Abstract
Animals must balance investments in different physiological activities to allow them to maximize fitness in the environments they inhabit. These adjustments among reproduction, growth and survival are mandated because of the competing high costs of each process. Seasonally breeding rodents generally bias their investments towards reproduction when environmental conditions are benign, but shift these investments towards processes that promote survival, including immune activity, when environmental conditions deteriorate. Because survival probability of non-tropical small mammals is generally low in winter, under certain circumstances, these animals may not allocate resources to survival mechanisms in an effort to produce as many offspring as possible in the face of increased probability of death. Such ‘terminal investments’ have been described in passerines, but there are few examples of such phenomena in small mammals. Here, we show that male Siberian hamsters (Phodopus sungorus) challenged with lipopolysaccharide (a component of gram-negative bacteria that activates the immune system) induced a small, but significant, retardation of seasonal regression of the reproductive system relative to saline-injected hamsters. This delayed reproductive regression likely reflects a strategy to maintain reproductive function when survival prospects are compromised by infection.
Keywords: photoperiodism, seasonality, Siberian hamsters, terminal investment, life history
1. Introduction
Organisms cannot simultaneously maximize all life-history traits promoting survival, growth and reproduction (Stearns 1992). Thus, organisms must distribute their resources among competing systems to maximize lifetime reproductive output in the habitats in which they live. One prediction from life-history theory, termed the ‘terminal investment hypothesis’, suggests that animals should invest more in current reproductive output if the chance of surviving to reproduce again are low (Clutton-Brock 1984). Although examples of this phenomenon have been documented in wild animals, the underlying mechanisms producing these outcomes remain largely unstudied.
Many non-tropical rodent species display annual variation in reproductive output. The energetic bottleneck of winter (e.g. increased thermoregulatory demands in the face of food scarcity) influences most mammals to minimize reproductive investment at this time of year to improve the likelihood that they survive to breed the following year (Bronson 1989). Many species use day length (photoperiod) to time these annual cycles. Photoperiod is a reliable and relatively noise-free environmental signal that provides seasonal information; thus, many of the physiological and behavioural responses to season can be simulated in the laboratory solely by manipulating photoperiod (Goldman & Nelson 1993). Among small mammals in the wild, decreasing photoperiods at the end of the summer induce reproductive regression in preparation for the upcoming winter (Prendergast et al. 2002). Siberian hamsters (Phodopus sungorus) are long-day breeding rodents that respond to changes in photoperiod. Transfer from long to short photoperiods in this species induces regression of the reproductive system including a significant reduction in testes mass and a concomitant decrease in steroid production and spermatogenesis (Prendergast et al. 2002).
We tested the hypothesis that simulated bacterial infection would block or attenuate reproductive regression in short days. To test this ‘terminal investment hypothesis’, we administered bacterial lipopolysaccharide (LPS) coincident with the transfer of Siberian hamsters from long to short days. LPS is a component of bacterial cell walls that activates the immune system and induces fever, but is not a replicating pathogen. We predicted that if hamsters interpreted LPS as a signal of infection, then they would maintain the integrity of their reproductive system to increase their chances of successful breeding before succumbing to infection.
2. Material and methods
(a) Animals
Siberian hamsters (P. sungorus) in this study were bred in our colony at the Ohio State University. Animals were individually housed in polypropylene cages in rooms with constant temperature (21±4 °C) and humidity (50±10%) and had ad libitum access to food (Harlan Teklad 8640 Rodent Diet, Indianapolis, IN) and filtered tap water. All studies were conducted with approval of the Ohio State University Institutional Animal Care and Use Committee and were conducted in compliance with all US animal welfare requirements.
(b) Experimental procedures
Hamsters were born and maintained in long day lengths (LD 16 : 8 lights off at 15.00 h eastern daylight time) until ca 60 days of age. At that point, they were anaesthetized with isoflurane vapours, weighed and baseline gonad size was assessed by measuring the length and width of the left testis (±0.1 mm) with analogue calipers. The product estimated testis volume (ETV) was calculated using the formula (testis width)2×(testis length), which is correlated (R>0.95) with testis mass (Gorman & Zucker 1995b) and is a valid indicator of reproductive condition. Following the testis measurements, hamsters were moved into a short day length (LD 8 : 16) room. Body mass and ETVs were assessed weekly for the remainder of the experiment. Three and 10 days after moving to short days, hamsters were injected with either saline or one of three doses (50 μg kg−1, 500 μg kg−1, or 1 mg kg−1, n=10 per group), of LPS. Food intake and body weights were collected preceding and following injections.
After six weeks of photoperiod treatment, hamsters were again anaesthetized, weighed, decapitated and trunk blood was collected. Reproductive tissues (testes, seminal vesicles, epididymis and inguinal fat pads) were removed, cleaned of connective tissue, then weighed. Both testes were removed from the tunica, minced with scissors and ground in a saline solution containing 0.05% Triton-X and 0.025 mM thimerosal for 25 s. Spermatid nuclei in the resulting homogenate were then counted on a haemocytometer. Blood was allowed to clot at room temperature for at least 30 min, clots were removed, samples spun at 3000 r.p.m. for 30 min at 4 °C, and sera were stored at −70 °C. Blood samples were then assayed for circulating testosterone concentrations with a double antibody I125 radioimmunoassay kit (MP Biomedicals, Irvine, California) according to the manufacturer's instructions. The minimum detection limit was 0.1 ng ml−1; samples falling below detection limit were conservatively assigned a value of 0.1 ng ml−1.
(c) Statistical analysis
Initial statistical analyses of the reproductive responses to photoperiod in LPS treated animals revealed no significant effects of dose. Therefore, we collapsed across dose producing two groups: (i) saline-injected (n=10) and (ii) LPS-injected (n=28). Food intake and body weight data were analysed with repeated measures ANOVA. Following a significant F score, multiple one-way ANOVAs were conducted on individual points. Tissue weights and testosterone concentrations were analysed with one-way ANOVAs. Tissue masses were corrected for body weight. When the variables were not normally distributed, they were log transformed. All mean differences were considered statistically significant if p<0.05.
3. Results
(a) Sickness behaviour
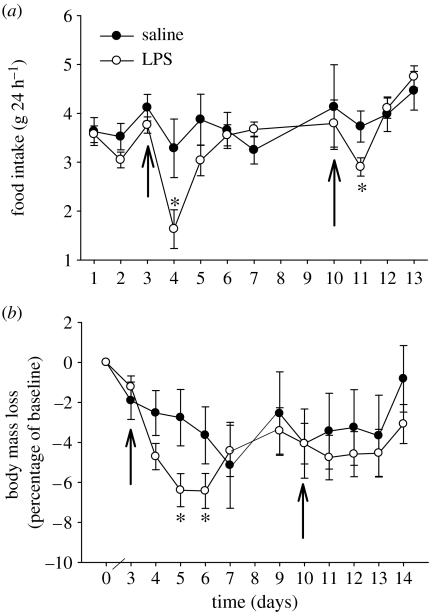
LPS induced sickness responses in Siberian hamsters in this study, in common with its effects in other species (Hart 1988). LPS reduced food intake (F1,27=3.067; p<0.05, figure 1a) following both the first and second injections. Overall, LPS reduced body mass (F1,30=2.847; p<0.05, figure 1b), although there was no significant mass loss following the second injection.
Figure 1.
LPS reduced food intake (a) and body weight (b) relative to saline injected animals. Arrows indicate LPS injections. Mean±s.e.m.; *p<0.05.
(b) Reproductive responses
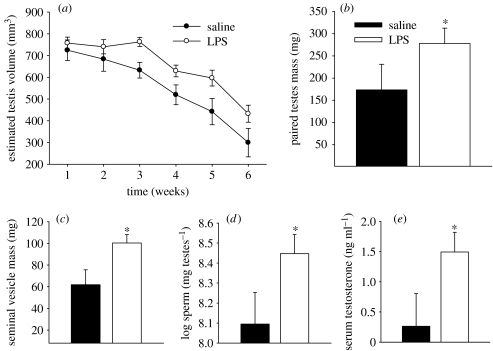
Treatment with LPS tended to slow the short day induced regression of the reproductive system. The repeated measures analysis of the ETVs was not significant, but the data are included for illustrative purposes (figure 2a). At the end of the experiment saline-injected animals had smaller testes (F1,27=4.6875, p<0.05, figure 2b), and seminal vesicles (F1,28=5.893, p<0.05, figure 2c), as well as lower serum testosterone concentrations than LPS treated hamsters (F1,28=8.644, p<0.01, figure 2d). Also, LPS injected animals had more spermatid nuclei (per milligram testis) than saline-injected hamsters (F1,27=5.325, p<0.01, figure 2e).
Figure 2.
Reproductive parameters, (a) estimated testis volumes, (b) paired testes mass, (c) seminal vesicle mass, (d) log transformed sperm per milligram testes and (e) serum testosterone concentrations. Mean±s.e.m.; *p<0.05.
4. Discussion
LPS treatment induced sickness responses in Siberian hamsters as indicated by the decrease in body mass and reduced food intake following injections. Reproductively, treatment with LPS at the transfer from long days to short attenuated the extent to which hamsters regressed their reproductive systems following six weeks of photoperiod treatment. Hamsters treated with LPS had larger testes and seminal vesicles, more testicular sperm, and higher testosterone concentrations at the end of the experiment (although compared to LD animals in other studies, all of these parameters were significantly reduced; Gorman & Zucker 1995a). These data suggest that immune challenge may bias investment away from survival and towards immediate reproduction in some circumstances.
Similar phenomena have been reported in birds, especially late in reproductive life. For instance, aged (5+ years) female collared flycatchers (Ficedula albicollis) invested more in brood care than younger females as measured by more frequent feeding bouts and higher daily energy expenditures. This strategy persisted despite a negative relationship between the number of young fledged and the survival of the mother to the next breeding season (Part et al. 1992). Also, female house sparrows (Passer domesticus) treated with Newcastle disease virus vaccine laid larger replacement clutches than those treated with a vehicle when initial clutches were first removed (Bonneaud et al. 2004). Reports of terminal investments in mammals have been comparatively rare. In the laboratory, male white-footed mice (Peromyscus leucopus) treated with sheep red blood cells displayed larger testes than control animals (Derting & Virk 2005).
Among seasonal breeding rodents, a proportion of individuals in the population do not regress reproductive organs in response to short days. This photoperiod non-responsiveness appears to be an alternative strategy by which a subset of animals take advantage of mild winters to continue breeding (Prendergast et al. 2001). This strategy can also be considered a form of terminal investment wherein animals might benefit from a mild winter with additional reproductive success, but during severe winters, they are less likely to survive (Prendergast et al. 2001). Importantly, the hamsters in this experiment did not completely fail to regress their reproductive tracts; rather, they delayed gonadal regression by several weeks.
These somewhat modest changes in reproductive status in hamsters in this study may be related to the type of immune challenge employed. Specifically, LPS is a component of bacterial cell walls; thus, it induces a robust acute phase response at the dose used the first time it is administered. Development of tolerance to LPS has been previously demonstrated in other species (Cavaillon 1995), which may explain the reduced responsiveness to the second injection of LPS in the hamsters studied here (i.e., a reduction in anorexia and body mass loss relative to changes after the first injection). Therefore, our intention to simulate a prolonged, rather than acute, infection may not have been fully successful.
Overall, the apparent shift in investment towards immediate reproduction in LPS-treated hamsters is unexpected given that the relationship between the immune and reproductive systems would lead to the opposite prediction. First, immune activity is also costly, so one might expect that reproductive tissue would regress more rapidly if maintenance of that tissue was expensive (Lochmiller & Deerenberg 2000). Second, LPS induces expression of proinflammatory cytokines that generally inhibit the reproductive neuroendocrine-axis at multiple anatomical levels (Rivier 1993). Day length information is transduced into a physiological signal via the nighttime secretion of melatonin. The melatonin signal alters the reproductive axis to inhibit the release of gonadotrophic hormones. From this point of view, it would seem that short photoperiods and immune challenge would have synergistic rather than opposing results. A proximate explanation for this counterintuitive result may involve the interference of LPS with the circadian production of melatonin. LPS administration can phase shift the circadian clock in house mice (Marpegan et al. 2005).
Our study is the first to demonstrate that an immune challenge attenuates short-day-induced gonadal regression in a seasonally breeding rodent, and it is among the first empirical examples of terminal investment in males of any species. Future studies will address both the proximate mechanisms underlying this phenomenon and the ultimate consequences of this shift in life history strategy.
Acknowledgments
This work was supported by National Institutes of Health (NIH) grants MH 57535 and MH 66144, as well as by National Science Foundation Grant IBN 04-16897.
References
- Bonneaud C, Mazuc J, Chastel O, Westerdahl H. Terminal investment induced by immune challenge and fitness traits associated with major histocompatibility complex in the house sparrow. Evolution. 2004;58:2823–2830. doi: 10.1111/j.0014-3820.2004.tb01633.x. [DOI] [PubMed] [Google Scholar]
- Bronson F.H. University of Chicago Press; Chicago, IL: 1989. Mammalian reproductive biology. [Google Scholar]
- Cavaillon J. The nonspecific nature of endotoxin tolerance. Trends Microbiol. 1995;3:320–324. doi: 10.1016/s0966-842x(00)88963-5. doi:10.1016/S0966-842X(00)88963-5 [DOI] [PubMed] [Google Scholar]
- Clutton-Brock T.H. Reproductive effort and terminal investment in iteroparous animals. Am. Nat. 1984;123:212–229. doi:10.1086/284198 [Google Scholar]
- Derting T.L, Virk M.K. Positive effects of testosterone and immunochallenge on energy allocation to reproductive organs. J. Comp. Physiol. B. 2005;175:543–556. doi: 10.1007/s00360-005-0015-1. doi:10.1007/s00360-005-0015-1 [DOI] [PubMed] [Google Scholar]
- Goldman B.D, Nelson R.J. Melatonin and seasonality in mammals. In: Yu H.S, Reiter R.J, editors. Melatonin: biosynthesis, physiological effects, and clinical applications. vol. 225–252. CRC; Boca Raton, FL: 1993. [Google Scholar]
- Gorman M.R, Zucker I. Seasonal adaptations of Siberian hamsters. II. Pattern of change in daylength controls annual testicular and body weight rhythms. Biol. Reprod. 1995a;53:116–125. doi: 10.1095/biolreprod53.1.116. doi:10.1095/biolreprod53.1.116 [DOI] [PubMed] [Google Scholar]
- Gorman M.R, Zucker I. Testicular regression and recrudescence without subsequent photorefractoriness in Siberian hamsters. Am. J. Physiol. 1995;269:R800–R806. doi: 10.1152/ajpregu.1995.269.4.R800. [DOI] [PubMed] [Google Scholar]
- Hart B.L. Biological basis of the behavior of sick animals. Neurosci. Biobehav. Rev. 1988;12:123. doi: 10.1016/s0149-7634(88)80004-6. doi:10.1016/S0149-7634(88)80004-6 [DOI] [PubMed] [Google Scholar]
- Lochmiller R.L, Deerenberg C. Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos. 2000;88:87–98. [Google Scholar]
- Marpegan L, Bekinschtein T.A, Costas M.A, Golombek D.A. Circadian responses to endotoxin treatment in mice. J. Neuroimmunol. 2005;160:102–109. doi: 10.1016/j.jneuroim.2004.11.003. doi:10.1016/j.jneuroim.2004.11.003 [DOI] [PubMed] [Google Scholar]
- Part T, Gustafsson L, Moreno J. Terminal investment and a sexual conflict in the collared flycatcher (Ficedula albicollis) Am. Nat. 1992;140:868–882. doi: 10.1086/285445. doi:10.1086/285445 [DOI] [PubMed] [Google Scholar]
- Prendergast B.J, Kriegsfeld L.J, Nelson R.J. Photoperiodic polyphenisms in rodents: neuroendocrine mechanisms, costs, and functions. Q. Rev. Biol. 2001;76:293–325. doi: 10.1086/393989. doi:10.1086/393989 [DOI] [PubMed] [Google Scholar]
- Prendergast B.J, Nelson R.J, Zucker I. Mammalian seasonal rhythms: behavior and neuroendrocrine substrates. In: Pfaff D.W, editor. Hormones, brain, and behavior. vol. 2. Academic Press; San Diego, CA: 2002. pp. 93–156. [Google Scholar]
- Rivier C. Neuroendocrine effects of cytokines in the rat. Rev. Neurosci. 1993;4:223–237. doi: 10.1515/revneuro.1993.4.3.223. [DOI] [PubMed] [Google Scholar]
- Stearns S.C. Oxford University Press; Oxford, UK: 1992. The evolution of life histories. [Google Scholar]