Abstract
Long-term effects of developmental conditions on health, longevity and other fitness components in humans are drawing increasing attention. In evolutionary ecology, such effects are of similar importance because of their role in the trade-off between quantity and quality of offspring. The central role of energy consumption is well documented for some long-term health effects in humans (e.g. obesity), but little is known of the long-term effects of rearing conditions on energy requirements later in life. We manipulated the rearing conditions in zebra finches (Taeniopygia guttata) using brood size manipulation and cross-fostering. It has previously been shown in this species that being reared in a large brood has negative fitness consequences, and that such effects are stronger in daughters than in sons. We show that, independent of mass, standard metabolic rate of 1-year-old birds was higher when they had been reared in a large brood, and this is to our knowledge the first demonstration of such an effect. Furthermore, the brood size effect was stronger in daughters than in sons. This suggests that metabolic efficiency may play a role in mediating the long-term fitness consequences of rearing conditions.
Keywords: metabolic programming, metabolic syndrome, brood size manipulation, developmental stress, Taeniopygia guttata
1. Introduction
In many species, including humans, offspring with low birth weight, low growth rate and/or low mass at independence have lower fitness prospects, and effects of developmental conditions can persist well into adulthood (Gebhardt-Henrich & Richner 1998; Lindström 1999; Lummaa & Clutton-Brock 2002). This well-documented phenomenon is the cause of the trade-off between quantity and quality of offspring, which is one of the cornerstones of life-history theory (Lessells 1991). However, despite its importance in life-history evolution, the mechanisms underlying growth effects on fitness prospects are not well known.
The importance of long-term consequences of developmental conditions has increasingly been recognized in the medical and biological sciences in recent years (e.g. Bateson et al. 2004; Gluckman et al. 2005). Although human studies on the physiological consequences of perinatal conditions revolve around energy metabolism (Desai & Hales 1997), the long-term effects of nutritional stress during development on metabolic rate and energy requirements later in life have been less studied in both humans and animals. However, individuals with higher metabolic rates will have to invest more into foraging and are potentially more vulnerable to fluctuations in food availability and environmentally triggered increased energy demands (e.g. climatic or social challenges). Thus, long-term effects of rearing conditions on energy requirements could substantially affect fitness.
We investigated the long-term effect of the rearing environment on standard metabolic rate (SMR) in zebra finches (Taeniopygia guttata). We measured SMR in resting post-absorptive birds at night, but at a temperature below the lower critical temperature. SMR constitutes a large part of total energy consumption, and is highly variable within populations (Speakman et al. 2003). What causes this variation is poorly understood, but SMR is a repeatable trait (e.g. Rønning et al. 2005), indicating that SMR is an individual characteristic that may be susceptible to genetic and environmental effects. To study the effects of developmental conditions, we manipulated brood size, which has previously been shown to modify growth in the nest and lifespan of offspring after independence (Deerenberg et al. 1996; de Kogel 1997). In zebra finches, daughters are more susceptible to nutritional stress than sons, either when induced using brood size manipulation (de Kogel 1997), or through food rationing (Martins 2004), and we therefore also compared the brood size effects on SMR between the sexes.
2. Material and methods
We used wild-type outbred zebra finches, housed in 80×40×40 cm cages on a 13.30 : 10.30 h L : D (lights on at 7:00 h) schedule at 20–22 °C and 35–50% humidity. All birds had ad libitum access to commercial tropical seed mixture, drinking water and cuttlebone supplemented three times a week with 3–4 g of egg food per bird, twice a week with branches of millet, and once a week with germinated tropical seeds. Chicks of 14 breeding pairs were cross-fostered when they were 3.9±1.6 days old to form eight small (2 or 3 chicks) and six large (5 or 6 chicks) broods. All chicks were cross-fostered, and there were no sibs within broods. There was some chick mortality before day 10 (two from small and one from large broods), and we used the brood size at day 10 to characterize the rearing conditions. Chicks were housed with their foster parents until independence (age 34.2±3.4 days), then in mixed-sex groups of four birds together with an adult male until age 70.2±3.4 days. Subsequently, the birds were housed in single-sex groups of 4 or 5 birds per cage. The 43 subjects (19 from small broods; 24 from large broods) were 13 months old when SMR was measured. Birds were reared in Leiden (The Netherlands) and moved to Groningen (The Netherlands) for the respirometry measurements, where they were housed under similar conditions and in the same social groups as in Leiden. Metabolic measurements started one week after the birds arrived in Groningen.
Metabolic rate was measured as described by Verhulst et al. (2005). SMR was defined as the lowest hourly running mean. Ambient temperature in the metabolic chambers was 22.16 °C (s.d.=0.30), which was within the range normally experienced in the holding cages. Birds were weighed when moved into and out of the metabolic chambers, and we used the mean of these values to indicate mass. Birds were placed in the (dark) metabolic chamber at 17:50 ±84 min. Metabolic rate was measured for two nights in each bird; the interval between successive measurements was 5.1 days (±1.0 day, range 4–7 days). SMR was independent of ambient temperature, the time birds were placed in the metabolic chamber, and (for the second measurement) the number of days elapsed between successive measurements. These variables were ignored in the analyses.
SMR depends on mass, which in turn is determined by body size and nutritional stores. However, effects of size (as estimated using tarsus length) and residual mass (residuals of regression of mass on size) on SMR were indistinguishable (analysis not shown). For simplicity, we therefore did not separate between size and ‘condition’ effects but simply corrected SMR for mass. Other measures of body size, such as wing length and beak dimensions, did not explain additional variation in mass (or SMR), and were therefore ignored.
Data were analysed using general linear mixed models (GLMMs), with ‘individual’ as random effect to accommodate the fact that the two measurements made on each individual cannot be considered statistically independent samples. For the same reason, we tested foster nest and nest of origin as random effect in the analyses. Repeatability was calculated following Lessells & Boag (1987), and the standard error (s.e.) of the repeatability was calculated following Becker (1984).
3. Results
Repeatability (r) of SMR was high at 0.77 (s.e.=0.06, F42,43=7.82, p<0.001). SMR depends on mass, which was highly repeatable, but repeatability remained high when SMR was corrected for mass (residuals of a regression of SMR on mass: r=0.55, s.e.=0.11, F42,43=3.48, p<0.001). This confirms that individuals used in this study could be characterized by their SMR, at least over the modest time-scale of our measurements.
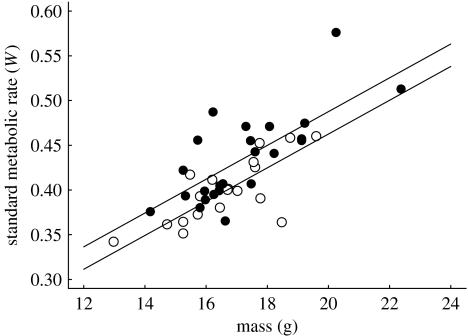
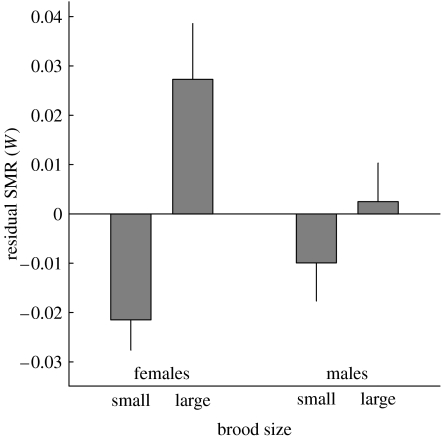
SMR was higher in birds reared in large broods when compared to birds reared in small broods (F1,43=7.7, p<0.01, GLMM including individual as random effect). Expansion of this model with other potentially relevant parameters did not change the results (table 1). SMR was significantly lower during the second measurement, but the brood size effect was independent of measurement number (i.e. there was no significant brood size×measurement number interaction). SMR was strongly dependent on mass, but the brood size effect on SMR cannot be explained by effects of mass. Firstly, mass during the SMR measurements was independent of manipulated brood size (F1,43=1.0, p=0.3). Body composition could depend on manipulated brood size, e.g. when birds reared in large broods were small but were fat as adults, but tarsus length at adulthood, a reliable measure of body size, was independent of brood size (F1,41=1.5, p=0.2). Secondly, the effect of brood size on SMR remained significant when mass was included in the mixed model (figure 1), and there was no interaction between brood size and mass (table 1). There was no sex difference in mass or size (tarsus length), and no significant effect of sex on SMR when added to the model (table 1). However, the brood size effect on SMR was stronger in daughters than in sons (figure 2; when added to the model in table 1, sex: F1,41=2.95, p=0.09, sex×brood size interaction: F1,41=4.09, p<0.05).
Table 1.
Results of the general linear mixed model analysis of SMR (W). (Series indicates first/second measurement (0 and 1, respectively). Sex is a dummy variable (0, female; 1, male). Top panel shows the model with all significant parameters, bottom panel shows the parameters that were not significant when added to the final model. Note that the significance of the effect of sex changes when the brood size×sex interaction is added together with sex—see text for details.)
| coefficient (s.e.) | F (d.f.) | p | |
|---|---|---|---|
| parameter | |||
| constant | 0.059 (0.044) | ||
| individuala | 2.41 (41, 41) | <0.005 | |
| series | −0.013 (0.005) | 7.29 (1, 41) | 0.01 |
| mass (g) | 0.019 (0.003) | 54.0 (1, 41) | <0.001 |
| brood size | 0.012 (0.004) | 8.89 (1, 41) | <0.005 |
| rejected terms | |||
| sex | −0.01 (0.01) | 0.89 (1, 41) | 0.4 |
| brood size×mass | 0.002 (0.002) | 1.3 (1, 40) | 0.3 |
| brood size×series | 0.003 (0.004) | 0.50 (1, 40) | 0.5 |
| foster brooda | 1.2 (12, 41) | 0.3 | |
| birth nesta | 1.0 (12, 41) | 0.4 |
Entered as random effect.
Figure 1.
SMR (W) in relation to mass and manipulated natal brood size (open circles: small broods, 2 or 3 chicks; filled circles: large broods, 5 or 6 chicks). Data shown are the averages per individual over the first and second measurement.
Figure 2.
Residual SMR (W, +s.e.) in relation to sex and manipulated brood size. Statistical analysis was based on raw data, but the averages shown here were calculated as follows: (i) residuals of regressions of SMR on mass were calculated separately for the first and second measurement, (ii) the mean residual was calculated per individual, and (iii) these means were averaged per group. From left to right, n=6, 8, 13 and 16.
4. Discussion
In adult birds of 1 year of age, SMR was approximately 9% higher in birds that had been reared in large broods when compared to birds reared in small broods (figure 1). To our knowledge, this is the first study to demonstrate a long-term effect of rearing conditions on metabolic efficiency independent of mass. (Vickers et al. (2000) reported a comparable effect using rodents, but their finding may be explained by the long-term effects of rearing conditions on mass that they also found.) The fact that effects on SMR persisted into adulthood suggests that early post-natal conditions can induce different metabolic phenotypes. The nestling phase seems to be a critical period during development when arising effects of rearing conditions are (at least partly) irreversible. Fitness consequences of rearing conditions may differ between the sexes (Trivers & Willard 1973; Råberg et al. 2005), and in the zebra finch it has been found that daughters are more susceptible to nutritional stress than sons (de Kogel 1997; Martins 2004). In line with these findings, we show that the brood size effect on SMR was significantly stronger in daughters (figure 2). Thus, our results indicate that long-term brood size effects on metabolic rate may be part of the mechanism causing the effects of brood size on fitness prospects.
Development of a functional explanation (Cuthill 2005) for the brood size effect on SMR is hampered by our lack of knowledge of the functional consequences of SMR variation (Williams & Vézina 2001; Speakman et al. 2004a). However, earlier data showed that birds reared in large broods (that we found to have a high SMR) live for a shorter time (de Kogel 1997). Furthermore, the brood size effect on SMR was found to be significantly stronger in daughters, in agreement with the sexual difference in brood size effect on lifespan (de Kogel 1997). However, our finding that a high SMR is associated with low-fitness prospects is inferred from the combination of different brood size manipulation studies, and there is a need for data that enable a direct test for an association between SMR and longevity. The negative association between SMR and longevity that emerges from our study in combination with the results of de Kogel (1997) contrasts with recent work on mice, where a positive association between SMR and lifespan was found (Speakman et al. 2004b). Understanding the causes of the taxonomic variation in these associations poses a challenge that could be an important step towards a better understanding of the relationship between lifespan and energy consumption.
Acknowledgements
This study was supported by an NWO-Vici grant to S.V. M.-J.H. was supported by an NWO-ALW grant to K.R. Animal experiments were carried out under license from the Animal Experiments Committee of the University of Leiden.
References
- Bateson P, et al. Developmental plasticity and human health. Nature. 2004;430:419–421. doi: 10.1038/nature02725. doi:10.1038/nature02725 [DOI] [PubMed] [Google Scholar]
- Becker W.A. A manual of quantitative genetics. Academic Enterprises; Pullman, WA: 1984. [Google Scholar]
- Cuthill I.C. The study of function in behavioural ecology. Anim. Biol. 2005;55:399–418. doi:10.1163/157075605774840923 [Google Scholar]
- de Kogel C.H. Long-term effects of brood size manipulation on morphological development and sex-specific mortality of offspring. J. Anim. Ecol. 1997;66:167–178. [Google Scholar]
- Deerenberg C, de Kogel C.H, Overkamp G.J.F. Cost of reproduction in the zebra finch Taeniopygia guttata: manipulation of brood size in the laboratory. J. Avian Biol. 1996;27:321–326. [Google Scholar]
- Desai M, Hales C.N. Role of fetal metabolism and infant growth in programming metabolism in later life. Biol. Rev. 1997;72:329–348. doi: 10.1017/s0006323196005026. doi:10.1017/S0006323196005026 [DOI] [PubMed] [Google Scholar]
- Gebhardt-Henrich S.G, Richner H. Causes of growth variation and its consequences for fitness. In: Starck J.M, Ricklefs R.E, editors. Avian growth and development. Evolution within the altricial–precocial spectrum. Oxford University Press; New York, NY: 1998. pp. 324–339. [Google Scholar]
- Gluckman P.D, Hanson M.A, Spencer H.G, Bateson P. Environmental influences during development and their later consequences for health and disease: implications for the interpretation of empirical studies. Proc. R. Soc. B. 2005;272:671–677. doi: 10.1098/rspb.2004.3001. doi:10.1098/rspb.2004.3001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessells C.M. The evolution of life histories. In: Krebs J.R, Davies N.B, editors. Behavioural ecology: an evolutionary approach. Blackwell Scientific Publications; Oxford, UK: 1991. pp. 32–68. [Google Scholar]
- Lessells C.M, Boag P.T. Unrepeatable repeatabilities: a common mistake. Auk. 1987;104:116–121. [Google Scholar]
- Lindström J. Early development and fitness in birds and mammals. Trends Ecol. Evol. 1999;14:343–349. doi: 10.1016/s0169-5347(99)01639-0. [DOI] [PubMed] [Google Scholar]
- Lummaa V, Clutton-Brock T. Early development, survival and reproduction in humans. Trends Ecol. Evol. 2002;17:141–147. doi:10.1016/S0169-5347(01)02414-4 [Google Scholar]
- Martins T.L.F. Sex-specific growth rates in zebra finch nestlings: a possible mechanism for sex ratio adjustment. Behav. Ecol. 2004;15:174–180. doi:10.1093/beheco/arg094 [Google Scholar]
- Råberg L, Stjernman M, Nilsson J.A. Sex and environmental sensitivity in blue tit nestlings. Oecologia. 2005;145:496–503. doi: 10.1007/s00442-005-0133-1. doi:10.1007/s00442-005-0133-1 [DOI] [PubMed] [Google Scholar]
- Rønning B, Moe B, Bech C. Long-term repeatability makes basal metabolic rate a likely heritable trait in the zebra finch Taeniopygia guttata. J. Exp. Biol. 2005;208:4663–4669. doi: 10.1242/jeb.01941. doi:10.1242/jeb.01941 [DOI] [PubMed] [Google Scholar]
- Speakman J.R, Ergon T, Cavanagh R, Reid K, Scantlebury D.M, Lambin X. Resting and daily energy expenditures of free-living field voles are positively correlated but reflect extrinsic rather than intrinsic effects. Proc. Natl Acad. Sci. USA. 2003;100:14 057–14 062. doi: 10.1073/pnas.2235671100. doi:10.1073/pnas.2235671100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speakman J.R, Król E, Johnson M.S. The functional significance of individual variation in basal metabolic rate. Phys. Biochem. Zool. 2004a;77:900–915. doi: 10.1086/427059. doi:10.1086/427059 [DOI] [PubMed] [Google Scholar]
- Speakman J.R, et al. Uncoupled and surviving: individual mice with high metabolism have greater mitochondrial uncoupling and live longer. Aging Cell. 2004b;3:87–95. doi: 10.1111/j.1474-9728.2004.00097.x. doi:10.1111/j.1474-9728.2004.00097.x [DOI] [PubMed] [Google Scholar]
- Trivers R.L, Willard D.E. Natural selection of parental ability to vary the sex ratio of offspring. Science. 1973;179:90–92. doi: 10.1126/science.179.4068.90. [DOI] [PubMed] [Google Scholar]
- Verhulst S, Riedstra B, Wiersma P. Brood size and immunity costs in zebra finches. J. Avian Biol. 2005;36:22–30. doi:10.1111/j.0908-8857.2005.03342.x [Google Scholar]
- Vickers M.H, Breier B.H, Cutfield W.S, Hofman P.L, Gluckman P.D. Fetal origins of hyperphagia, obesity, and hypertension and postnatal amplification by hypercaloric nutrition. Am. J. Physiol. Endocrinol. Metab. 2000;279:E83–E87. doi: 10.1152/ajpendo.2000.279.1.E83. [DOI] [PubMed] [Google Scholar]
- Williams T.D, Vézina F. Reproductive energy expenditure, intraspecific variation, and fitness. Curr. Ornithol. 2001;16:355–405. [Google Scholar]