Abstract
Differential allocation of reproductive effort towards offspring of attractive mates is a form of post-copulatory mate choice. Although differential allocation has been demonstrated in many taxa, its evolutionary implications have received little attention. Theory predicts that mate choice will lead to a positive genetic correlation between female preference and male attractiveness. This prediction has been upheld for pre-copulatory mate choice, but whether such a relationship between male attractiveness and female differential allocation exists has never been tested. Here, we show that both female pre-copulatory mate choice and post-copulatory differential allocation are genetically associated with male attractiveness in house crickets, Acheta domesticus. Daughters of attractive males mated sooner and laid more eggs when paired with larger males. These forms of mate choice are strongest in large females, suggesting that costs decrease with increasing female size. The genetic association between attractiveness and differential allocation suggests potential for differential allocation to become exaggerated by coevolutionary runaway processes in an analogous manner to pre-copulatory choice. Sexual selection is thus likely to be stronger than predicted by pre-copulatory choice alone.
Keywords: differential allocation, mate choice, indirect benefits, Acheta domesticus, genetic correlation
1. Introduction
It is increasingly apparent that female mate choice is expressed in several stages (Thornhill 1983; Sheldon 2000). In addition to pre-copulatory choice, females may also favour preferred males after copulation (Cunningham & Russell 2000). Differential allocation (Burley 1988; Sheldon 2000) is one form of post-copulatory choice in which females lay more eggs or increase the investment in each egg when mated to an attractive male (de Lope & Møller 1993; Wedell 1996; Eberhard 2000).
The importance of differential allocation to sexual selection has historically been underestimated (de Lope & Møller 1993; Eberhard 2000). Most theories of mate choice assume that female reproductive investment is unrelated to the expression of male sexual traits. However, differential allocation may increase the opportunity for, and the strength of, selection operating on attractive male traits (Sheldon 2000). If females invest more in the offspring of preferred males then differential allocation could lead to increased quality of offspring sired by preferred males (Fisher 1930; Sheldon 2000), and influence the genetic benefits that preferred males confer on their offspring (de Lope & Møller 1993). If there is genetic variation in male ability to stimulate female differential allocation and in female ability to allocate differentially, we predict that a positive genetic correlation between male attractiveness and differential allocation will arise in the same way as for pre-copulatory choice (Fisher 1930; Lande 1981). As a result, indirect selection mediated through this genetic covariance could facilitate the evolution of both male attractiveness and female allocation strategies.
Here, we test for a genetic association between male attractiveness and both pre-copulatory choice and differential allocation in female house crickets, Acheta domesticus. In this species, there are three temporally discreet opportunities for females to exercise choice. First, females seek out males (often larger males) on the basis of male advertisement call chirp rate (Gray 1997). Second, females mount (and subsequently mate with) attractive males sooner than unattractive males in close-range, no-choice trials (Savage et al. 2004; Head et al. 2005). Last, females mated to larger, more attractive, males lay more eggs than those mated to unattractive males, at a direct cost to female survival and lifetime fecundity (Head et al. 2005). This cost is partly ameliorated by the high resemblance between fathers and sons in their attractiveness (Head et al. 2005) suggesting that evolution of mate choice via indirect benefits is likely to be important in A. domesticus.
2. Material and methods
We paired the daughters of attractive and unattractive males with randomly selected stock males. We then measured pre-copulatory mate choice and differential allocation strategies of these daughters.
(a) Sire attractiveness
Parentals were obtained as final-instar nymphs from a commercial cricket breeder (Pisces Enterprises). Virgin nymphs were reared in single-sex tubs with constant access to food (Friskies Go-Cat senior) and water until eclosion. Adults were maintained in single-sex cultures for a further 10 days to ensure sexual maturity. To obtain sires that were either attractive or unattractive to females we ran a two-round tournament based on the time taken for a female to mount a male as outlined in Head et al. (2005). As in other studies (e.g. Gray 1997) attractive sires were larger than unattractive sires, although this difference was not significant (mean±s.e.: attractive, 305.250±7.178; unattractive, 297.967±7.911, tt8=0.682, p=0.497).
Each selected sire (40 attractive and 40 unattractive) was paired for life with a random sexually mature female (i.e. dam) in a plastic container (7×7×5 cm) with food, water and a Petri-dish of moist sand for egg-laying. If a sire died (4 of 40 attractive and 10 of 40 unattractive sires), he was replaced with another male of corresponding attractiveness. Food, water and sand were replaced every 7 days.
Eggs were collected weekly and monitored for hatchlings. From each weekly collection 50 hatchlings per female were separated into two boxes (20×13×13 cm), each containing 25 nymphs. Once mature, offspring were weighed and sexed. Daughters were housed individually in similar containers to their parents.
(b) The mate choice decisions of daughters
We measured pre- and post-copulatory choice of 20 attractive and 15 unattractive families with a range of 8–45 daughters per family (a total of 292 daughters sired by attractive males and 294 daughters sired by unattractive males). We measured pre-copulatory mate choice of each daughter by pairing her with a random male from stock 10 days after eclosion. Time to mounting was recorded for the first 90 min, with daughters not mounting in this interval given a time of 91 min. Daughters were left with males for 12 h to allow mating. Males were then removed and daughters were given a Petri dish of sand in which to lay their eggs. We collected and counted eggs after 7 days.
(c) Statistical analysis
To test if daughters sired by attractive or unattractive males differed in their mate choice, we employed a sequential model building approach used for response surface designs containing both quantitative (partners weight, daughters weight) and qualitative (sire attractiveness, family within sire attractiveness) variables. We constructed separate models for our two dependent variables; the time it took a daughter to mount her partner (i.e. pre-copulatory choice) and the number of eggs a daughter laid (i.e. differential allocation). Details of the model-building approach and results thereof are presented in electronic supplementary material.
We used response surface analysis to estimate linear, quadratic and correlational effects on mate choice and non-parametric thin-plate splines to visualize these response surfaces (see electronic supplementary material). Because model-building identified significant differences between response surfaces for daughters depending on sire attractiveness, we estimated and visualized these surfaces separately for daughters of attractive and unattractive males.
3. Results and discussion
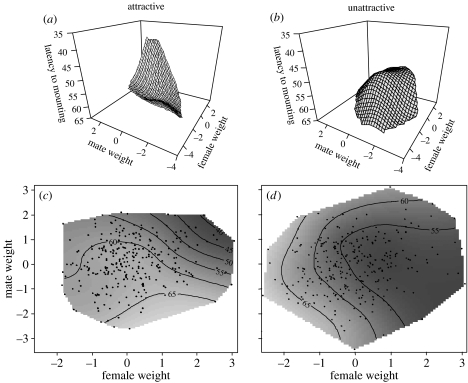
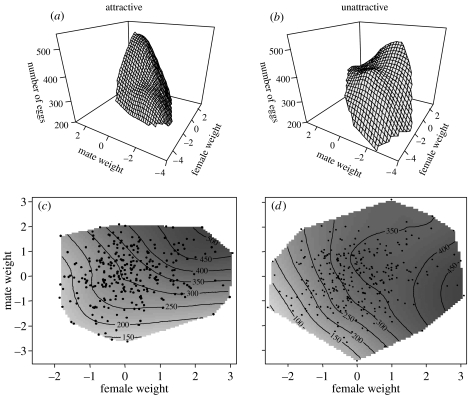
Daughters' mate choice decisions are genetically associated with their sires' attractiveness, the only factor that differed between our treatments. The significant treatment differences between the response surfaces describing the effects of a daughter's size and her mate's size on her latency to mounting (figure 1) and the number of eggs she subsequently laid (figure 2) were due to the interaction between sires' attractiveness and the correlational term (daughter's size×her mates' size) for both pre-copulatory mate choice (latency to mounting, partial F-test, F1,545=6.090, p=0.014) and post-copulatory differential allocation (number of eggs laid, partial F-test, F1,545=12.915, p=0.000; see electronic supplementary material, table S1, for full comparison of surfaces). Large daughters sired by attractive males mated more rapidly with (figure 1a,c) and laid more eggs for (figure 2a,c) large (attractive) males (table 1), whereas all daughters of unattractive males tended to mate more rapidly with (figure 1b,d) and laid more eggs for (figure 2b,d) intermediate sized partners (table 1). Thus, female reproductive effort in relation to male phenotype closely matches pre-copulatory choice (compare figure 1 with figure 2), as predicted by differential allocation theory (Burley 1988; Sheldon 2000).
Figure 1.
Pre-copulatory mate choice (latency to mounting) for (a and c) daughters sired by attractive males, (b and d) daughters sired by unattractive males. Response surfaces (a and b) illustrate the shape of female preferences with regard to their own size and the size of their mate, while contours (c and d) show where individual female preferences lay on this surface. Male and female weights are standardized across treatments.
Figure 2.
Post-copulatory differential allocation (number of eggs) for (a and c) daughters sired by attractive males, (b and d) daughters sired by unattractive males. Response surfaces (a and b) illustrate the shape of female preferences with regard to their own size and the size of their mate, while contours (c and d) show where individual female preferences lay on this surface. Male and female weights are standardized across treatments.
Table 1.
Multiple regression analysis for pre-copulatory (latency to mounting) and post-copulatory (number of eggs) mate choice of daughters sired by attractive and unattractive males.
| attractive | unattractive | |||||||
|---|---|---|---|---|---|---|---|---|
| β | t | P | β | t | P | |||
| latency to mounting | linear | daughter's weight | 0.378 | 0.161 | 0.873 | −3.919 | −1.917 | 0.056 |
| mate's weight | −2.853 | −1.347 | 0.179 | −0.112 | −0.055 | 0.956 | ||
| quadratic | daughter's weight | −1.956 | −1.250 | 0.212 | 1.203 | 0.879 | 0.380 | |
| mate's weight | 0.223 | 0.129 | 0.897 | 2.544 | 1.849 | 0.065 | ||
| correlational | daughter×mate | −5.754 | −2.497 | 0.013 | 0.962 | 0.476 | 0.635 | |
| number of eggs | linear | daughter's weight | 53.525 | 4.007 | 0.000 | 61.421 | 5.336 | 0.000 |
| mate's weight | 44.392 | 3.697 | 0.000 | 19.644 | 1.721 | 0.086 | ||
| quadratic | daughter's weight | −16.725 | −1.884 | 0.061 | −9.211 | −1.196 | 0.233 | |
| mate's weight | −7.915 | −0.809 | 0.419 | −16.039 | −2.071 | 0.039 | ||
| correlational | daughter×mate | 36.556 | 2.797 | 0.006 | −23.940 | −2.103 | 0.036 | |
Our finding upholds an important theoretic prediction (Fisher 1930; Lande 1981) that choice (in this case expressed as differential allocation) covaries genetically with male attractiveness. Genetic covariation between male attractiveness and female pre-copulatory mating preferences has been demonstrated in a handful of cases (e.g. Blows 1999; Iyengar et al. 2002). No such genetic covariation between attractiveness and post-copulatory choice has ever been reported. The significance of our findings is that sexual selection on male attractiveness may affect indirect selection on both pre-copulatory mating preferences and post-copulatory differential allocation, raising the novel possibility of Fisher–Zahavi (Fisher 1930; Zahavi 1975) coevolution between male attractiveness and differential allocation.
The genetic association between sire attractiveness and female choice may be due to linkage disequilibrium and/or pleiotropy. Our method underestimates the strength of linkage disequilibrium, which is halved by our use of random matings to generate daughters (Lande 1981). Pleiotropy between attractiveness and differential allocation may be direct (the same genes expressed in males and females) or indirect (genes that influence sire attractiveness directly also influence daughter's behaviour via induced maternal effects in the dam; Moore & Pizzari 2005). Regardless, our conclusion of a genetic covariance between sire's attractiveness and daughter's differential allocation holds. Both direct and indirect mechanisms should facilitate the coevolution of male attractiveness and female choice (Moore & Pizzari 2005). While Moore & Pizzari (2005) do not explicitly refer to instances of differential allocation, they suggest that differential allocation may be viewed as an extended phenotype that is influenced by interactions with the phenotype of the male (Sheldon 2000). How a relationship between female differential allocation and male attractiveness can extend multiple generations requires further research.
Increased fecundity of daughters may be due to manipulation by males. If male manipulation is important then our results show that females differ in susceptibility to males depending on their sire's attractiveness/manipulative ability. Our conclusions regarding the genetic association between male attractiveness and female choice hold regardless of whether increased egg deposition is considered a form of active female choice or male manipulation.
Only large females allocated more eggs when mated to males of their preferred phenotype (table 1, figure 2). This may occur if the costs of differential allocation are dependent on female size, and females trade the benefits of choice against costs of stronger preferences (Jennions & Petrie 1997). If this is the case, variation in female quality will lead to large (good condition) females expressing stronger mate preferences than small (poor condition) females (Hingle et al. 2001). In our experiment, large females sired by attractive males expressed different preferences for male size than large females sired by unattractive males, suggesting larger females may be better able to withstand the costs of being choosy and of allocating differentially (Jennions & Petrie 1997) and thus of expressing their preferences which are dependent on their sire's attractiveness.
Theoretical models predict that the indirect benefits of mate choice are negligible compared to the direct costs (Kirkpatrick & Barton 1997). Previously, we have shown that female A. domesticus mated to attractive males have increased net fitness, despite substantial direct costs (Head et al. 2005). The results we present here provide further evidence for indirect benefits of mating with attractive males in this species and of allocating differentially. By upholding the prediction that daughters of attractive males allocate resources differentially toward offspring of attractive males, our findings show that indirect selection is likely to influence the coevolution of both pre-copulatory choice and post-copulatory differential allocation.
Acknowledgments
We are grateful to Michael Jennions, Simon Griffith, Helen Rodd, Charlotte Kvarnemo, Scott Sakaluk, Genny Kozak and Allen Moore for comments on this manuscript. This work was supported by an ARC grant to J.H. and R.B. and an Australian Postgraduate Award to M.L.H. J.H. was funded by a NERC Fellowship during the writing of this manuscript.
Footnotes
Present address: Centre for Ecology and Conservation, The University of Exeter in Cornwall, Tremough Campus, Cornwall TR10 9EZ, UK.
Supplementary Material
References
- Blows M.W. Evolution of the genetic covariance between male and female components of mate recognition: an experimental test. Proc. R. Soc. B. 1999;266:2169–2174. doi: 10.1098/rspb.1999.0904. doi:10.1098/rspb.1999.0904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burley N. The differential-allocation hypothesis: an experimental test. Am. Nat. 1988;132:611–628. doi:10.1086/284877 [Google Scholar]
- Cunningham E.J.A, Russell A.F. Egg investment is influenced by male attractiveness in the mallard. Nature. 2000;404:74–76. doi: 10.1038/35003565. doi:10.1038/35003565 [DOI] [PubMed] [Google Scholar]
- de Lope F, Møller A.P. Female reproductive effort depends on the degree of ornamentation of their mates. Evolution. 1993;47:1152–1160. doi: 10.1111/j.1558-5646.1993.tb02142.x. [DOI] [PubMed] [Google Scholar]
- Eberhard W.G. Criteria for demonstrating postcopulatory female choice. Evolution. 2000;54:1047–1050. doi: 10.1111/j.0014-3820.2000.tb00105.x. [DOI] [PubMed] [Google Scholar]
- Fisher R.A. Oxford University Press; Oxford, UK: 1930. The genetical theory of natural selection. [Google Scholar]
- Gray D.A. Female house crickets, Acheta domesticus, prefer the chirps of large males. Anim. Behav. 1997;54:1553–1562. doi: 10.1006/anbe.1997.0584. doi:10.1006/anbe.1997.0584 [DOI] [PubMed] [Google Scholar]
- Head M.L, Hunt J, Jennions M.D, Brooks R. The indirect benefits of mating with attractive males outweigh the direct costs. PLoS Biol. 2005;3:e33. doi: 10.1371/journal.pbio.0030033. doi:10.1371/journal.pbio.0030033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingle A, Fowler K, Pomiankowski A. The effect of transient food stress on female mate preference in the stalk-eyed fly Cyrtodiopsi dalmanni. Proc. R. Soc. B. 2001;268:1239–1244. doi: 10.1098/rspb.2001.1647. doi:10.1098/rspb.2001.1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyengar V.K, Reeve H.K, Eisner T. Paternal inheritance of a female moth's mating preference. Nature. 2002;419:830–832. doi: 10.1038/nature01027. doi:10.1038/nature01027 [DOI] [PubMed] [Google Scholar]
- Jennions M.D, Petrie M. Variation in mate choice and mating preferences—a review of causes and consequences. Biol. Rev. 1997;72:283–327. doi: 10.1017/s0006323196005014. doi:10.1017/S0006323196005014 [DOI] [PubMed] [Google Scholar]
- Kirkpatrick M, Barton N.H. The strength of indirect selection on female mating preferences. Proc. Natl Acad. Sci. USA. 1997;94:1282–1286. doi: 10.1073/pnas.94.4.1282. doi:10.1073/pnas.94.4.1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lande R. Models of speciation by sexual selection on polygenic traits. Proc. Natl Acad. Sci. USA. 1981;78:3721–3725. doi: 10.1073/pnas.78.6.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore A.J, Pizzari T. Quantitative genetic models of sexual conflict based on interacting phenotypes. Am. Nat. 2005;165:S88–S97. doi: 10.1086/429354. doi:10.1086/429354 [DOI] [PubMed] [Google Scholar]
- Savage K.E, Hunt J, Jennions M.D, Brooks R. Male attractiveness covaries with fighting ability but not with prior fight outcome in house crickets. Behav. Ecol. 2004;16:196–200. doi:10.1093/beheco/arh143 [Google Scholar]
- Sheldon B.C. Differential allocation: tests, mechanisms and implications. Trends Ecol. Evol. 2000;15:397–402. doi: 10.1016/s0169-5347(00)01953-4. doi:10.1016/S0169-5347(00)01953-4 [DOI] [PubMed] [Google Scholar]
- Thornhill R. Cryptic female choice and its implications in the scorpionfly Harpobittacus nigriceps. Am. Nat. 1983;122:765–788. doi:10.1086/284170 [Google Scholar]
- Wedell N. Mate quality affects reproductive effort in a paternally investing species. Am. Nat. 1996;148:1075–1088. doi:10.1086/285972 [Google Scholar]
- Zahavi A. Mate selection—a selection for a handicap. J. Theor. Biol. 1975;53:205–214. doi: 10.1016/0022-5193(75)90111-3. doi:10.1016/0022-5193(75)90111-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.