Abstract
Using a combination of stable isotope analysis of δ13C and δ15N and long-term census data on population abundances for meiofauna in tropical aquatic rock pools, we provide evidence that species which exhibit greater variation in δ13C, an indication of a greater range of distinct carbon sources in their diet, have more stable populations than species with lower variation in δ13C. This link between increased isotope variability and reduced population variability, however, did not hold for δ15N. This suggests that increases in population stability were due to non-omnivorous feeding on multiple carbon sources within a trophic level rather than omnivorous feeding on multiple carbon sources across trophic levels. Our findings corroborate MacArthur's original hypothesis that populations that can access a greater range of resources are more stable than those which consume a more restricted range of resources.
Keywords: generality, omnivory, population dynamics, rock pools, stable isotope analysis, zooplankton
1. Introduction
In a seminal paper, MacArthur (1955) proposed that:
The amount of choice which energy has in following the paths up through the food web is a measure of the stability of the community (Odum 1953). To see this…suppose one species is abnormally uncommon. For this to have minimum effect upon the rest of the community, each predator of the species should have a large number of alternate foods to reduce the pressure on the scarce species and yet maintain their own abundance at very nearly the original level.
(MacArthur 1955, p. 334)
In MacArthur's (1955) framework, community stability arises from the increased stability of populations. This is a distinctly different perspective from current claims that community stability arises from compensatory effects of populations (e.g. Tilman 1999, reviewed in McCann 2000). MacArthur's model suggests that choice in the pathways of energy flow is key to stability. Increasing species richness may affect the number of feeding pathways in two ways. First, such an increase could increase omnivorous generality, i.e. feeding on multiple species at more than one trophic level (see Coll & Guershon 2002). Second, an increase in species richness could increase the number of distinct feeding pathways leading to any one species from a single trophic level by increasing non-omnivorous generality. Recent empirical studies show that populations can be stabilized by increasing species richness (Petchey et al. 2002; Kolasa & Li 2003; Valone & Hoffman 2003; Romanuk & Kolasa 2004; Vogt. et al. 2006), but the mechanisms (e.g. prey reliability) responsible for this stabilizing effect lack clear empirical support.
We analysed stable isotopes for invertebrates in tropical rock pools and compared variability in δ13C and δ15N (s.d.) to the species' variability in abundance (c.v.) over 49 rock pools and 10 years of annual sampling. Stable isotopes of carbon and nitrogen have a long history of use in food web studies (Peterson & Fry 1987). Stable isotope ratios are expressed in delta (δ) notation, defined as the parts per thousand or ‘per mille’ deviation from a standard material, which is Pee Dee belemnite limestone for δ13C and atmospheric nitrogen for δ15N, where δ13C or δ15N=([R.sample]/[R.standard]−1)×1000 m, where R=13C/12C or 15N/14N (Vander Zanden & Rasmussen 1999). δ13C indicates the sources of carbon assimilated by a consumer and generally does not fractionate as energy moves up the food web in contrast to δ15N which typically increases 3.4‰ per trophic level (Vander Zanden & Rasmussen 1999). If populations of rock pool fauna are stabilized by increased trophic generality, δ15N and δ13C may be less variable for species with lower population variability. Furthermore, the type of generality responsible for increased stability may be distinguished between omnivorous and non-omnivorous generality with the former being reflected in a wider range of δ15N and the latter being reflected in a wider range of δ13C.
2. Material and methods
(a) Stable isotope analysis
In January 2003, we collected tissue samples for stable isotope analysis (SIA). There are 72 species that have previously been classified as obligate rock pool species (Romanuk & Kolasa 2002). We collected individuals of 10 of these species, which account for approximately 80% of the total abundance of the metacommunity, for SIA (table 1). Owing to the small size of most species, i.e. 60 μm to 5 mm, each sample consisted of multiple individuals and for most species individuals were obtained from multiple pools. Our goal was to collect enough tissue for at least three separate composite samples for each species. However, we found no systematic effect of sample size on s.d. of either δ15N or δ13C. Organisms were left alive for 24 h to clear their gut contents and then frozen until analysis, where they were freeze-dried, ground into powder, acidified with 0.1N HCl to remove the bicarbonate and analysed by continuous flow mass spectrometry using an elemental analyser in line with a GV Instruments IsoPrime mass spectrometer at the UQAM–McGill GEOTOP facility. Analysis was conducted according to a protocol developed by Carmen & Fry (2002) as outlined in Limén & Marty (2004).
Table 1.
List of species used in the stable isotope analysis (SIA), number of SIA samples, mean SIA values, standard deviations (s.d.) of SIA values, species variability (c.v.), occupancy (the number of pools a species was collected (49 pools×10 sampling dates)) and total species abundance.
| s.d. | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| species | taxon | n | δ13C | δ15N | c.v. | occupancy | total abundance | ||
| δ13C | δ15N | ||||||||
| Heterocypris sp. | Ostracoda | 5 | −22.714 | 6.375 | 0.186 | 0.307 | 2.768 | 93 | 7891 |
| Candona sp. | Ostracoda | 3 | −24.654 | 8.056 | 0.433 | 0.077 | 1.117 | 46 | 2930 |
| Cypricercus sp. | Ostracoda | 3 | −21.306 | 6.849 | 0.200 | 0.428 | 1.788 | 53 | 6287 |
| Cypridopsis cf. mariae Rome | Ostracoda | 3 | −22.191 | 5.666 | 0.423 | 0.273 | 1.788 | 103 | 39 354 |
| Orthocyclops modestus | Copepoda | 2 | −25.023 | 5.809 | 0.032 | 0.411 | 3.508 | 256 | 40 146 |
| Ceriodaphnia rigaudi | Cladocera | 3 | −27.642 | 2.504 | 0.365 | 0.148 | 1.704 | 72 | 32 780 |
| Leidigia leidigi | Cladocera | 3 | −25.029 | 3.890 | 0.376 | 0.276 | 0.933 | 71 | 866 |
| Dasyhelea sp. | Diptera | 3 | −21.991 | 2.836 | 0.087 | 0.062 | 2.577 | 79 | 2283 |
| Culex sp. | Diptera | 3 | −19.982 | 4.016 | 0.240 | 0.286 | 1.242 | 104 | 1660 |
| oligochaete | Oligochaeta | 3 | −15.989 | 5.453 | 0.020 | 0.323 | 2.565 | 68 | 3635 |
(b) Population dynamics
Population dynamics were assessed as the variability in total population abundance for each of the 10 species summed across 49 rock pools. Ten annual samples collected in December or January from 1989 to 2004 were used in the analysis. Collection methods have been reported previously (Romanuk & Kolasa 2002, 2004).
(c) Statistical analysis
Variability in δ15N and δ13C for each species was calculated as the s.d. in δ15N and δ13C across all samples. Population variability was calculated as c.v. in abundance for each species over all rock pools and dates.
3. Results
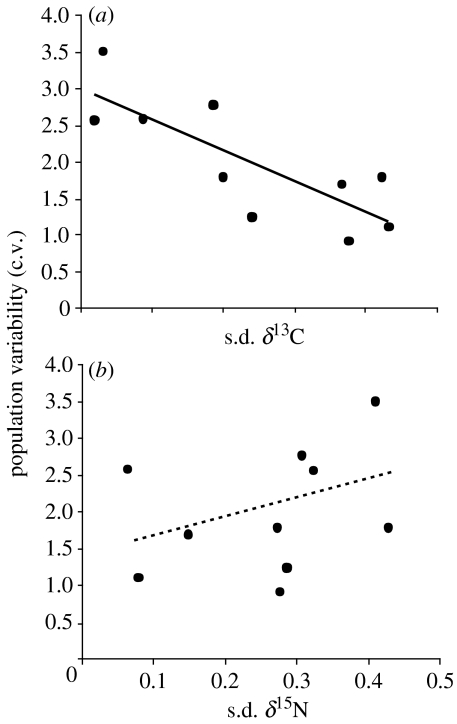
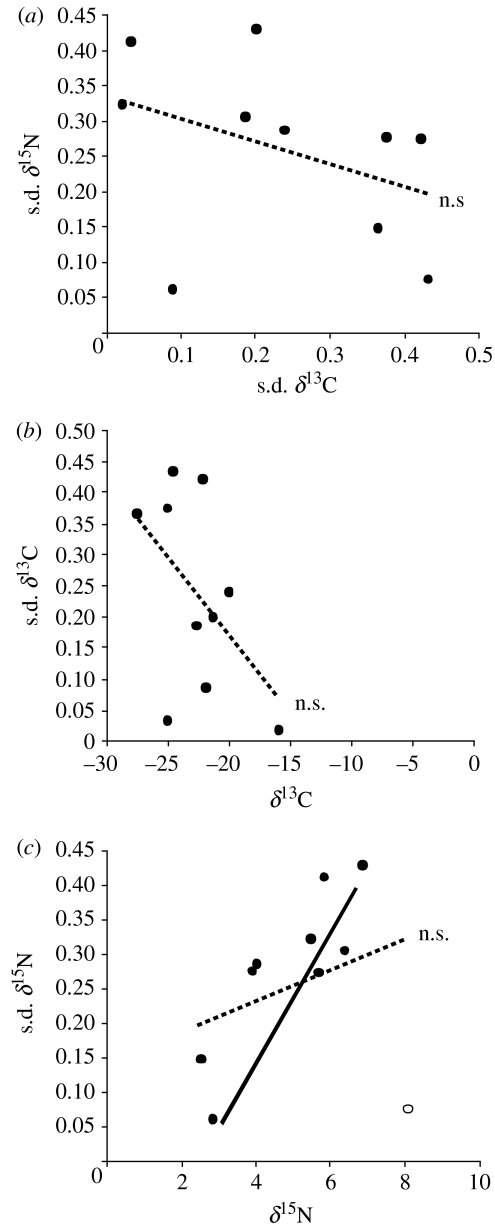
As hypothesized, the mean c.v. of population abundance decreased significantly with increases in the s.d. of δ13C (r2=0.651, p=0.004; figure 1a). Interestingly, this pattern was not repeated for δ15N (figure 1b). To ensure that these results were not due to the structure of the data or other underlying correlations, we tested a number of relationships between population c.v., mean and s.d. of SIA values, and correlations between SIA values themselves. There was no relationship between mean population c.v. and mean δ13C (p=0.655) or δ15N (p=0.882) and no relationship between mean δ13C and s.d. δ13C (p=0.12) or mean δ15N and s.d. δ15N (p=0.374; figure 2b,c). However, when the ostracod Candona sp. was removed from the latter analysis, s.d. of δ15N increased with mean δ15N (r2=0.729, p=0.003, n=9; figure 2c). These analyses show that population variability was unrelated to the mean value of the actual carbon source (e.g. freshwater versus marine inputs, Fry & Sherr 1984) and that there was no trend of SIA values varying according to mean carbon source. It is possible, however, that s.d. of δ15N increases with mean δ15N. This would suggest that as trophic level increases, species can access resources from a wider range of trophic positions. However, we found that s.d. of δ13C and s.d. of δ15N did not covary (p=0.385; figure 2a), confirming that these two components of energy flow were independent for these samples.
Figure 1.
More stable species access a wider range of carbon sources. (a) Relationship between range of carbon sources (s.d. of δ13C) and species variability (c.v.) and (b) relationship between range of nitrogen sources (s.d. of δ15N) and species variability (c.v.). Non-significant regression lines are shown (hatched line; n.s.).
Figure 2.
(a) Relationship between s.d. of δ13C and s.d. of δ15N. Non-significant regression line is shown (hatched line; n.s.). Relationship between (b) Mean δ13C and s.d. of δ13C and (c) Mean δ15N and s.d. of δ15N. When Candona sp. is removed from the analysis (black line) s.d. of δ15N increases significantly with mean δ15N. Non-significant regression lines are shown (hatched line; n.s.).
4. Discussion
This study provides, to our knowledge, the first clear empirical evidence that populations that access a greater range of resources are more stable than those which consume a more restricted range of resources. Furthermore, in rock pool communities, it appears that non-omnivorous, rather than omnivorous, generality stabilizes populations. Interestingly, these results complement and converge with more general observations that habitat generalists tend to be less variable than habitat specialists (Waltho & Kolasa 1994; Kolasa & Li 2003).
Our results also reveal a putative mechanism for why some populations in multi-trophic communities appear to be stabilized by species richness as well as corroborate the predictions of MacArthur (1955) that a ‘restricted diet lowers stability’. However, as MacArthur further states:
[a]restricted diet is what is essential for efficiency…efficiency and stability are the two features required for survival under natural selection. Efficiency enables individual animals to outcompete others, but stability allows individual communities to outsurvive less stable ones. From this, it seems reasonable that natural selection operates for maximum efficiency subject to certain necessary stability.
(MacArthur 1955, p. 535)
In order for populations to persist through time, there must be a balance between consumption efficiency (assumed to decline with increasing ability to access multiple resources) and stability (which may generally increase with increasing ability to access multiple resources). However, if several resources are accessed from within a prey trophic level (non-omnivorous generality) as opposed to across trophic levels (omnivorous generality) then the decline in consumption efficiency may be less than accessing resources from multiple trophic levels. This means that the food web structure that enhances population stability is one that increases the number of pathways connecting a consumer to its prey trophic level. This idea suggests that a return to the study of the number of links between trophic levels (Cohen & Briand 1984; Martinez 1994), a set of structural food web properties that have been excluded from more recent analyses of food web structure (e.g. Williams & Martinez 2000; Cattin et al. 2004), may be warranted.
While the ubiquity and potentially stabilizing role of omnivorous generality has been widely recognized (Agrawal 2003; but see Vandermeer 2006) less attention has been paid to non-omnivorous generality, despite its potential importance. In an analysis of omnivory in large complex food webs, Williams & Martinez (2004) found most species were restricted to consumption of adjacent rather than disparate trophic levels. This type of structure results in linearized food webs that appear to be more likely to experience trophic cascades (Pace et al. 1999; Schmitz et al. 2000; Thibault & Loreau 2003) and have higher interaction strengths that can destabilize population dynamics (McCann et al. 1998). However, the number of species within trophic levels increases much faster than the number of trophic levels as species are added to a community (Williams & Martinez 2004). This linearity and restricted food web height increases the potential for non-omnivorous generality to stabilize population dynamics.
In conclusion, our results provide significant evidence that non-omnivorous generality stabilizes populations of rock pool meiofauna. The ability to exploit horizontal diversity appears to contribute to the population stability of consumers, and this may be an, as of yet, unrecognized further benefit of high levels of species richness in food webs. Horizontal diversity may be especially relevant under highly variable environmental conditions, where alternative pathways could become differentially available through time.
Acknowledgments
This work was supported by a National Science and Research Council of Canada (NSERC) Postdoctoral Fellowship to T.N.R, an NSERC operating grant to J.K., an NSERC Discovery grant to B.E.B. and US National Science Foundation grants to N.D.M. from the Information Technology Research (ITR-0326460) and Biological Databases and Informatics (DBI-0234980) programs. We thank Richard Vogt for assistance in the field and Maria Maezo and Allain Barnett for assistance back at the lab.
References
- Agrawal A.A. Why omnivory? Ecology. 2003;84:2521. [Google Scholar]
- Carman K.R, Fry B. Small-sample methods for δ13C and δ15N analysis of the diets of meiofaunal species using natural-abundance and tracer-addition isotope techniques. Mar. Ecol. Prog. Ser. 2002;240:85–92. [Google Scholar]
- Cattin M.F, Bersier L.F, Banasek-Richter C, Baltensperger R, Gabriel J.P. Phylogenetic constraints and adaptation explain food web structure. Nature. 2004;427:835–839. doi: 10.1038/nature02327. doi:10.1038/nature02327 [DOI] [PubMed] [Google Scholar]
- Cohen J.E, Briand F. Trophic links of community food webs. Proc. Natl Acad. Sci. USA. 1984;81:4105–4109. doi: 10.1073/pnas.81.13.4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coll M, Guershon M. Omnivory in terrestrial arthropods: mixing plant and prey diets. Annu. Rev. Entomol. 2002;47:267–297. doi: 10.1146/annurev.ento.47.091201.145209. doi:10.1146/annurev.ento.47.091201.145209 [DOI] [PubMed] [Google Scholar]
- Fry B, Sherr E.B. d13C measurements as indicators of carbon flow in marine and freshwater ecosystems. Contrib. Mar. Sci. 1984;27:13–47. [Google Scholar]
- Kolasa J, Li L.B. Removing the confounding effect of habitat specialization reveals the stabilizing contribution of diversity to species variability. Proc. R. Soc. B. 2003;270(Suppl.):S198–S201. doi: 10.1098/rsbl.2003.0059. doi:10.1098/rsbl.2003.0059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limén, H. & Marty, J. 2004 Stable carbon and nitrogen isotope analysis on small-sized samples: a protocol for preparation and analysing microscopic organisms. GV Instruments, Application Note, AN13. (http://www.geotop.uqam.ca)
- MacArthur R.H. Fluctuation of animal populations and a measure of community stability. Ecology. 1955;36:533–536. [Google Scholar]
- Martinez N.D. Scale-dependent constraints on food-web structure. Am. Nat. 1994;144:935–953. doi:10.1086/285719 [Google Scholar]
- McCann K. The diversity–stability debate. Nature. 2000;405:228–233. doi: 10.1038/35012234. doi:10.1038/35012234 [DOI] [PubMed] [Google Scholar]
- McCann K, Hastings A, Huxel G.R. Weak trophic interactions and the balance of nature. Nature. 1998;395:794–798. doi:10.1038/27427 [Google Scholar]
- Odum E.P. W. B. Saunders; Philadelphia, PA: 1953. Fundamentals of ecology. [Google Scholar]
- Pace M.L, Cole J.J, Carpenter S.R, Kitchell J.F. Trophic cascades revealed in diverse systems. Trends Ecol. Evol. 1999;14:483–488. doi: 10.1016/s0169-5347(99)01723-1. doi:10.1016/S0169-5347(99)01723-1 [DOI] [PubMed] [Google Scholar]
- Petchey O.L, Casey T.J, Jiang L, McPhearson P.T, Price J. Species richness, environmental fluctuations, and temporal change in total community biomass. Oikos. 2002;99:231–240. doi:10.1034/j.1600-0706.2002.990203.x [Google Scholar]
- Peterson B.J, Fry B. Stable isotopes in ecosystem studies. Annu. Rev. Ecol. Syst. 1987;18:293–320. doi:10.1146/annurev.es.18.110187.001453 [Google Scholar]
- Romanuk T.N, Kolasa J. Environmental variability alters the relationship between richness and variability of community abundances in aquatic rock pool microcosms. Ecoscience. 2002;9:55–62. [Google Scholar]
- Romanuk T.N, Kolasa J. Population variability is lower in diverse rock pools when the obscuring effects of local processes are removed. Ecoscience. 2004;11:455–462. [Google Scholar]
- Schmitz O.J, Hamback P.A, Beckerman A.P. Trophic cascades in terrestrial systems: a review of the effects of carnivore removals on plants. Am. Nat. 2000;155:141–153. doi: 10.1086/303311. doi:10.1086/303311 [DOI] [PubMed] [Google Scholar]
- Thibault E, Loreau M. Food-web constraints on biodiversity–ecosystem functioning relationships. Proc. Natl Acad. Sci. USA. 2003;100:14 949–14 954. doi: 10.1073/pnas.2434847100. doi:10.1073/pnas.2434847100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilman D. The ecological consequences of changes in biodiversity. Ecology. 1999;80:1455–1474. [Google Scholar]
- Valone T.J, Hoffman C.D. Population stability is higher in more diverse annual plant communities. Ecol. Lett. 2003;6:90–95. doi:10.1046/j.1461-0248.2003.00406.x [Google Scholar]
- Vandermeer J. Omnivory and the stability of food webs. J. Theor. Biol. 2006;238:497–504. doi: 10.1016/j.jtbi.2005.06.006. doi:10.1016/j.jtbi.2005.06.006 [DOI] [PubMed] [Google Scholar]
- Vander Zanden M.J, Rasmussen J.B. Primary consumer δ13C and δ15N and the trophic position of aquatic consumers. Ecology. 1999;80:1395–1404. [Google Scholar]
- Vogt R.J, Romanuk T.N, Kolasa J. Richness–variability relationships in multi-trophic aquatic microcosms. Oikos. 2006;113:55–66. [Google Scholar]
- Waltho N, Kolasa J. Organization of instabilities in multispecies systems: a test of hierarchy theory. Proc. Natl Acad. Sci. USA. 1994;91:1682–1685. doi: 10.1073/pnas.91.5.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R.J, Martinez N.D. Simple rules yield complex food webs. Nature. 2000;404:180–183. doi: 10.1038/35004572. doi:10.1038/35006555 [DOI] [PubMed] [Google Scholar]
- Williams R.J, Martinez N.D. Limits to trophic similarity and omnivory in complex food webs: theory and data. Am. Nat. 2004;163:458–468. doi: 10.1086/381964. doi:10.1086/381964 [DOI] [PubMed] [Google Scholar]