Abstract
Predation and microbial infections are the major causes of natural mortality for early life stages of oviparous species. The parental traits reducing the effects of predation are rather well described, whereas antimicrobial mechanisms enhancing offspring survival are largely unexplored. In this paper, we report that a male sexually dimorphic trait, the anal glands, of the redlip blenny (Ophioblennius atlanticus atlanticus) and the peacock blenny (Salaria pavo), two fish species with paternal egg care, produce a mucus enriched with antimicrobial substances. Histological and histochemical analyses showed that the anal glands of these species are characterized by the massive presence of mucus-secreting cells. Anal gland extracts, from both the hydrophilic and the hydrophobic protein fraction, exhibited a lysozyme-like activity. Field observations demonstrated that redlip blenny males, while performing egg care, rub the anal region over the nest internal surface, probably facilitating the transfer of mucus to eggs. These results strongly indicate that this sexually dimorphic trait is involved in egg defence against microbial infections.
Keywords: Blenniidae, sexually dimorphic trait, parental care, antimicrobial activity, anal glands
1. Introduction
In oviparous animals, the benefits of parental care and the quality of the sex performing egg care have mainly been described in relation to the morphological and/or behavioural traits reducing the effects of predation, while almost no attention has been given to the relationship between parental investment and microbial infection (Clutton-Brock 1991). Indeed, although many antimicrobials have been identified in different animal taxa (Brogden 2005), their possible role in reproduction has been poorly investigated and primarily related to the maternal transfer of immune factors to eggs (Yousif et al. 1994; Marchini et al. 1997).
The epidermal mucus of fishes plays a significant role in their protection against pathogenic infections and antimicrobials have been found associated with and released from the epidermal mucus-secreting cells of several species (Cole et al. 1997; Ellis 2001). Recently, in a species performing paternal care, the skin mucus has also been demonstrated to protect developing eggs (Knouft et al. 2003). Considering the widespread occurrence of male parental care in fish (Clutton-Brock 1991), if the adult epidermal mucus can protect the eggs deposited on the substrate against pathogens, morphological and/or behavioural traits facilitating or enhancing the transfer of mucus to eggs should be expected in the breeding males of many species.
In several species of Blenniidae, a family of demersal spawners with paternal care, males exhibit a peculiar sexually dimorphic trait: a pair of globular structures on the most anterior anal fin rays, the anal glands (Zander 1975). Previous studies have indicated that these organs are well developed in nesting males during the breeding season (Giacomello & Rasotto 2005) but remain rudimentary in males engaged in opportunistic mating tactics (Gonçalves et al. 1996; Neat et al. 2003). Information on the morphology and physiology of these organs is scanty, however, they have been hypothesized to play a role in pheromone production or in the release of antimicrobials to eggs (Zander 1975). While field studying the mating system of some blennies, it came to our attention that breeding males rub the anal–urogenital region over the nest internal surface during spawning (see electronic supplementary material), a behaviour associated with ejaculation in Gobiidae (Marconato et al. 1996).
If the anal glands represent a likely location from which to release a protective mucus onto developing eggs, then it should be expected that (i) they show a high concentration of mucus-secreting cells; (ii) their secretions exhibit antimicrobial activity; and (iii) males perform rubbing behaviour not only during spawning, but also while performing parental care. We tested the predictions (i) and (ii) on the redlip blenny, Ophioblennius atlanticus atlanticus, and on the peacock blenny, Salaria pavo, two polygynous species whose larger males exhibit larger anal glands (figure 1a) and attain the highest mating success (Oliveira et al. 1999; Giacomello 2004). The third prediction was tested, with field observations, in the redlip blenny, where the large shelters used by nesting males facilitate the record of rubbing behaviour.
Figure 1.
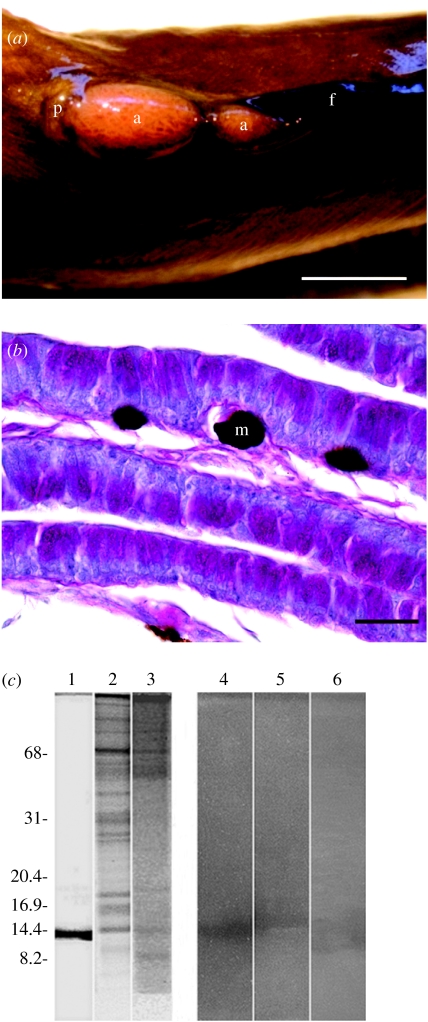
Anal glands of male blenniid fish produce a mucus enriched with antimicrobials. (a) Anal glands in a nesting male of O. atlanticus atlanticus. p, genital papilla; a, anal gland; f, anal fin. Scale bar, 0.5 cm. (b) Transverse section of anal gland of O. atlanticus atlanticus (PAS staining). Secreting cells appear pink stained. m, melanophore. Scale bar, 20 μm. (c) Non-reducing SDS-PAGE (15% polyacrylamide gel) on anal glands extracts. Left panel, SYPRO Ruby stain; right panel, gel overlaid with M. lysodeikticus. Lanes 1 and 4, hen egg lysozyme, 1 μg and 25 ng, respectively; lanes 2 and 5, hydrophobic extract from O. atlanticus atlanticus, 16 and 60 μg, respectively; lanes 3 and 6, hydrophobic extract from S. pavo, 8 and 26 μg, respectively. On the left, the molecular mass of protein markers is expressed in kDa.
2. Material and methods
(a) Field observations
Behavioural observations on 15 nesting males (total length, TL=14.2–22.0 cm) of O. atlanticus atlanticus were conducted in Faial Island (Azores, Portugal), by scuba diving, during the breeding season 2003. Repeated 5 min focal observations were made (1–7 of each male), recording the time the male spent rubbing the anal–urogenital region over the nest internal surface and the number of rubbing acts, both during spawning time (6.30–8.30) and outside it (10.00–20.00). The mean rubbing time (±s.e.) and frequency of rubbing (±s.e., number of rubbing acts per 5 min) were calculated for each individual.
(b) Fish samples
Breeding males of O. atlanticus atlanticus, in Faial Island, and S. pavo, in the Lagoon of Venice (Italy), were collected in 2004. Animals were anaesthetised with MS222 (Sandoz), measured (TL) and rinsed with filtered seawater to reduce the bacterial contamination on body surface. Anal glands were removed. After surgery all animals fully recovered and were released into the field.
(c) Histology and histochemistry
Anal glands of O. atlanticus atlanticus (TL=14.5–21.0 cm, n=5) and S. pavo (TL=10.9–14.5, n=2) were processed and stained in order to reveal mucosubstances as in Neat et al. (2003).
(d) Anal gland extracts
After excision glands were immediately frozen and lyophilized. Glands from 1 to 4 animals (4–19 mg dried weight per male) were suspended in 0.3 ml 100 mM Na–phosphate buffer at pH 6.8 (PB) and homogenized with Eppendorf micropestles in ice for 15 min. The hydrophilic extract consisted of the three supernatants obtained by three runs of centrifugation (all centrifuge steps were performed at 12 000g for 90 min at 4 °C) and resuspension of the pellet with 0.3 ml PB. The pellet underwent 10 runs of resuspension with 1.8 ml PB and centrifugation, discarding supernatants. The pellet was then extracted with 0.1 ml 0.04% Lithium Dodecyl Sulphate (LiDS, Sigma) in PB by magnetic stirring at 4 °C for 2 h and centrifuged. This step was repeated four times. The four supernatants (hydrophobic extract) were pooled together and ultrafiltered with Millipore Microcon YM-3 filters onto which PB was added, so as to obtain a final concentration of LiDS (less than 0.004%) not lytic on Micrococcus lysodeikticus (control tests not shown). The protein content of the hydrophilic and hydrophobic extract, determined according to Bradford (1976) using bovine serum albumin (BSA, Sigma) as standard, was about 350 μg total protein per gland pair in S. pavo and 1000 μg total protein per gland pair in O. atlanticus atlanticus, for the former, and about 10 μg in S. pavo and 600 μg in O. atlanticus atlanticus, for the latter.
(e) Assays for lysozyme-like activity and electrophoreses
Lysozyme is a major component of innate immunity and the standard substrate for assaying its activity is M. lysodeikticus cells (Jolles & Jolles 1984). The lysozyme-like activity of anal glands was assayed on plates (Marchini et al. 1993) prepared with 0.1% suspension of M. lysodeikticus dried cells (Sigma). Mid-dilution samples from hydrophilic and hydrophobic extracts, containing 15–240 or 2.5–160 μg total protein, respectively, were applied to 2 mm-wells in a volume of 3–10 μl. Plates were incubated at room-temperature (RT) overnight. Hen egg white lysozyme (LYS, Sigma) was used as positive control. SDS-PAGE was performed in a discontinuous pH gel system (Laemmli 1970), but in non-reducing conditions. Samples were loaded in double in the gel. Runs were carried out at 18 mA for 2 h at 4 °C. After electrophoresis, one part of the gel was stained with SYPRO Ruby gel stain (Molecular Probes), while the other part was renaturated (Saul et al. 1990), omitting β-mercaptoethanol. The gel was equilibrated with PB for 10 min at RT and overlaid with a 0.1% suspension of M. lysodeikticus in PB plus 0.7% agar (Difco). Low molecular weight proteins (Promega), BSA and LYS were used as molecular mass markers. BSA and LYS were also used as negative and positive control of lytic activity. Acidic native electrophoresis was also performed (Gabriel 1971) and over-layered as above.
3. Results
Nesting males of the redlip blenny rubbed the anal-urogenital region over the eggs not only during reproductive time (mean rubbing time ±s.e. 13.42±3.24 s per 5 min; frequency of rubbing ±s.e. 2.06±0.46), but also while guarding the eggs (mean rubbing time 1.17±0.76 s per 5 min; frequency of rubbing ±s.e. 0.18±0.11). Anal glands of the parental males of O. atlanticus atlanticus and S. pavo were constituted by extensive integument folds, characterized by the massive presence of columnar cells with a cytoplasm containing granular material. Histochemical analyses demonstrated that the gland cells contained a mixture of both sulphated and non-sulphated mucins (figure 1b). The mucous secretions of the anal glands exhibited antimicrobial activity, as shown by the analysis of different gland extracts. In both species, 120 μg of the hydrophilic fraction protein and 40 μg of the hydrophobic fraction protein was able to produce a lysis halo (5 mm diameter) on M. lysodeikticus plates, showing that gland extracts had a lysozyme-like activity. We performed a non-reducing SDS-PAGE, followed by overlaying the gel with M. lysodeikticus, with hydrophobic gland extracts, and we observed that the activity was associated with a band of apparent molecular weight of 14 kDa, which is compatible with that of hen egg white lysozyme (figure 1c). This result was supported by the outcome of an acidic native electrophoresis, in which the anal gland protein migrated similarly to lysozyme, in both species (not shown).
4. Discussion
Our results provide evidence that the anal glands of blenny males consist of secreting cells, releasing a mucus enriched with bacteriolytic substances. The rubbing behaviour, performed throughout the day by nesting males, combined with the presence of antimicrobial substances indicates that the mucus produced by the anal glands is transferred to eggs, presumably providing protection from microbial colonization. Although the extent and efficacy of this protection have to be quantified, the blenny anal glands are a sexually dimorphic trait that appears to be involved in the production of antimicrobials as a component of parental care.
Indications of the occurrence in breeding adults of morphological and/or behavioural traits involved in the application of antimicrobial compounds to laid eggs have been reported only in two insects (Marchini et al. 1997; Ravi Ram & Wolfner 2005) and in a fish (Knouft et al. 2003). However, in the insects the antimicrobials appear to serve the purpose of protecting both the adults and the eggs. Indeed in the medfly Ceratitis capitata (Marchini et al. 1997) and the fruitfly Drosophila melanogaster (Lung et al. 2001) antibacterial proteins are produced by the reproductive accessory glands of the females in the former and of the males, that transfer them to females during mating, in the latter. Male and female reproductive tracts are subject to microbial invasion and mating provides an additional opportunity for infection. In insects, these compounds probably protect the male's and/or female's reproductive tract and can be bound to the surface of the eggs during deposition. On the contrary in the percid fish Etheostoma crossopterum, as in blenny fish, the coating of eggs with mucus appears to be a component of paternal care. Indeed, in this species, the inhibition of microbial infection on laid eggs has been attributed to the delivery, by nesting males, of antimicrobial compounds produced by a fleshy dorsal skin area (Knouft et al. 2003). However, at present, there is no information about the possible occurrence of a rubbing-like behaviour of this area on the eggs.
The anal glands are a male sexually dimorphic trait showing a conspicuous intra-sexual variability in size (Gonçalves et al. 1996; Neat et al. 2003; Giacomello 2004; Giacomello & Rasotto 2005) but the possible influence of female choice on their development remains puzzling. In species where male parental care plays a crucial role in offspring survival, females are expected to assess mates selecting those traits that are reliably associated with parental ability (Trivers 1972). In blennies male mating success is known to either depend on nest characteristics or on both nest and male traits, such as body or orbital tentacle sizes (reviewed in Giacomello & Rasotto 2005). The contribution of anal gland secretion to parental care performance suggests that their development too, being an honest indicator of paternal quality, might enhance male attractiveness.
Acknowledgements
The Department of Oceanography and Fisheries of the University of Azores provided logistic support for the work on the redlip blenny. M. De Girolamo was an invaluable partner in collecting data and images on the redlip blenny. C. Montecucco, C. Petersen and A. Pilastro gave helpful comments on the manuscript. Our research adheres to the Animal Behaviour Society Guidelines for the Use of Animals in Research and comply with the current Italian laws on animals.
Supplementary Material
A nesting male of Ophioblennius atlanticus atlanticus rubs the anal-urogenital region over the nest internal surface
References
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. doi:10.1016/0003-2697(76)90527-3 [DOI] [PubMed] [Google Scholar]
- Brogden K.A. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005;3:238–250. doi: 10.1038/nrmicro1098. doi:10.1038/nrmicro1098 [DOI] [PubMed] [Google Scholar]
- Clutton-Brock T.H. Princeton University Press; New Jersey, NJ: 1991. The evolution of parental care. [Google Scholar]
- Cole A.M, Weis P, Diamond G. Isolation and characterization of pleurocidin, an antimicrobial peptide in the skin secretions of winter flounder. J. Biol. Chem. 1997;272:12 008–12 013. doi: 10.1074/jbc.272.18.12008. doi:10.1074/jbc.272.18.12008 [DOI] [PubMed] [Google Scholar]
- Ellis A.E. Innate host defense mechanisms of fish against viruses and bacteria. Dev. Comp. Immunol. 2001;25:827–839. doi: 10.1016/s0145-305x(01)00038-6. doi:10.1016/S0145-305X(01)00038-6 [DOI] [PubMed] [Google Scholar]
- Gabriel O. Locating enzymes on gels. Methods Enzymol. 1971;22:578–604. [Google Scholar]
- Giacomello, E. 2004 Sexual dimorphism and male mating success in two blenny species. Ph.D. Thesis, University of Padova.
- Giacomello E, Rasotto M.B. Sexual dimorphism and male mating success in the tentacled blenny, Parablennius tentacularis (Teleostei: Blenniidae) Mar. Biol. 2005;147:1221–1228. doi:10.1007/s00227-005-0023-4 [Google Scholar]
- Gonçalves E.J, Almada V.C, Oliveira R.F, Santos A.J. Female mimicry as a mating tactic in males of the blenniid fish Salaria pavo. J. Mar. Biol. Assoc. UK. 1996;76:529–538. [Google Scholar]
- Jolles P, Jolles J. What's new in lysozyme research? Always a model system, today as yesterday. Mol. Cell Biochem. 1984;63:165–189. doi: 10.1007/BF00285225. [DOI] [PubMed] [Google Scholar]
- Knouft J.H, Page L.M, Plewa M.J. Antimicrobial egg cleaning by the fringed darter (Perciformes: Percidae: Etheostoma crossopterum): implications of a novel component of parental care in fishes. Proc. R. Soc. B. 2003;270:2405–2411. doi: 10.1098/rspb.2003.2501. doi:10.1098/rspb.2003.2501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U.K. Cleavage of structural proteins during the assembly of the head of Bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. doi:10.1038/227680a0 [DOI] [PubMed] [Google Scholar]
- Lung O, Kuo L, Wolfner M.F. Drosophila males transfer antibacterial proteins from their accessory gland and ejaculatory duct to their mates. J. Insect Physiol. 2001;47:617–622. doi: 10.1016/s0022-1910(00)00151-7. doi:10.1016/S0022-1910(00)00151-7 [DOI] [PubMed] [Google Scholar]
- Marchini D, Giordano P.C, Amons R, Bernini L.F, Dallai R. Purification and primary structure of ceratotoxin A and B, two antibacterial peptides from the female reproductive accessory glands of the medfly Ceratitis capitata (Insecta: Diptera) Insect Biochem. Mol. Biol. 1993;23:591–598. doi: 10.1016/0965-1748(93)90032-n. doi:10.1016/0965-1748(93)90032-N [DOI] [PubMed] [Google Scholar]
- Marchini D, Marri L, Rosetto M, Manetti A.G.O, Dallai R. Presence of antibacterial peptides on the laid egg chorion of the medfly Ceratitis capitata. Biochem. Biophys. Res. Commun. 1997;240:657–663. doi: 10.1006/bbrc.1997.7694. doi:10.1006/bbrc.1997.7694 [DOI] [PubMed] [Google Scholar]
- Marconato A, Rasotto M.B, Mazzoldi C. On the mechanism of sperm release in three gobiid fishes (Teleostei: Gobiidae) Environ. Biol. Fishes. 1996;46:321–327. doi:10.1007/BF00005009 [Google Scholar]
- Neat F.C, Locatello L, Rasotto M.B. Reproductive morphology in relation to alternative male reproductive tactics in Scartella cristata. J. Fish Biol. 2003;62:1381–1391. doi:10.1046/j.1095-8649.2003.00122.x [Google Scholar]
- Oliveira R.F, Almada V.C, Forsgren E, Gonçalves E.J. Temporal variation in male traits, nest aggregations and mating success in the peacock blenny. J. Fish Biol. 1999;54:499–512. doi:10.1111/j.1095-8649.1999.tb00631.x [Google Scholar]
- Ravi Ram K, Wolfner M.F. Fates and targets of male accessory gland proteins in mated female Drosophila melanogaster. Insect Biochem. Mol. Biol. 2005;35:1059–1071. doi: 10.1016/j.ibmb.2005.05.001. doi:10.1016/j.ibmb.2005.05.001 [DOI] [PubMed] [Google Scholar]
- Saul D.J, Williams L.C, Grayling R.A, Chamley L.W, Love D.R, Bergquist P.L. CelB, a gene coding for a bifunctional cellulase from the extreme thermophile Caldocellum saccharolyticum. Appl. Environ. Microbiol. 1990;56:3117–3124. doi: 10.1128/aem.56.10.3117-3124.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivers R.L. Parental investment and sexual selection. In: Campbell B, editor. Sexual selection and the descent of man. Aldine; Chicago, IL: 1972. pp. 136–179. [Google Scholar]
- Yousif A.N, Albright L.J, Evelyn T.P.T. In vitro evidence for the antibacterial role of lysozyme in salmonid eggs. Dis. Aquat. Org. 1994;19:15–19. [Google Scholar]
- Zander C.D. Secundary sex characteristics of blennioid fishes (Perciformes) Publ. Stn. Zool. Napoli. 1975;39:717–727. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A nesting male of Ophioblennius atlanticus atlanticus rubs the anal-urogenital region over the nest internal surface