Abstract
In non-disabled (ND) individuals, reflexes are modulated by influences related to physiologic state (e.g., posture, joint position, load) and activation history. Repeated activation of the H-reflex results in post-activation depression (PAD) of the response amplitude. The modulation associated with physiologic state and activation history is suppressed or abolished in individuals with spinal cord injury (SCI). While posture is known to affect H-reflex amplitude and PAD in non-disabled individuals, the effect of posture on PAD in SCI individuals is not known. Further, while the amount of PAD is also known to be influenced by the stimulus rate and by the amplitude of the evoked reflex, the interaction of posture with stimulus parameters has not been previously investigated in either group. We investigated differences in PAD of the soleus H-reflex between SCI subjects and ND subjects during sitting versus supported standing. Subjects were tested using paired conditioning-test stimulus pulses of 2.5 sec and 5 sec interpulse intervals (ISI) and with stimulus intensity adjusted to evoke reflex responses of 20% and 40% of the maximum motor response. We found standing posture to be associated with significantly less PAD in SCI subjects compared to ND subjects. In both groups, shorter ISIs and smaller reflex amplitudes were associated with greater PAD of the H-reflex. These results indicate that postural influences on post-activation modulation, while present, are impaired in individuals with chronic incomplete SCI.
Keywords: standing, load, reflex modulation, rate-dependent depression
Appropriate modulation of spinal reflex activity contributes to the production of purposeful movement. The soleus H-reflex is influenced by supraspinal, homonymous, and heteronymous modulation, as well as intrinsic motoneuronal properties (see Misiaszek [1]). Accordingly, this reflex is influenced by the state of the physiologic system, with facilitation or inhibition induced by factors such as joint position [2], muscle activity [3;4], limb load [5;6], and phase of movement [7;8]. The H-reflex is also modulated by posture, wherein standing posture is associated with depressed H-reflex amplitudes relative to sitting [9-12].
Factors such as activation history and stimulus amplitude also influence H-reflex amplitude. Prior activation of the H-reflex in seated non-disabled (ND) subjects is associated with post-activation depression (PAD) of reflex amplitude in a rate-dependent manner. Shorter interstimulus intervals (ISIs) produce greater depression of reflex amplitude, with depression persisting for up to 8 sec [13;14]. In addition to activation history, stimulus amplitude (i.e., size of the test reflex) can influence the degree of post-activation depression, with smaller H-reflexes (i.e., lower stimulus intensities) being more sensitive to depression [15;16].
Functionally, PAD is thought to be important to prevent reactivation of the stretch reflex under dynamic conditions wherein a muscle is subjected to repeated stretch, (e.g., as is the soleus in the terminal stance phase of walking. [17;18]. In ND individuals, posture influences PAD of the soleus H-reflex, with greater depression during standing compared to sitting [19;20]. There are also interactions among the influences of posture and activation history; the H-reflex is less sensitive to high-frequency depression (ISIs of up to 400 ms; 10Hz) in standing compared to seated or prone position [21].
Mechanisms underlying reflex modulation associated with PAD appear to be defective in individuals with spinal cord injury (SCI) or stroke [22-25]. In these individuals, the loss of PAD of the Ia circuit is thought to permit cyclic reactivation of the stretch reflex, manifesting clinically as clonus [26;27]. Clonic muscle activity contributes to the spastic gait patterns observed in SCI individuals, and thereby impedes function. The purpose of this study was to assess differences in responses to postural condition, activation history and reflex size as they relate to the modulation of H-reflex amplitude in individuals with motor-incomplete SCI (iSCI) and ND subjects.
Experiments were conducted on 5 individuals with iSCI (aged 19-63 years, mean±SD: 37±12.5 years) and 7 neurologically healthy volunteers (aged 22-54 years, mean±SD: 32±9.3 years). All iSCI subjects had chronic injury (>1year) due to trauma, and were classified as ASIA C [28] (some movement distal to injury level, but majority of muscles unable to move joint through full range of movement against gravity). All subjects gave written informed consent to participate in this study, which was approved by the University of Miami Institutional Review Board. See Table for iSCI subject demographics.
Table .
Demographic data for subjects with MI-SCI
| Subject number | Gender | Age (years) | Injury level | Duration of Injury (yrs) | Lower extremity motor score | Medication |
|---|---|---|---|---|---|---|
| 1 | M | 42 | C8 | 4 | 8 | Tz (16 mg) |
| 2 | M | 22 | C4 | 2.5 | 9 | none |
| 3 | M | 27 | T5 | 1.5 | 3 | Bc (80 mg) |
| 4 | F | 41 | C5 | 20 | 10 | Bc (20 mg) |
| 5 | F | 53 | T10 | 12 | 12 | none |
Demographic data for subjects with SCI. Injury level indicates most distal level of intact motor innervation. Lower extremity motor scores represent sum of muscle test scores from key muscles of the more affected lower extremity [20]. C=cervical injury, T=thoracic injury, M=male, F=female, Bc=baclofen, Tz=tizanidine. Medication doses indicated are per day.
To assess effects of posture, subjects were tested using paired-pulse stimulation to the posterior tibial nerve (PTN) under each of two conditions: sitting (SIT) and supported standing (SS). In the SIT condition, subjects sat on a padded seat with the knee, ankle, and hip joints at 90 degrees of flexion, and the trunk supported by a backrest. In the SS condition, subjects stood in a standing frame (Easy Stand 5000, Altimate Medical, Inc., Morton, MN) that provided support from the ischial tuberosity to just above the occiput. We selected the supported standing condition as iSCI subjects are unable to stand without support for prolonged periods. Further, it allowed us to eliminate postural perturbations associated with soleus activation [29], as postural instability is associated with decreased H-reflex amplitude [29;30]. Lower extremity load in SS condition was within 5% of the load measured in unsupported standing. While in the standing frame, subjects were instructed to stand quietly and remain still. To assess effects of activation history, subjects were tested under each of two ISI conditions: 2.5 sec and 5 sec. These frequencies were selected based on pilot testing wherein ISIs of < 2 sec evoked excessive clonus in the SS condition in iSCI subjects, and those >5 sec were associated with negligible H-reflex depression. To assess effects of reflex size, stimulus intensity was adjusted to elicit an H-reflex with each of two amplitudes: 20% and 40% of Mmax. To ensure reflex amplitude was a true reflection of motoneuron recruitment level, we analyzed only subjects from whom both small (20 ± 5% Mmax) and large (40 ± 5% Mmax) H-reflexes could be evoked without an accompanying M-wave. ISI and reflex size conditions were presented in random order with an average of 12 (±3) trials for each combination of conditions. All EMG responses were recorded from the more affected (i.e., weaker) lower. The H-reflex was obtained by stimulating the posterior tibial nerve (PTN) with a cathode in the popliteal fossa in the location required the lowest stimulus intensity. The anode was placed at the medial patella. The recording electrodes were placed over the soleus muscle just distal to the belly of the medial gastrocnemius muscle and medial to the Achilles tendon (interelectrode distance = 3mm). Stimulus intensity was incrementally increased to obtain an H-reflex recruitment curve, and further increased to evoke a maximum motor response (Mmax). A 10 second delay was imposed between the test stimulus of one pair and conditioning stimulus of the subsequent pair [13]. The EMG signal was amplified, band pass-filtered, and sampled at 2 kHz (Signal 2, version 4, Cambridge Electronic Design, UK). Data were recorded and analyzed offline using Signal 2, version 4 (Cambridge Electronic Design, UK).
Statistical analysis included two-way factorial analysis of variance with within-subject repeated measures on factors of postural condition, ISI and reflex size. Post-hoc testing was done using Bonferroni analysis (Statistical Package for the Social Sciences, SPSS Inc, Chicago, IL). Additionally, RMS (root-mean square) amplitude of background EMG of the TA and SOL was measured; EMG in all conditions was normalized to EMG in the SIT condition. Summary data for all conditions is given in Figure 1.
Figure 1.
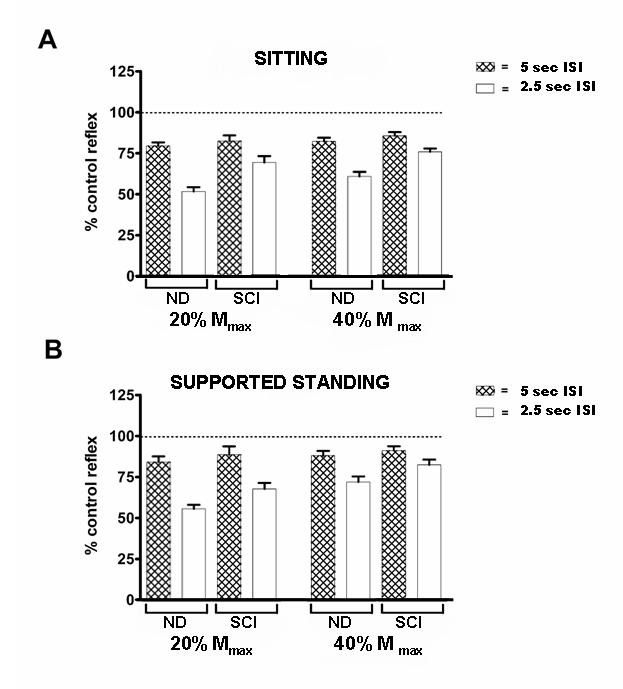
The effect of ISI, amplitude of the evoked reflex and postural condition on the post-activation depression (PAD) of the soleus H-reflex. All data is expressed as a percentage of the control reflex under the same postural condition. Hatched bars indicate reflexes evoked with ISIs of 5 sec, open bars indicate reflexes evoked with 2.5 sec ISIs. Error bars indicate the standard error of the mean (SEM)
Statistical analysis showed a significant interaction between variables of group and ISI (p < 0.001), signifying the response to postural and test amplitude conditions was different between groups depending on test ISI. Data inspection indicated that there was minimal PAD in either group with the 5 sec ISI. The PAD observed with the 2.5 sec ISI was significantly greater for ND subjects compared to iSCI subjects (p < 0.001). When only the 2.5 second ISI was included along with variables of reflex size and postural condition, iSCI subjects showed 17.5% less depression through all conditions compared to ND subjects. This indicated significant between-group differences in influence of both postural condition and reflex size when tested with 2.5 sec ISIs. Therefore, the remainder of results will focus on between-group differences observed under the 2.5 sec ISI test condition.
The factor associated with the largest between-group difference was standing posture. In SS (with 2.5 sec ISI, including both 20% and 40% reflex), the mean test reflex was depressed to 60.4% of conditioning reflex amplitude in the ND subjects compared to 78.4% for iSCI subjects, reflecting a 18% difference between groups. This between-group difference was statistically significant (p = 0.008). In SIT (with 2.5 sec ISI, including responses elicited at both 20% and 40% reflex size), the mean test reflex was depressed to 54.5% of conditioning reflex amplitude in the ND subjects compared to 71.7% for iSCI subjects, reflecting a 17.2% difference between groups. This between-group difference was statistically significant (p = 0.013). Within-group differences in influence of postural condition in the ND group for SIT compared to SS showed significantly less PAD in the SS condition (p = 0.016). In iSCI subjects, the influence of postural condition was also significant (p = 0.038), although to a lesser extent. Figure 2 gives data for one individual with iSCI (Subject 3) showing response obtained in SIT compared to SS conditions.
Figure 2.
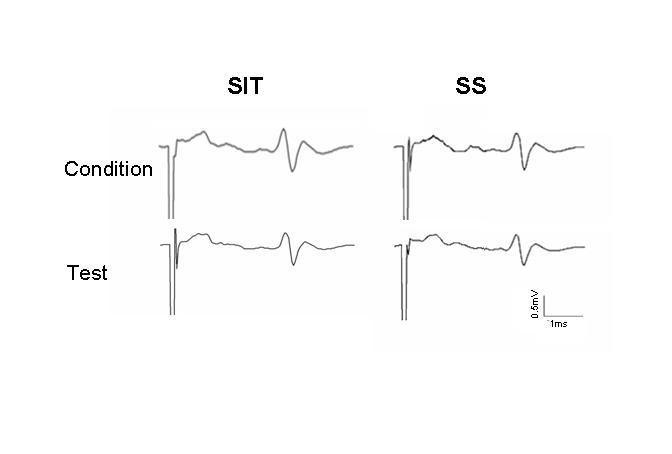
Postural condition has minimal effect on PAD in iSCI subjects. Both conditioning and test reflexes are shown. In this exemplary subject, there is no PAD of the test reflex under conditions of small reflex size (20% Mmax) and short interpulse interval (2.5 ISI) that were most effective in inducing PAD in ND subjects.
There were also large between-group differences in the 20% reflex size (with 2.5 sec ISI, including both SIT and SS conditions). ND subjects exhibited 16.5% greater PAD than iSCI subjects with the 20% reflex size. The mean test reflex was depressed to 51.7% and 68.2% of conditioning reflex amplitude, respectively, for ND and iSCI subjects; this difference was statistically significant (p = 0.024). Reflexes of lower amplitudes (20% Mmax) were more sensitive than reflexes of larger amplitude (40% Mmax) to PAD of H-reflex amplitude. This difference in PAD between reflexes of different size was present in both ND (p < 0.001) and iSCI (p = 0.008) subjects.
The primary finding of this study is that while an effect of posture on PAD was observed in both groups, with less PAD in standing compared to sitting, individuals with iSCI show significantly less PAD during standing than ND individuals. This is consistent with alteration of modulatory influences observed in other reflex circuits that are also presumably regulated via presynaptic inhibitory mechanisms [23;24;31-33]. Perez and Field-Fote [31] demonstrated that, during standing, the influence of the Ia reciprocal inhibition on soleus H-reflex amplitude is significantly less in SCI individuals compared to ND individuals. Others have shown that, in SCI individuals, modulatory influences on soleus H-reflex associated with repeated common peroneal nerve stimulation [23], stretch [24;32], and quadriceps activation [33], are suppressed. Apparently at odds with these findings is the work of Kawashima et al. [11] who showed that posture-dependent modulation of the H-reflex in SCI individuals is enhanced compared to ND individuals. These authors demonstrated that the magnitude of the H-reflex amplitude in supported standing is depressed to a greater degree relative to sitting, in individuals with motor-complete SCI compared to ND individuals. This may suggest that posture-related mechanisms regulating H-reflex amplitude are different than those regulating H-reflex PAD.
Differences in injury severity may offer an alternative explanation for the observed differences between increased efficacy of posture-dependent modulation of H-reflex amplitude in SCI individuals observed by Kawashima et al.[11] and decreased efficacy of posture-dependent modulation of H-reflex PAD observed in our study. Subjects in our study had incomplete (ASIA C) injuries while those in the study by Kawashima et al.[11] had complete SCI. Lee et al.[34] demonstrated that rats with complete spinal cord transection exhibit greater (i.e., more normal) PAD than do those with mild or moderate contusion injuries.
There is increasing evidence to suggest that reflex modulation may be different in complete versus incomplete SCI, but this is an area of considerable controversy in the literature. While some authors have reported increased normalized H-reflex excitability in both individuals with complete injury and those with incomplete injury [35], there is evidence to support the argument that there may be differences in the responses among individuals that is attributable to severity of injury. Schindler-Ivens and Shields [36] reported that subjects with motor-complete SCI exhibit H-reflex amplitudes, thresholds and gains that are not different from ND individuals. In individuals with motor-complete cervical or thoracic SCI, Hiersemenzel et al.[37] demonstrated that, while normalized H-reflex responses were greater in the individuals with tetraplegia, overall reflex amplitudes were not different from those of ND individuals. However, studies of incomplete SCI have come to the opposite conclusion, finding that normalized H-reflex amplitudes are increased in SCI individuals compared to ND individuals [38;39].
In parallel to the disparity observed in H-reflex amplitude, H-reflex modulation in response to loading may be different in individuals with motor-complete injury compared to incomplete injury. Knikou and Rymer [3] demonstrated, in individuals with complete SCI, that mechanical loading of the sole of the foot depressed soleus H-reflex excitability. However, Phadke et al.[38] recently showed that lower extremity loading does not alter H-reflex excitability in individuals with incomplete SCI. These observed differences between individuals with complete versus incomplete SCI are consistent with clinical observations that spasticity is more problematic in individuals with incomplete injury compared to complete injury.
Supraspinal influences are thought to be the primary source of presynaptic inhibition of Ia afferent terminals, and therefore the presumed origin of posture-related H-reflex depression [40;41]. Accordingly, impairment of presynaptic inhibition is thought to underlie the loss, or suppression, of modulation in those with SCI. Studies to clarify the role of supraspinal influence on H-reflex modulation [20;42] have been equivocal. Cowan et al.[42], using electrical cortical stimulation in seated subjects, identified short-latency inhibition to the H-reflex even during voluntary soleus contraction. Conversely, Goulart et al.[20], using transcranial magnetic stimulation, identified an early phase of soleus H-reflex facilitation that was present in supine, sitting and standing. A second phase of facilitation observed in supine and seated positions was absent in standing. While the contribution of supraspinal influences on presynaptic H-reflex modulation remains unclear, if these influences constitute the primary source of PAD of the H-reflex, then differences between ND and SCI subjects in the amount of descending input likely underlie the observed differences in PAD identified in the present study.
Despite evidence that supraspinal influences are essential to posture-related H-reflex modulation, a case can be made that segmental contributions to are equally as important. In individuals with complete SCI, in whom little supraspinal input would be expected, there is still depression of the H-reflex associated with supported standing compared to sitting [11]. Kawashima et al. [11] argued that supported standing provides a condition wherein supraspinal influences are negligible, and therefore reflex modulation arises from the segmental influences. Indeed, lower extremity segmental influences related to muscle and joint receptors have been shown to influence soleus H-reflex amplitude [5;43-46]. Nakazawa et al [5] examined the effects of loading the knee or ankle joint of ND subjects while standing in a reduced-gravity environment (i.e., water tank). Load applied to either joint was associated with significant depression of H-reflex amplitude compared to the unloaded condition, implicating a role for joint afferents in modulation of H-reflex excitability. Abbruzzese et al [43] assessed the influences of cutaneous and muscle afferent input on soleus H-reflex excitability under prone and standing conditions. They concluded that foot muscle afferents exert inhibitory influences on soleus excitability. If segmental influences are the most significant source of posture-dependent H-reflex modulation, then differences observed between ND and SCI subjects in the present study are likely attributable to adaptive changes in the regulation of spinal cord circuitry in chronic SCI.
Finally, it is important to note that three of five subjects in our study took antispasticity medication. Because these are centrally-acting agents it is possible that they had some influence on reflex responses to stimulation in these subjects. However, examination of the data identified no obvious differences in responses among subjects taking and those not taking medications. Further, when data were plotted to inspect for relationships between PAD values and lower extremity motor scores or duration of injury, no such relationships were apparent.
In summary, our findings indicate that there is a relationship between chronically-compromised descending control and mechanisms responsible for posture-related PAD. These results are consistent with the view that altered modulation observed in those with chronic SCI may be due to adaptive changes in the regulation of spinal reflex circuitry [23;47;48]. While our methods do not allow us to identify mechanisms underlying this suppression of modulation, they are surely attributable to the fragmenting and/or distortion of descending inputs to the spinal cord that results in alteration of the relationship between descending input and spinal reflex circuits. These findings contribute to the growing evidence that state-dependent modulation of spinal reflex activity is disrupted in individuals with chronic iSCI.
Acknowledgements
We gratefully acknowledge the technical contributions of Mohd T. Kahn. The study was supported by NIH grant # HD41487 to EFF and by The Miami Project to Cure Paralysis.
Reference List
- 1.Misiaszek JE. The H-reflex as a tool in neurophysiology: its limitations and uses in understanding nervous system function. Muscle Nerve. 2003;28(2):144–160. doi: 10.1002/mus.10372. [DOI] [PubMed] [Google Scholar]
- 2.Knikou M, Rymer Z. Effects of changes in hip joint angle on H-reflex excitability in humans. Exp. Brain Res. 2002;143:149–159. doi: 10.1007/s00221-001-0978-4. [DOI] [PubMed] [Google Scholar]
- 3.Knikou M, Conway BA. Reflex effects of induced muscle contraction in normal and spinal cord injured subjects. Muscle Nerve. 2002;26:374–382. doi: 10.1002/mus.10206. [DOI] [PubMed] [Google Scholar]
- 4.Romano C, Schieppati M. Reflex excitability of human soleus motoneurones during voluntary shortening or lengthening contractions. J Physiol. 1987;390:271–284. doi: 10.1113/jphysiol.1987.sp016699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakazawa K, Miyoshi T, Sekiguchi H, Nozaki D, Akai M, Yano H. Effects of loading and unloading of lower limb joints on the soleus H-reflex in standing humans. Clin. Neurophysiol. 2004;115:1296–1304. doi: 10.1016/j.clinph.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 6.Knikou M, Conway BA. Modulation of soleus H-reflex following ipsilateral mechanical loading of the sole of the foot in normal and complete spinal cord injured humans. Neurosci. Lett. 2001;303:107–110. doi: 10.1016/s0304-3940(01)01718-9. [DOI] [PubMed] [Google Scholar]
- 7.Edamura M, Yang JF, Stein RB. Factors that determine the magnitude and time course of human H-reflexes in locomotion. J Neurosci. 1991;11:420–427. doi: 10.1523/JNEUROSCI.11-02-00420.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hiraoka K. Phase-dependent modulation of the soleus H-reflex during rhythmical arm swing in humans. Electromyogr. Clin Neurophysiol. 2001;41:43–47. [PubMed] [Google Scholar]
- 9.Koceja DM, Trimble MH, Earles DR. Inhibition of the soleus H-reflex in standing man. Brain Res. 1993;629:155–158. doi: 10.1016/0006-8993(93)90495-9. [DOI] [PubMed] [Google Scholar]
- 10.Koceja DM, Markus CA, Trimble MH. Postural modulation of the soleus H reflex in young and old subjects. Electroencephalogr. Clin. Neurophysiol. 1995;97:387–393. doi: 10.1016/0924-980x(95)00163-f. [DOI] [PubMed] [Google Scholar]
- 11.Kawashima N, Sekiguchi H, Miyoshi T, Nakazawa K, Akai M. Inhibition of the human soleus Hoffman reflex during standing without descending commands. Neurosci. Lett. 2003;345:41–44. doi: 10.1016/s0304-3940(03)00485-3. [DOI] [PubMed] [Google Scholar]
- 12.Dietz V, Quintern J, Berger W. Corrective reactions to stumbling in man: functional significance of spinal and transcortical reflexes. Neurosci. Lett. 1984;44:131–135. doi: 10.1016/0304-3940(84)90070-3. [DOI] [PubMed] [Google Scholar]
- 13.Crone C, Nielsen J. Methodological implications of the post activation depression of the soleus H-reflex in man. Exp. Brain Res. 1989;78:28–32. doi: 10.1007/BF00230683. [DOI] [PubMed] [Google Scholar]
- 14.Hultborn H, Illert M, Nielsen J, Paul A, Ballegaard M, Wiese H. On the mechanism of the post-activation depression of the H-reflex in human subjects. Exp. Brain Res. 1996;108:450–462. doi: 10.1007/BF00227268. [DOI] [PubMed] [Google Scholar]
- 15.Crone C, Hultborn H, Mazieres L, Morin C, Nielsen J, Pierrot-Deseilligny E. Sensitivity of monosynaptic test reflexes to facilitation and inhibition as a function of the test reflex size: a study in man and the cat. Exp. Brain Res. 1990;81:35–45. doi: 10.1007/BF00230098. [DOI] [PubMed] [Google Scholar]
- 16.Floeter MK, Kohn AF. H-reflexes of different sizes exhibit differential sensitivity to low frequency depression. Electroencephalogr. Clin. Neurophysiol. 1997;105:470–475. doi: 10.1016/s0924-980x(97)00032-5. [DOI] [PubMed] [Google Scholar]
- 17.Edamura M, Yang JF, Stein RB. Factors that determine the magnitude and time course of human H-reflexes in locomotion. J Neurosci. 1991;11:420–427. doi: 10.1523/JNEUROSCI.11-02-00420.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Capaday C, Stein RB. Amplitude modulation of the soleus H-reflex in the human during walking and standing. J Neurosci. 1986;6:1308–1313. doi: 10.1523/JNEUROSCI.06-05-01308.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trimble MH, Du P, Brunt D, Thompson FJ. Modulation of triceps surae H-reflexes as a function of the reflex activation history during standing and stepping. Brain Res. 2000;858:274–283. doi: 10.1016/s0006-8993(00)01956-9. [DOI] [PubMed] [Google Scholar]
- 20.Goulart F, Valls-Sole J, Alvarez R. Posture-related changes of soleus H-reflex excitability. Muscle Nerve. 2000;23:925–932. doi: 10.1002/(sici)1097-4598(200006)23:6<925::aid-mus13>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 21.Mynark RG, Koceja DM, Lewis CA. Heteronymous monosynaptic Ia facilitation from supine to standing and its relationship to the soleus H-reflex. Int. J. Neurosci. 1997;92:171–186. doi: 10.3109/00207459708986400. [DOI] [PubMed] [Google Scholar]
- 22.Aymard C, Katz R, Lafitte C, Lo E, Penicaud A, Pradat-Diehl P, Raoul S. Presynaptic inhibition and homosynaptic depression: a comparison between lower and upper limbs in normal human subjects and patients with hemiplegia. Brain. 2000;123(Pt 8):1688–1702. doi: 10.1093/brain/123.8.1688. [DOI] [PubMed] [Google Scholar]
- 23.Schindler-Ivens S, Shields RK. Low frequency depression of H-reflexes in humans with acute and chronic spinal-cord injury. Exp. Brain Res. 2000;133:233–241. doi: 10.1007/s002210000377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nielsen J, Petersen N, Ballegaard M, Biering-Sorensen F, Kiehn O. H-reflexes are less depressed following muscle stretch in spastic spinal cord injured patients than in healthy subjects. Exp. Brain Res. 1993;97:173–176. doi: 10.1007/BF00228827. [DOI] [PubMed] [Google Scholar]
- 25.Taylor S, Ashby P, Verrier M. Neurophysiological changes following traumatic spinal lesions in man. J Neurol Neurosurg. Psychiatry. 1984;47:1102–1108. doi: 10.1136/jnnp.47.10.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masakado Y, Kagamihara Y, Takahashi O, Akaboshi K, Muraoka Y, Ushiba J. Post-activation depression of the soleus H-reflex in stroke patients. Electromyogr. Clin Neurophysiol. 2005;45:115–122. [PubMed] [Google Scholar]
- 27.Koelman JH, Bour LJ, Hilgevoord AA, van Bruggen GJ, Ongerboer d. V. Soleus H-reflex tests and clinical signs of the upper motor neuron syndrome. J Neurol Neurosurg Psychiatry. 1993;56:776–781. doi: 10.1136/jnnp.56.7.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.American Spinal Injury Association . International Standards for Neurological Classification of Spinal Cord Injury. Chicago: 2000. [DOI] [PubMed] [Google Scholar]
- 29.Trimble MH, Koceja DM. Effect of a reduced base of support in standing and balance training on the soleus H-reflex. Int. J Neurosci. 2001;106:1–20. doi: 10.3109/00207450109149734. [DOI] [PubMed] [Google Scholar]
- 30.Llewellyn M, Yang JF, Prochazka A. Human H-reflexes are smaller in difficult beam walking than in normal treadmill walking. Exp. Brain Res. 1990;83:22–28. doi: 10.1007/BF00232189. [DOI] [PubMed] [Google Scholar]
- 31.Perez MA, Field-Fote EC. Impaired posture-dependent modulation of disynaptic reciprocal Ia inhibition in individuals with incomplete spinal cord injury. Neurosci. Lett. 2003;341:225–228. doi: 10.1016/s0304-3940(03)00183-6. [DOI] [PubMed] [Google Scholar]
- 32.Xia R, Rymer WZ. Reflex reciprocal facilitation of antagonist muscles in spinal cord injury. Spinal Cord. 2005;43:14–21. doi: 10.1038/sj.sc.3101656. [DOI] [PubMed] [Google Scholar]
- 33.Faist M, Ertel M, Berger W, Dietz V. Impaired modulation of quadriceps tendon jerk reflex during spastic gait: differences between spinal and cerebral lesions. Brain. 1999;122(Pt 3):567–579. doi: 10.1093/brain/122.3.567. [DOI] [PubMed] [Google Scholar]
- 34.Lee JK, Emch GS, Johnson CS, Wrathall JR. Effect of spinal cord injury severity on alterations of the H-reflex. Exp. Neurol. 2005;196:430–440. doi: 10.1016/j.expneurol.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 35.Nakazawa K, Kawashima N, Akai M. Enhanced stretch reflex excitability of the soleus muscle in persons with incomplete rather than complete chronic spinal cord injury. Arch. Phys. Med. Rehabil. 2006;87:71–75. doi: 10.1016/j.apmr.2005.08.122. [DOI] [PubMed] [Google Scholar]
- 36.Schindler-Ivens SM, Shields RK. Soleus H-reflex recruitment is not altered in persons with chronic spinal cord injury. Arch. Phys. Med. Rehabil. 2004;85:840–847. doi: 10.1016/j.apmr.2003.08.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hiersemenzel LP, Curt A, Dietz V. From spinal shock to spasticity: neuronal adaptations to a spinal cord injury. Neurology. 2000;54:1574–1582. doi: 10.1212/wnl.54.8.1574. [DOI] [PubMed] [Google Scholar]
- 38.Phadke CP, Wu SS, Thompson FJ, Behrman AL. Soleus H-reflex modulation in response to change in percentage of leg loading in standing after incomplete spinal cord injury. Neurosci. Lett. 2006;403:6–10. doi: 10.1016/j.neulet.2006.04.058. [DOI] [PubMed] [Google Scholar]
- 39.Trimble MH, Behrman AL, Flynn SM, Thigpen MT, Thompson FJ. Acute effects of locomotor training on overground walking speed and H-reflex modulation in individuals with incomplete spinal cord injury. J Spinal Cord. Med. 2001;24:74–80. doi: 10.1080/10790268.2001.11753558. [DOI] [PubMed] [Google Scholar]
- 40.Faist M, Dietz V, Pierrot-Deseilligny E. Modulation, probably presynaptic in origin, of monosynaptic Ia excitation during human gait. Exp. Brain Res. 1996;109:441–449. doi: 10.1007/BF00229628. [DOI] [PubMed] [Google Scholar]
- 41.Katz R, Meunier S, Pierrot-Deseilligny E. Changes in presynaptic inhibition of Ia fibres in man while standing. Brain. 1988;111(Pt 2):417–437. doi: 10.1093/brain/111.2.417. [DOI] [PubMed] [Google Scholar]
- 42.Cowan JM, Day BL, Marsden C, Rothwell JC. The effect of percutaneous motor cortex stimulation on H reflexes in muscles of the arm and leg in intact man. J Physiol. 1986;377:333–347. doi: 10.1113/jphysiol.1986.sp016190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abbruzzese M, Rubino V, Schieppati M. Task-dependent effects evoked by foot muscle afferents on leg muscle activity in humans. Electroencephalogr. Clin Neurophysiol. 1996;101:339–348. doi: 10.1016/0924-980x(96)95682-9. [DOI] [PubMed] [Google Scholar]
- 44.Egawa K, Oida Y, Kitabatake Y, Maie H, Mano T, Iwase S, Miwa C. Postural modulation of soleus H-reflex under simulated hypogravity by head-out water immersion in humans. Environ. Med. 2000;44:117–120. [PubMed] [Google Scholar]
- 45.Morita H, Petersen N, Christensen LO, Sinkjaer T, Nielsen J. Sensitivity of H-reflexes and stretch reflexes to presynaptic inhibition in humans. J Neurophysiol. 1998;80:610–620. doi: 10.1152/jn.1998.80.2.610. [DOI] [PubMed] [Google Scholar]
- 46.Bove M, Trompetto C, Abbruzzese G, Schieppati M. The posture-related interaction between Ia-afferent and descending input on the spinal reflex excitability in humans. Neurosci. Lett. 2006;397:301–306. doi: 10.1016/j.neulet.2005.12.049. [DOI] [PubMed] [Google Scholar]
- 47.Katz RT, Rovai GP, Brait C, Rymer WZ. Objective quantification of spastic hypertonia: correlation with clinical findings. Arch Phys Med Rehabil. 1992;73(4):399–347. doi: 10.1016/0003-9993(92)90007-j. [DOI] [PubMed] [Google Scholar]
- 48.Calancie B, Broton JG, Klose KJ, Traad M, Difini J, Ayyar DR. Evidence that alterations in presynaptic inhibition contribute to segmental hypo- and hyperexcitability after spinal cord injury in man. Electroencephalogr. Clin Neurophysiol. 1993;89:177–186. doi: 10.1016/0168-5597(93)90131-8. [DOI] [PubMed] [Google Scholar]


