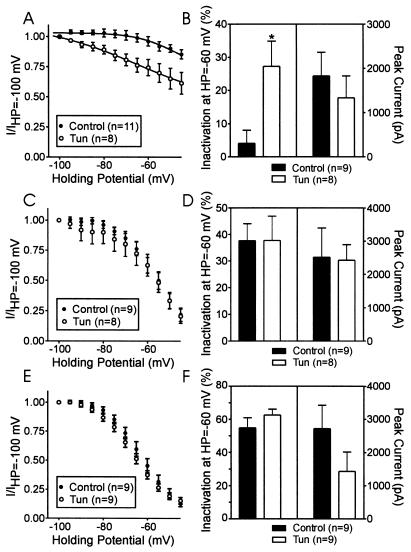
The bovine β2a subunit contains two cysteines in the N-terminal region, which, like the rat brain β2a, may be palmitoylated. In contrast, β2b differs from β2a only in the N-terminal region; in β2b, the 16-aa N terminus of β2a is replaced by an unrelated sequence of 43 aa, which contain no cysteines. If the effects of tunicamycin on Ca2+ channel inactivation are due to decreased palmitoylation of the β2a subunit, tunicamycin should have similar effects on cloned channels containing β2a subunits but no effect on channels containing β2b subunits. Fig. 4 demonstrates that tunicamycin shifted in the hyperpolarizing direction, compared with control, the steady state inactivation properties of recombinant N-type channels made by coexpressing α1B, β2a, and α2δ in tsA cells (Fig. 4A). When the percentage of inactivation observed at HP = −60 mV as compared with HP = −100 mV is plotted, it is clear that tunicamycin induced a significant increase in inactivation (4.09 ± 3.97%, control; 27.29 ± 7.6%, tunicamycin; Fig. 4B). Tunicamycin did not affect the current amplitude significantly (Fig. 4B) nor the voltage-dependence of activation (not shown). In contrast, tunicamycin had no effect on steady state inactivation properties or peak current amplitude of N-type Ca2+ channels constructed by coexpressing α1B, β2b, and α2δ (Fig. 4 C and D) or by channels constructed by coexpressing α1B and α2δ with a palmitoylation-deficient β2a subunit, where the palmitoylation sites at Cys3 and Cys4 were altered to Ser (Fig. 4 E and F). As expected, channels made from these mutant β2a(Cys3,4Ser) subunits exhibited robust inactivation (21). [Although the tunicamycin exposures did not significantly reduce current amplitude, there was a consistent trend toward smaller current amplitudes, a process which may involve membrane targeting (19).]
Figure 4.
Tunicamycin shifted the steady state inactivation properties of recombinant N-type Ca2+ channels in tsA cells transfected with the bovine α1B, bovine β2a, and α2δ subunits. (A) Steady-state inactivation properties for a group of control cells and cells treated with tunicamycin. (B) Comparison of inactivation at HP = −60 mV compared with HP = −100 mV and peak current size with and without tunicamycin treatment, in the same group of cells as shown in A. (C) Steady-state inactivation properties for control cells transfected with the bovine α1B, bovine β2b, and α2δ and identical cells treated with tunicamycin. (D) Comparison of inactivation at HP = −60 mV compared with HP = −100 mV and peak current size with and without tunicamycin treatment in the same group of cells as shown in C. (E) Steady-state inactivation properties for control cells transfected with the bovine α1B, bovine β2a(Cys3,4Ser), and α2δ and identical cells treated with tunicamycin. (F) Comparison of inactivation at HP = −60 mV compared with HP = −100 mV and peak current size with and without tunicamycin treatment in the same group of cells as shown in E.
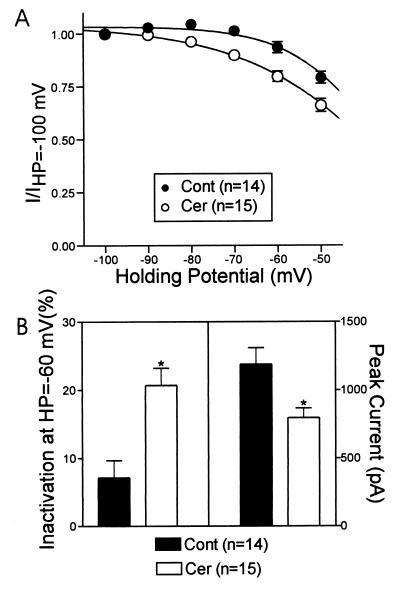
Tunicamycin is a nucleoside antibiotic that can inhibit both protein glycosylation and palmitoylation (22, 23, 27). Cerulenin is another compound that inhibits palmitoylation but with no known effects on glycosylation (28). Fig. 5 shows that incubation of cells with cerulenin (98.5 μM) increased the steady-state inactivation of the Ca2+ currents in bovine chromaffin cells at all holding potentials greater than −80 mV (Fig. 5A). Cerulenin increased the amount of inactivation at HP = −60 mV by about 3-fold (Fig. 5B), similar to the effects of tunicamycin. In contrast, cerulenin significantly reduced the peak current size (Fig. 5B) but, like tunicamycin, had no effect on the voltage-dependence of the I–V relationship (not shown).
Figure 5.
Cerulenin, another compound that blocks palmitoylation, shifted the steady state inactivation properties of chromaffin cells in the hyperpolarizing direction. (A) Steady-state inactivation properties for a group of control cells and cells treated with cerulenin. (B) Comparison of inactivation at HP = −60 mV compared with HP = −100 mV and peak current size with and without cerulenin treatment.
Discussion
Previous studies in heterologous expression systems have shown that Ca2+ currents exhibit the least inactivation when the β2a subunit is included as part of the channel, regardless of which α1 subunit is expressed (2, 21). Mutagenesis studies have mapped this property of the β2a subunit to the N terminus at the site of two cysteines that are palmitoylated; preventing β2a palmitoylation by substitution of serine for cysteine in β2a(Cys3,4Ser) subunits affects channel availability at different holding potentials (21). β2b subunits are very similar to β2a except their N-terminal region does not have any cysteines required for palmitoylation. Coexpression of either β2b or β2a(Cys3,4Ser) with α1A, α1B, or α1E typically produces Ca2+ currents with robust inactivation. In our current study, we found that there was considerable variation in the inactivation properties of recombinant N-type channels, even though it appeared likely that the channel subunit composition remained constant. This variable inactivation suggested that dynamic palmitoylation may regulate channel inactivation in transfected tsA cells. Because similar data were obtained with native Ca2+ channels in bovine chromaffin cells, it appeared possible that dynamic palmitoylation could alter these channels, thereby affecting secretion.
Because it is known that several signaling proteins are regulated by dynamic palmitoylation, we wanted to test the hypothesis that dynamic palmitoylation might regulate Ca2+ channel function. Tunicamycin is a nucleoside antibiotic that inhibits protein glycosylation and palmitoylation (22, 23, 27). Tunicamycin altered channel properties in both the heterologous and native systems; it selectively increased steady-state inactivation of Ca2+ channels in bovine chromaffin cells and heterologously expressed N-type channels containing β2a subunits but not β2b or β2a(Cys3,4Ser) subunits. These results suggest that the effects of tunicamycin are due to inhibition of posttranslational palmitoylation and point to an additional mode of regulation of Ca2+ channels. It appears that not all of the β2a subunits, in either chromaffin cells or in tsA cells, were unpalmitoylated in the presence of tunicamycin. There was much less steady-state inactivation present in chromaffin cells or tsA cells treated with tunicamycin compared with data from tsA cells using the palmitoylation-deficient β2a(Cys3,4Ser) mutant subunit. Alternatively, chromaffin cells may simultaneously express multiple types of β subunits, which can give rise to some of the differences observed.
Compared with other lipid modifications, palmitoylation is unique in its reversibility and, in some cases, appears to be regulated and reversible in a manner similar to phosphorylation. This modification is seen in a variety of substrates, including some membrane-bound receptors and ion channels, and intracellular proteins localized to the plasma membrane, such as Ras and the Gα subunits of heterotrimeric G proteins. Interestingly, several proteins involved in neurotransmitter release are also palmitoylated, e.g., synaptosome-associated protein of 25 kDa (SNAP-25) and synaptotagmin I (29–32). Delineating the role of palmitoylation in protein function has been difficult, but, in some proteins, palmitoylation is clearly linked to initial membrane targeting; thereafter other mechanisms may be involved in maintaining membrane localization (29, 33). Palmitoylation may also target certain proteins to membrane subdomains (for review, see ref. 17). In some cases, palmitoylation affects protein–protein interactions; e.g., palmitoylation of Gsα increases its affinity for the βγ subunit (34). A well-documented example of dynamic regulation of palmitoylation is seen after β2-adrenergic receptor stimulation, which results in increased palmitate turnover of both the β2-adrenergic receptor and Gsα, but the physiological significance of this increased turnover is unknown (35, 36). Difficulties encountered in these types of studies of palmitoylation include low abundance of target proteins, low specific activity of tritiated palmitate, and inability to correlate palmitate protein levels with activity. Furthermore, most studies have been done in cell-free systems or by using site-directed mutagenesis of the modified cysteines; in these systems it is not feasible to examine reversible palmitoylation. Patterson and Skene (22, 23) have demonstrated the use of tunicamycin as a specific, cell-permeable inhibitor of dynamic palmitoylation and described methods to distinguish its effects on palmitoylation versus those on glycosylation. One such study demonstrated that tunicamycin rapidly and reversibly inhibited growth cone-mediated neurite extension in PC12 cells and dorsal root ganglion, suggesting a role for dynamic fatty acylation in neurite elongation (22).
The role of the palmitoylated cysteines in the β2a subunit of Ca2+ channels has been examined with mutagenesis studies. Hosey and colleagues (19, 37, 38) first identified the sites of palmitoylation and examined their role in membrane targeting of β2a in tsA cells. Interestingly, their results demonstrate that, whereas palmitoylation is necessary for membrane targeting of the β2a subunit when expressed alone, palmitoylation is not necessary for plasma membrane localization when the β2a subunit is coexpressed with the α1C subunit. In addition, these authors reported that palmitoylation-deficient β2a containing L-type channels produced dramatically smaller ionic currents with no change in gating current compared with their palmitoylated counterparts. Although our results suggest that there may be a trend toward diminished current amplitude, they were not significant. It is possible that tunicamycin inhibited palmitoylation of only a fraction of the expressed subunits in our experiments, perhaps not enough to see a reduction in current size. Longer tunicamycin treatments (16 h) reduced the size of the ionic currents in bovine chromaffin cells by about 25%, but whether this reduced current size is due to inhibition of palmitoylation or glycosylation is unclear. Qin et al. (21) have also used site-directed mutagenesis to study the role of palmitoylation of the β2a subunit in Ca2+ channel function. Their experiments, which were done in Xenopus oocytes, demonstrated that palmitoylation-deficient β2a subunits left-shifted the steady-state inactivation curves of α1E channels compared with α1E channels containing normal β2a subunits or α1E channels alone. The study by Qin et al. (21) is in agreement with our results using tunicamycin and supports the possibility that Ca2+ channels containing β2a subunits may be regulated by palmitoylation.
Our experiments were initiated, in part, because of the variable inactivation we observed in bovine chromaffin cell Ca2+ channels. Several mechanisms exist that could account for this variability besides regulated inactivation, and these include changes in channel subunit composition, localization, or interactions. There is precedent for regulated changes in β subunit expression in development and in differentiation (39). Also, there is evidence that suggests that preferential association may exist between certain subunit combinations (2). We believe based on our cloning and reverse transcription-PCR results (unpublished observations) that multiple α1B and β subunits exist within populations of bovine chromaffin cells, but whether this heterogeneity exists within single cells is unknown. Certainly, one or more of these mechanisms may account for the variable inactivation in bovine chromaffin cell Ca2+ channels.
Bovine chromaffin cells express three types of Ca2+ channels, and each is regulated by different mechanisms. The N- and P/Q-type channels are differentially modulated by G proteins and subject to negative feedback control by endogenous neurotransmitters (40). N- and P/Q-type channels are also regulated by proteins involved in exocytosis. For instance, expression of syntaxin-1A reduces N- and P/Q-type channel availability (7, 41). The L-type (or facilitation) channel, which is normally quiescent, is activated by receptors coupled to protein kinase A (42). The activity of all three channels controls catecholamine secretion in these cells, which allows them to respond appropriately to extracellular stimuli. In addition to regulation by G proteins, phosphorylation, and interactions with exocytotic machinery, these channels are subject to other less well-understood types of regulation. Each α1 subunit combines with a β subunit that contributes significantly to its biophysical properties. Ca2+ channels containing β2a subunits may be subject to an additional mode of regulation. It is intriguing to speculate that dynamic regulation of voltage-dependent inactivation via palmitoylation may be another mechanism for regulating Ca2+ channel function although physiological regulators of palmitoylation are still unknown.
Acknowledgments
We thank Dr. Marlene Hosey for her kind gift of the β2a(Cys3,4Ser) subunit and Chien-Yuan Pan for preparing the chromaffin cells. This work was supported by a National Institutes of Health (National Institute of Neurological Disorders and Stroke) grant to A.P.F.
Abbreviations
- HP
holding potential
- ω-Cgtx GVIA
ω-conotoxin GVIA
- ICa
calcium current
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.160589697.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.160589697
References
- 1.Dunlap K, Luebke J I, Turner T J. Trends Neurosci. 1995;18:89–98. [PubMed] [Google Scholar]
- 2.Walker D, De Waard M. Trends Neurosci. 1998;21:148–154. doi: 10.1016/s0166-2236(97)01200-9. [DOI] [PubMed] [Google Scholar]
- 3.Hirning L D, Fox A P, McCleskey E W, Olivera B M, Thayer S A, Miller R J, Tsien R W. Science. 1988;239:57–61. doi: 10.1126/science.2447647. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi T, Momiyama A. Nature (London) 1993;366:156–158. doi: 10.1038/366156a0. [DOI] [PubMed] [Google Scholar]
- 5.Turner T J, Adams M E, Dunlap K. Proc Natl Acad Sci USA. 1993;90:9518–9522. doi: 10.1073/pnas.90.20.9518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wheeler D B, Randall A, Tsien R W. Science. 1994;264:107–111. doi: 10.1126/science.7832825. [DOI] [PubMed] [Google Scholar]
- 7.Bezprozvanny I, Scheller R H, Tsien R W. Nature (London) 1995;378:623–626. doi: 10.1038/378623a0. [DOI] [PubMed] [Google Scholar]
- 8.el Far O, Charvin N, Leveque C, Martin-Moutot N, Takahashi M, Seagar M J. FEBS Lett. 1995;361:101–105. doi: 10.1016/0014-5793(95)00156-4. [DOI] [PubMed] [Google Scholar]
- 9.Leveque C, el Far O, Martin-Moutot N, Sato K, Kato R, Takahashi M, Seagar M J. J Biol Chem. 1994;269:6306–6312. [PubMed] [Google Scholar]
- 10.Sheng Z H, Rettig J, Takahashi M, Catterall W A. Neuron. 1994;13:1303–1313. doi: 10.1016/0896-6273(94)90417-0. [DOI] [PubMed] [Google Scholar]
- 11.Sheng Z H, Rettig J, Cook T, Catterall W A. Nature (London) 1996;379:451–454. doi: 10.1038/379451a0. [DOI] [PubMed] [Google Scholar]
- 12.Artalejo C R, Adams M E, Fox A P. Nature (London) 1994;367:72–76. doi: 10.1038/367072a0. [DOI] [PubMed] [Google Scholar]
- 13.Artalejo C R, Perlman R L, Fox A P. Neuron. 1992;8:85–95. doi: 10.1016/0896-6273(92)90110-y. [DOI] [PubMed] [Google Scholar]
- 14.Stanley E F, Goping G. J Neurosci. 1991;11:985–993. doi: 10.1523/JNEUROSCI.11-04-00985.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olcese R, Qin N, Schneider T, Neely A, Wei X, Stefani E, Birnbaumer L. Neuron. 1994;13:1433–1438. doi: 10.1016/0896-6273(94)90428-6. [DOI] [PubMed] [Google Scholar]
- 16.Castellano A, Perez-Reyes E. Biochem Soc Trans. 1994;22:483–488. doi: 10.1042/bst0220483. [DOI] [PubMed] [Google Scholar]
- 17.Resh M D. Biochim Biophys Acta. 1999;1451:1–16. doi: 10.1016/s0167-4889(99)00075-0. [DOI] [PubMed] [Google Scholar]
- 18.Dunphy J T, Linder M E. Biochim Biophys Acta. 1998;1436:245–261. doi: 10.1016/s0005-2760(98)00130-1. [DOI] [PubMed] [Google Scholar]
- 19.Chien A J, Gao T, Perez-Reyes E, Hosey M M. J Biol Chem. 1998;273:23590–23597. doi: 10.1074/jbc.273.36.23590. [DOI] [PubMed] [Google Scholar]
- 20.Birnbaumer L, Qin N, Olcese R, Tareilus E, Platano D, Costantin J, Stefani E. J Bioenerg Biomembr. 1998;30:357–375. doi: 10.1023/a:1021989622656. [DOI] [PubMed] [Google Scholar]
- 21.Qin N, Platano D, Olcese R, Costantin J L, Stefani E, Birnbaumer L. Proc Natl Acad Sci USA. 1998;95:4690–4695. doi: 10.1073/pnas.95.8.4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patterson S I, Skene J H. J Cell Biol. 1994;124:521–536. doi: 10.1083/jcb.124.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patterson S I, Skene J H. Methods Enzymol. 1995;250:284–300. doi: 10.1016/0076-6879(95)50079-0. [DOI] [PubMed] [Google Scholar]
- 24.Cahill A L, Hurley J H, Fox A P. J Neurosci. 2000;20:1685–1693. doi: 10.1523/JNEUROSCI.20-05-01685.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fox A P, Nowycky M C, Tsien R W. J Physiol. 1987;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cox D H, Dunlap K. J Gen Physiol. 1994;104:311–336. doi: 10.1085/jgp.104.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buckley B J, Whorton A R. Am J Physiol. 1997;273:C1298–C1305. doi: 10.1152/ajpcell.1997.273.4.C1298. [DOI] [PubMed] [Google Scholar]
- 28.Schlesinger M J, Malfer C. J Biol Chem. 1982;257:9887–9890. [PubMed] [Google Scholar]
- 29.Gonzalo S, Linder M E. Mol Biol Cell. 1998;9:585–597. doi: 10.1091/mbc.9.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vogel K, Roche P A. Biochem Biophys Res Commun. 1999;258:407–410. doi: 10.1006/bbrc.1999.0652. [DOI] [PubMed] [Google Scholar]
- 31.Chapman E R, An S, Barton N, Jahn R. J Biol Chem. 1994;269:27427–27432. [PubMed] [Google Scholar]
- 32.Chapman E R, Blasi J, An S, Brose N, Johnston P A, Sudhof T C, Jahn R. Biochem Biophys Res Commun. 1996;225:326–332. doi: 10.1006/bbrc.1996.1174. [DOI] [PubMed] [Google Scholar]
- 33.Chamberlain L H, Burgoyne R D. Biochem J. 1998;335:205–209. doi: 10.1042/bj3350205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iiri T, Backlund P S, Jr, Jones T L, Wedegaertner P B, Bourne H R. Proc Natl Acad Sci USA. 1996;93:14592–14597. doi: 10.1073/pnas.93.25.14592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mouillac B, Caron M, Bonin H, Dennis M, Bouvier M. J Biol Chem. 1992;267:21733–21737. [PubMed] [Google Scholar]
- 36.Degtyarev M Y, Spiegel A M, Jones T L. J Biol Chem. 1993;268:23769–23772. [PubMed] [Google Scholar]
- 37.Chien A J, Carr K M, Shirokov R E, Rios E, Hosey M M. J Biol Chem. 1996;271:26465–26468. doi: 10.1074/jbc.271.43.26465. [DOI] [PubMed] [Google Scholar]
- 38.Gao T, Chien A J, Hosey M M. J Biol Chem. 1999;274:2137–2144. doi: 10.1074/jbc.274.4.2137. [DOI] [PubMed] [Google Scholar]
- 39.Vance C L, Begg C M, Lee W L, Haase H, Copeland T D, McEnery M W. J Biol Chem. 1998;273:14495–14502. doi: 10.1074/jbc.273.23.14495. [DOI] [PubMed] [Google Scholar]
- 40.Currie K P, Fox A P. Neuron. 1996;16:1027–1036. doi: 10.1016/s0896-6273(00)80126-9. [DOI] [PubMed] [Google Scholar]
- 41.Rettig J, Sheng Z H, Kim D K, Hodson C D, Snutch T P, Catterall W A. Proc Natl Acad Sci USA. 1996;93:7363–7368. doi: 10.1073/pnas.93.14.7363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Artalejo C R, Ariano M A, Perlman R L, Fox A P. Nature (London) 1990;348:239–242. doi: 10.1038/348239a0. [DOI] [PubMed] [Google Scholar]