Abstract
Imprinting provides precocial offspring with an efficient means to optimize their subsequent behaviours. We discovered food imprinting using a sophisticated invertebrate model: the cuttlefish. We showed that early juveniles preferred the prey to which they have been visually familiarized, when the amount of information was sufficient and only if such familiarization occurred during a short sensitive period. We also demonstrated that the effects of visual food imprinting overcame those of the first food ingested. Our study shows that visual imprinting is a critical process in animals, surpassing more direct reward experiences that occur outside the critical exposure period.
Keywords: food imprinting, prey selection, cuttlefish, learning, ontogenesis
1. Introduction
Imprinting was one of the first phenomena tackled by the field of ethology. Beyond a theoretical interest, it has considerable ecological and evolutionary significance (Immelmann 1975). Originally, it was studied in precocial birds and referred to the formation of social bonds between offspring and parent (Lorenz 1935). Imprinting meets particular criteria different from other kinds of learning. First, it occurs even without any obvious reinforcement (this point was widely debated). Second, it occurs during a sensitive period, typically shortly after birth or hatching. Third, imprinting is thought to be persistent into adulthood (Lorenz 1935; Immelmann 1975; Bolhuis 1991). Subsequently, the term ‘imprinting’ has been applied to a wide variety of behaviours (e.g. habitat preference, song learning or host imprinting) and investigations have also been carried out with insects (Jaisson 1975), fish (Russock 1991) and mammals (Horn 1985). Food imprinting was originally suggested to occur in the snapping turtle, Chelydra serpentina, which showed a preference for the food first experienced early in the post-hatching period (Burghardt & Hess 1966; Burghardt 1967). Whether food imprinting really occurred is still controversial, partly because in these and other studies (Punzo 2002), the establishment of food preference was based on food ingestion (i.e. on a possible association between the food item and positive reinforcement). Since imprinting could be distinct from associative learning, subsequent studies on other animals reported a primacy effect for food preference but did not mention whether imprinting had occurred (Allen & Littleford 1955; Capretta 1969; Rabinowitch 1969; Apfelbach 1973; Burghardt 1992).
Newly hatched cuttlefish, Sepia officinalis, innately recognize, prefer and capture shrimp-like prey throughout the first week of life (Wells 1958, 1962; Darmaillacq et al. 2004b). Nevertheless, visually exposing crabs—a naturally non-preferred prey—to juvenile cuttlefish for 5 h immediately after hatching is sufficient to change this innate preference. The switch in preference occurs without reinforcement (i.e. no consumption of the prey), and is detectable only three days later, when the cuttlefish first feed (Darmaillacq et al. 2006). This result is apparently paradoxical, since previous studies failed to show even a 24 h retention of an associative learning task in cuttlefish before the third month of life (Messenger 1973; Dickel et al. 2000, 2001). Taken together, these results suggest that the establishment of prey preference and the acquisition of associative learning are separate processes. Here, we examined food imprinting considering the original criteria for classical imprinting, using an invertebrate model—the cuttlefish. Since induction of prey preference without food reinforcement has already been demonstrated (Darmaillacq et al. 2006), we aimed to determine whether the other two criteria for imprinting could be met. The first two experiments assessed the sensitive period for food imprinting, and a third experiment investigated the persistence of the early experience on subsequent prey preference.
2. Material and methods
Newborn cuttlefish hatch gradually throughout the night (Paulij et al. 1991). Consequently, hatchlings used in the following experiments were collected immediately at sunrise (ca 6.00; day 0) and individually isolated in opaque black plastic tanks (7×8×8 cm) provided with running sea water (20±1 °C). They were then left to acclimatize for 2 h, before the start of the experiment, under day light conditions. Unless otherwise specified, cuttlefish were not fed until the end of the experiment. In each experiment, the eggs were obtained from a single egg-laying bout from one female.
Sepia officinalis hatchlings do not benefit from any parental care. They hatch with yolk reserves that allow them not to eat and hence to survive until they can ingest their first meal (von Boletzky 1975, 2003; Dickel et al. 1997). This short period of time after hatching is crucial to their survival and is likely to be a sensitive period for food imprinting to occur. According to Bateson & Hinde (1987), the term sensitive period, ‘implies a sharply defined phase of susceptibility; if the relevant experience is provided before or after the period, no long-term effects are supposed detectable’. First, to determine the minimal exposure time required to change innate preference, five crabs, Carcinus sp. (carapace width 2–3 mm), were put into the tank of each cuttlefish (n=95, mean dorsal mantle length, DML±s.e.m.=8.6±0.4 mm,) for 15, 30, 60, 90 or 120 min, starting at 8.00, after the 2 h period of acclimatization. We also tested the effect of the number of prey on the familiarization efficiency by presenting 5 or 20 crabs for 1 h to each juvenile (n=35). At the end of the exposure period, the crabs were counted to verify that no cuttlefish had eaten, and were gently removed. Control cuttlefish had no crab-exposure at hatching. The feeding preference of each cuttlefish was assessed on day 3 in a two-way choice test between crabs and shrimps, Crangon crangon (detailed in Darmaillacq et al. 2004b). Subsequently, to determine the period at which a visual crab exposure was the most effective, we tested 54 cuttlefish (DML=8.5±0.2 mm,) in the following experiment. After the 2 h acclimatization period, they were assigned to one of four groups, successively familiarized with five crabs for a 2 h period beginning on their first day of life, from 8.00 to 16.00. Their feeding preference was subsequently tested on day 3 in a two-way choice test (mentioned earlier).
Imprinting should be persistent (Immelmann 1975). To test this hypothesis for food imprinting, cuttlefish (n=29, DML=9.0±0.1 mm,) were familiarized with five crabs for 5 h on day 0 and then were given a single shrimp of suitable size (total length 8 mm), on day 3 (n=15). Control cuttlefish were only given a shrimp on day 3 (n=14). Their feeding preference was assessed on day 7.
The choice tests were performed by an experimenter blind to the experimental status of the animals. Cuttlefish were tested individually and only once. All data were analysed with non-parametric tests (Siegel & Castellan 1988) and computed using StatXact-6. Differences between the values were considered significant at p<0.05. All tests were two-tailed.
3. Results
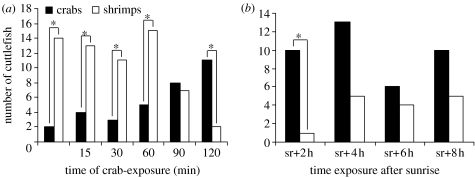
When exposure time was 60 min or less, juveniles significantly preferred shrimps to crabs (15 min: χ2=4.76, p<0.05; 30 min: χ2=4.57, p<0.05; 60 min: χ2=5, p<0.05; figure 1a), just as did cuttlefish with no crab exposure (χ2=9, p<0.01; figure 1a). When exposure time was 90 min, juveniles showed no significant preference (χ2=0.07, p=0.8); however, at 120 min exposure, they significantly preferred crabs to shrimps (χ2=6.23, p<0.05; figure 1a). Furthermore, cuttlefish tended to prefer crabs to shrimps when the exposure time was 60 min, if the number of crabs was increased from 5 to 20 (Fisher's exact test, p<0.05).
Figure 1.
Preference for crabs and shrimps by 3-day-old cuttlefish. (a) After no crab exposure (n=16) and after different lengths of a five-crab exposure: 15 min (n=17), 30 min (n=14), 60 min (n=20), 90 min (n=15) or 120 min (n=13). (b) By period of familiarization, post-hatching. The first day of life was divided into 2 h periods, 2 h after sunrise (sr), during which cuttlefish were exposed to five crabs (sr+2 h, n=11; sr+4 h, n=18; sr+6 h, n=10; sr+8 h, n=15). χ2-exact test: *p<0.05.
A significant preference for crabs was demonstrated only by cuttlefish familiarized with crabs within 2 h after sunrise (χ2=7.36, p<0.01; figure 1b). The familiarization efficiency appeared lower in the 4–6 h window after sunrise (χ2=3.56, p=0.059).
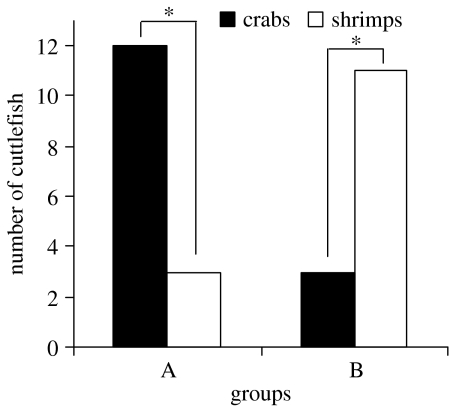
Cuttlefish familiarized with five crabs for 5 h and then given a shrimp on day 3 significantly preferred crabs over shrimps on day 7 (χ2=5.4, p<0.05; figure 2), while control cuttlefish that only ate one shrimp on day 3 significantly preferred shrimps to crabs (χ2=4.57, p<0.05).
Figure 2.
Preference for crabs and shrimps by 7-day-old cuttlefish. In group A, cuttlefish were familiarized with crabs for 5 h post-hatching and then first fed one shrimp, on day 3 (n=15); in group B, cuttlefish without crab exposure were fed one shrimp on day 3 (n=14). χ2-exact test: *p<0.05.
4. Discussion
First, our results show that the efficiency of the familiarization depends on the length of the exposure as well as the quantity of prey exposed, i.e. the information flow perceived during the familiarization. Then, we have shown that cuttlefish significantly preferred crabs only when such familiarization occurred during a 2–4 h window, which gradually terminates within 6 h after sunrise. These results suggest the existence of a sensitive period that closes early within the first day of life, whatever the hour of the night the cuttlefish have hatched. Second, in this study, we pointed out that the primacy of the early familiarization clearly outweighed untrained preferences. This effect could account for lasting feeding preferences observed in adult cuttlefish (Darmaillacq et al. 2004a).
On the basis of three main criteria—absence of reinforcement, sensitive period and primacy effect of early familiarization—we can state that food imprinting occurs in S. officinalis. Cuttlefish will eat prey other than the imprinted one, but preferentially select the imprinted prey, as for mate preference established through sexual imprinting (Immelmann 1975). Since there are possible changes in food availability, rigid invariability in prey selection is likely to be disadvantageous. Accordingly, preferring the prey to which they have been visually familiarized could allow early juveniles to maximize their chances of survival immediately after hatching. Food imprinting in animals that do not benefit from parental care constitutes an advantageous compromise between selecting food by trial/error learning—which can be long and risky—and by innate processes that restrict the young to feed on only one kind of prey.
Acknowledgments
We thank the staff of INSU-CNRS, the CREC, S. Goutte and C. Perrier for their technical assistance. We are very grateful to J. G. Boal and N. Shashar for fruitful discussions and to G. Burghardt for his valuable comments.
Footnotes
Present address: Interuniversity Institute for Marine Sciences of Eilat, PO Box 469, 88103 Eilat, Israel.
Supplementary Material
This figure shows that the amount of information perceived during the sensitive period is crucial in food imprinting: when the number of crabs was increased from 5 to 20 during the exposure time, the cuttlefish tend to prefer crabs to shrimps
References
- Allen J.F, Littleford R.A. Observations on the feeding habits and growth of immature diamondback terrapins. Herpetologica. 1955;11:77–80. [Google Scholar]
- Apfelbach R. Olfactory sign stimulus for prey selection in polecats (Putorius putorius L.) Z. Morphol. Tiere. 1973;33:270–273. doi: 10.1111/j.1439-0310.1973.tb02095.x. [DOI] [PubMed] [Google Scholar]
- Bateson P.P.G, Hinde R.A. Developmental changes in sensitivity to experience. In: Bornstein M.H, editor. Sensitive periods in development: interdisciplinary perpectives. Lawrence Erlbaum; Hillsdale, NJ: 1987. pp. 19–34. [Google Scholar]
- Bolhuis J.J. Mechanisms of avian imprinting: a review. Biol. Rev. 1991;66:303–345. doi: 10.1111/j.1469-185x.1991.tb01145.x. doi:10.1086/417244 [DOI] [PubMed] [Google Scholar]
- Burghardt G.M. The primacy effect of the first feeding experience in the snapping turtle. Psychon. Sci. 1967;7:383–384. [Google Scholar]
- Burghardt G.M. Prior exposure to prey cues influences chemical prey preference and prey choice in neonatal garter snakes. Anim. Behav. 1992;44:787–789. [Google Scholar]
- Burghardt G.M, Hess E.H. Food imprinting in the snapping turtle, Chelydra serpentina. Science. 1966;151:108–109. doi: 10.1126/science.151.3706.108. [DOI] [PubMed] [Google Scholar]
- Capretta P.J. The establishment of food preferences in chicks Gallus gallus. Anim. Behav. 1969;17:229–231. doi:10.1016/0003-3472(69)90006-2 [Google Scholar]
- Darmaillacq A.-S, Dickel L, Chichery M.-P, Agin V, Chichery R. Rapid taste aversion learning in adult cuttlefish, Sepia officinalis. Anim. Behav. 2004a;68:1291–1298. doi:10.1016/j.anbehav.2004.01.015 [Google Scholar]
- Darmaillacq A.-S, Chichery R, Poirier R, Dickel L. Effect of early feeding experience on subsequent prey preference by cuttlefish, Sepia officinalis. Dev. Psychobiol. 2004b;45:239–244. doi: 10.1002/dev.20034. doi:10.1002/dev.20034 [DOI] [PubMed] [Google Scholar]
- Darmaillacq A.-S, Chichery R, Shashar N, Dickel L. Early familiarization overrides innate feeding preference in newly-hatched Sepia officinalis cuttlefish. Anim. Behav. 2006;71:511–514. doi:10.1016/j.anbehav.2005.04.019 [Google Scholar]
- Dickel L, Chichery M.-P, Chichery R. Postembryonic maturation of the vertical lobe complex and early development of predatory behaviour in the cuttlefish (Sepia officinalis) Neurobiol. Learn. Mem. 1997;67:150–160. doi: 10.1006/nlme.1996.3754. doi:10.1006/nlme.1996.3754 [DOI] [PubMed] [Google Scholar]
- Dickel L, Boal J.G, Budelmann B.U. The effect of early experience on learning and memory in cuttlefish. Dev. Psychobiol. 2000;36:101–110. doi: 10.1002/(sici)1098-2302(200003)36:2<101::aid-dev2>3.0.co;2-l. doi:10.1002/(SICI)1098-2302(200003)36:2<101::AID-DEV2>3.0.CO;2-L [DOI] [PubMed] [Google Scholar]
- Dickel L, Chichery M.-P, Chichery R. Increase of learning abilities and maturation of the vertical lobe complex during postembryonic development in the cuttlefish, Sepia. Dev. Psychobiol. 2001;39:92–98. doi: 10.1002/dev.1033. doi:10.1002/dev.1033 [DOI] [PubMed] [Google Scholar]
- Horn G. Clarendon Press; Oxford, UK: 1985. Memory, imprinting, and the brain. [Google Scholar]
- Immelmann K. Ecological significance of imprinting and early learning. Annu. Rev. Ecol. Syst. 1975;6:15–37. doi:10.1146/annurev.es.06.110175.000311 [Google Scholar]
- Jaisson P. L'imprégnation dans l'ontogenèse du comportement de soin aux cocons chez la jeune fourmi rousse (Formica polyctena Först.) Behaviour. 1975;52:1–37. [PubMed] [Google Scholar]
- Lorenz K. Der Kumpan in der Umwelt des Vögels. J. Ornithol. 1935;83:137–213. doi:10.1007/BF01905355 See also pp. 289–413. [Google Scholar]
- Messenger J.B. Learning performance and brain structure: a study in development. Brain Res. 1973;58:519–523. doi: 10.1016/0006-8993(73)90025-5. doi:10.1016/0006-8993(73)90025-5 [DOI] [PubMed] [Google Scholar]
- Paulij W.P, Herman P.M.J, Roozen M.E.F, Denucé J.M. The influence of photoperiodicity on hatching of Sepia officinalis. J. Mar. Biol. Assoc. UK. 1991;71:665–678. [Google Scholar]
- Punzo F. Food imprinting and subsequent prey preference in the lynx spider, Oxyopes salticus Hentz (Aranea: Oxyopidae) Behav. Process. 2002;58:177–181. doi: 10.1016/s0376-6357(02)00031-1. doi:10.1016/S0376-6357(02)00031-1 [DOI] [PubMed] [Google Scholar]
- Rabinowitch V. The role of experience in the development and retention of seed preferences in zebra finches. Behaviour. 1969;33:222–236. [Google Scholar]
- Russock H.I. Effect of age at exposure and the exposure-test interval on the preferential behaviour of Oreochromis mossambicus fry to maternal models. Anim. Behav. 1991;42:688–690. [Google Scholar]
- Siegel S, Castellan N.J. 2nd edn. McGraw-Hill; Boston, MA: 1988. Nonparametric statistics for the behavioral sciences. [Google Scholar]
- von Boletzky S. A contribution to the study of yolk absorption in the Cephalopoda. Z. Morphol. Tiere. 1975;80:229–246. [Google Scholar]
- von Boletzky S. Biology of early life stages in cephalopod molluscs. Adv. Mar. Biol. 2003;44:143–203. doi: 10.1016/s0065-2881(03)44003-0. [DOI] [PubMed] [Google Scholar]
- Wells M.J. Factors affecting reactions to Mysis by newly hatched Sepia. Behaviour. 1958;13:96–111. [Google Scholar]
- Wells M.J. Early learning in Sepia. Symp. Zool. Soc. Lond. 1962;8:149–159. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This figure shows that the amount of information perceived during the sensitive period is crucial in food imprinting: when the number of crabs was increased from 5 to 20 during the exposure time, the cuttlefish tend to prefer crabs to shrimps