Abstract
Biological asymmetries are important elements of the structure and function of many living organisms. Using the Plio–Pleistocene fossil record of crab predation on morphologically similar pairs of right- and left-handed snail species, we show here for the first time, contrary to traditional wisdom, that rare left-handed coiling promotes survival from attacks by right-handed crabs. This frequency-dependent result influences the balance of selection processes that maintain left-handedness at the species level and parallels some social interactions in human cultures, such as sports that involve dual contests between opponents of opposite handedness.
Keywords: sinistral, shell-coiling, predation, survival advantage, evolution, biological asymmetry
1. Introduction
From the twists of molecules to the internal organs of humans, conspicuous biological asymmetries pervade nature (McManus 2002). The vast majority of marine snails, for instance, have shells that coil dextrally—to the right when oriented with the shell apex pointing upwards and the aperture facing the observer. Although left-handed (sinistral) individuals are known in many normally dextral species, characteristically sinistral species are exceptionally rare (Arthur 2000; Vermeij 2002).
Little attention has been given to the adaptive consequences of these rare reversals in shell coiling. Handedness is known to affect reproductive success (Ueshima & Asami 2003); dextral snails often have difficulty in mating with conspecific sinistral individuals, making it likely that the maintenance of handedness is associated with sexual selection for compatibility during mating (Asami et al. 1998). Sinistrality is commonly assumed, however, to have no survival-related advantages after hatching, being entirely equivalent in functional terms with right-handedness (Vermeij 1975, 2002, 2004; Gould et al. 1985).
Left-handedness probably originated as a species-level character in two common western Atlantic snail groups, whelks (Busycon) and cone shells (Conus), during the Pliocene some 3.5–4.0 Myr ago (Vermeij 2002). Since the origin of sinistrality in these two clades, ecologically and morphologically similar dextral and sinistral whelk species have coexisted sympatrically, while the trait was lost in cones with the extinction of Conus adversarius about 1.8 Myr ago (figure 1; table 1). The excellent fossil record of these two groups provides a unique opportunity to test the hypothesis that shell-coiling direction is selectively neutral after hatching.
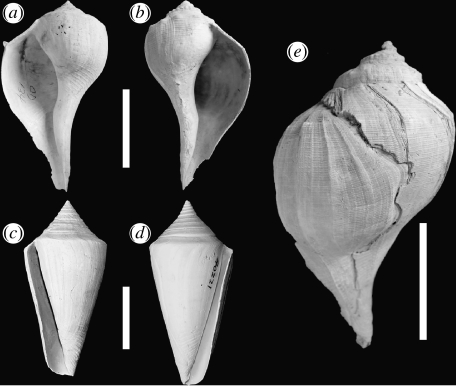
Figure 1.
(a–d) Sinistral and dextral species pairs of whelks and cones and (e) crab-induced repair scar on shell of Busycon carica. (a) Sinistrofulgur adversarium; (b and e) Busycon carica; (c) C. adversarius (PRI 52916-1); and (d) Conus cf. largillierti (PRI 40221-1). (a, b and e) scale bar, 50 mm; (c and d) scale bar, 20 mm.
Table 1.
Samples of dextral (d) and sinistral (s) pairs of whelks and cone shells examined, including taxon names, locality names, stratigraphic information, and sample size, number of shells with more than one repair scar and mean shell lengths of specimens in each sample.
| taxa | locality | formation (age) | sample size; number shells with at least one repair; mean shell length (mm) |
|---|---|---|---|
| Sinistrofulgur adversarium (s) | Shallotte, NC | upper Waccamaw Fm. (Lower Pleistocene) | s: 80, 5, 90.8 |
| Busycon carica (d) | d: 35, 12, 94.2 | ||
| Sinistrofulgur adversarium (s) | Old Dock, NC | early Waccamaw Fm. (Plio–Pleistocene) | s: 75, 14, 93.3 |
| Busycon carica (d) | d: 41, 11, 90.9 | ||
| Sinistrofulgur contrarium (s) | Rozier Farm, NC | Duplin Fm. (late Pliocene) | s: 27, 2, 79.4 |
| Busycon maximum (d) | d: 18, 3, 81.7 | ||
| Sinistrofulgur contrarium (s) | Natural Well, NC | Duplin Fm. (late Pliocene) | s: 59, 11, 65.5 |
| Busycon maximum (d) | d: 14, 4, 65.8 | ||
| Sinistrofulgur contrarium (s) | Kirby Farm Pond, SC | Duplin Fm. (late Pliocene) | s: 24; 3; 66.3 |
| Busycon maximum (d) | d: 31, 12, 70.4 | ||
| Conus adversarius (s) | Acme, NC | lower Waccamaw Fm. (Plio–Pleistocene) | s: 39, 17, 30.9 |
| Conus cf. largillierti (d) | d: 65, 31, 27.9 | ||
| Conus adversarius (s) | Old Dock, NC | lower Waccamaw Fm. (Plio–Pleistocene) | s: 10, 3, 41.6 |
| Conus cf. largillierti (d) | d: 38, 23, 36.1 | ||
| Conus adversarius (s) | Crescent Beach, SC | lower Waccamaw Fm. (Plio–Pleistocene) | s: 113, 35, 29.2 |
| Conus cf. largillierti (d) | d: 540, 199, 25.6 | ||
| Conus adversarius (s) | La Belle, FL | Caloosahatchee Fm. (Plio–Pleistocene) | s: 168, 72, 29.1 |
| Conus cf. largillierti (d) | d: 244, 93, 23.3 | ||
| Conus adversarius (s) | Lumber River, NC | Duplin Fm. (late Pliocene) | s: 22, 8, 45.9 |
| Conus cf. largillierti (d) | d: 42, 24, 45.1 | ||
| Conus adversarius (s) | Quality Aggregates, FL | Tamiami Fm. (late Pliocene) | s: 56, 11, 43.3 |
| Conus cf. largillierti (d) | d: 31, 13, 40.9 |
Crabs are the major enemies of both whelks and cones (Magalhaes 1948; Currey & Kohn 1976; Zipser & Vermeij 1980; Dietl 2003), but they, as with so many other predators, are not always successful in killing their prey (Vermeij 1982). They often leave tell-tale signs of failed attacks as jagged scars on the shells of their prey (figure 1). If handedness has no survival-related advantages, we predicted that the incidence of individuals with crab predation scars should not vary significantly between dextral and sinistral species.
2. Material and methods
We calculated the incidence of shell repair for samples of five whelk and six cone species pairs (totalling 1772 specimens) as the percentage of individuals in a sample with at least one repair scar (Alexander & Dietl 2003; table 1; electronic supplementary material). Since the frequency of shell repair is often size dependent, we also limited our analysis to comparisons of samples with similar mean shell lengths to avoid any potential size-related bias (Dietl 2003; table 1). We only counted major scars that appeared as jagged relief on the surface of the body whorl (Dietl 2003). We assumed that members of each species pair had similar behaviours, growth rates and habitat preferences, such that encounter rates with crab predators in the environment were comparable. These assumptions are reasonable for whelks given what is known about their biology and ecology (Magalhaes 1948; Kent 1983; Kraeuter et al. 1989; Dietl 2003), but are more speculative in nature for the studied cone species.
3. Results
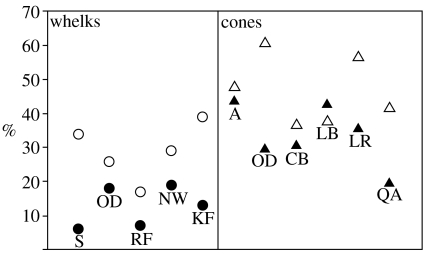
Dextral whelk and cone species typically have higher frequencies of shell repair than sinistral species (figure 2); only one of 11 samples showed the opposite pattern—higher repair frequencies in the left-handed species of the pair. We reject our null hypothesis that scars are present in equal proportions in left- and right-handed snails (binomial test, p<0.05).
Figure 2.
Percentage of whelk and cone shells with at least one repair scar. Open circle, dextral whelk; filled circle, sinistral whelk; open triangle, dextral cone; filled triangle, sinistral cone. We obtained a similar pattern, when we estimated the number of scars per shell for each sample, which takes into consideration multiple attacks by predators on individuals. Abbreviations pertain to locality samples: S=Shallotte, NC; OD=Old Dock, NC; RF=Rozier Farm, NC; NW=Natural Well, NC; KF=Kirby Farm Pond, SC; A=Acme, NC; CB=Crescent Beach, SC; LB=La Belle, FL; LR=Lumber River, NC and QA=Quality Aggregates, FL.
4. Discussion
Surprisingly, the results do not support the hypothesis that shell-coiling direction is selectively neutral. A higher incidence of death for left-handed snails that were attacked by crabs seems unlikely to explain this pattern given the similar morphologies (i.e. shell defences against predators) of left- and right-handed whelks and cones (figure 1). These results suggest that left-handed coiling may be advantageous for escaping crab predation.
We suggest that handedness is important when snails encounter laterally asymmetric shell-peeling crab predators, which were common enemies of subtropical American whelks and cones during the Plio–Pleistocene (Dietl 2003; Portell & Agnew 2004). Sinistrality provides a competitive edge in survivorship because the asymmetric claw that crab predators use to peel their prey's shell is typically on the right-hand side of their bodies (Ng & Tan 1985; Shigemiya 2003). This tendency for right-handedness in crab predators leaves them at a disadvantage in breaking the shell lip of sinistrally coiled snails (Ng & Tan 1985). This disadvantage would be especially pronounced in the Calappidae, which use a unique set of adventitious protuberances on their right claws to peel the shell of their prey (Shoup 1968).
The ‘sinistral advantage’ identified here thus occurs at the interface of interaction between a predator and its prey, and is not realized unless species of opposite handedness interact. We can think of two possibilities, albeit not mutually exclusive, to explain this advantage.
First, our preliminary observations of the box crab Calappa flammea attacking whelks (Busycon carica and Sinistrofulgur sinistrum) suggest that the frequency of shell repair was probably higher in dextral species because sinistral species are more difficult for right-handed crabs to manipulate into a position to start the shell-peeling process. Three crabs (85–95 mm in carapace width) were observed to pick up individuals of the left-handed S. sinistrum (43–48 mm shell length) and abandon them prior to inflicting shell damage that would later have to be repaired by the surviving snail. However, right-handed B. carica of similar size were readily opened by the same crabs. This behaviour makes sense in light of what we know about the shell-peeling behaviour of calappids.
Calappids typically orient the shell of their prey with the aperture facing up and the spire of the shell pointing away from the crab's body, which positions the lip or edge of the aperture on the right-hand side (Shoup 1968). This positioning allows the crab to insert the large tooth on its right dactyl into the snail's aperture to break the shell. The shell is then rolled towards the tooth of the right dactyl in an anticlockwise direction for another round of shell peeling (Shoup 1968). A left-handed snail, however, presents some novel challenges. First, the lip of the aperture is now on the left-hand side, making it difficult for the crab to insert its right dactyl into the aperture to break the shell. In this position, the prey's shell also has to be rolled in the opposite direction than a right-handed snail. Only if the prey's shell is flipped around so that the spire of the shell now points towards the crab's body would the lip of the sinistral shell aperture be oriented like a dextral shell prior to the initiation of peeling by the crab. In this orientation, however, the crab has less control over the prey's shell, which is for the most part directed away from the crab's body. This behaviour of not wasting time and energy in attempting to peel the snail's shell if more easily captured prey are available is very common among many taxa of crabs that preferentially select prey of various sizes and shapes (Thomas & Himmelman 1988).
A second possibility is that the handedness advantage may stem simply from the fact that left-handed prey are less commonly encountered than right-handed prey. This advantage parallels some social interactions in human cultures that result when right- and left-handed individuals compete, especially in sports or fights involving dual confrontations (interactive contests such as boxing, tennis, fencing and baseball), where left-handers occasionally enjoy an advantage over their right-handed opponents (Grouios et al. 2000; McManus 2002; Gould 2003; Brooks et al. 2004; Faurie & Raymond 2005). Crab predators ‘know’ much less about sinistral prey than sinistral prey know about their predators. This information inequality gives sinistral prey a competitive edge. In other words, from the left-handed snail's point of view, nothing is different in an interaction with its predator—right-handedness is the typical form for most of the predatory crab species that a sinistral snail might encounter in its lifetime. Right-handed crabs on the other hand do not share this luxury. Most prey they encounter in a lifetime are right-handed such that they have less experience manipulating sinistral prey should they encounter them. This advantage provides a historical role for frequency-dependent success of the prey in escaping predation in the maintenance of handedness by natural selection.
If selectively advantageous, why is left-handedness then so rare, especially in tropical seas where crab predation is ecologically important? We do not have an answer to this reasonable question, but we find some solace in knowing that we are not alone in our puzzlement. D'Arcy Wentworth Thompson captured the state of our understanding, which seems appropriate even today, when he wrote:
But why, in the general run of shells, all the world over, in the past and in the present, one direction of twist is so overwhelmingly commoner than the other, no man knows (Thompson 1943, p. 821).
Our findings do emphasize, however, that when reversals in shell coiling lead to the formation of new species, they may have important and unexpected adaptive consequences that lead to interesting relationships between selection and morphological asymmetry. The survival advantage of left-handedness suggests that natural and sexual selection processes act to reinforce rather than oppose (Darwin 1871) each other in maintaining species-level sinistrality in snails. In the balance of selection processes, the survival advantage of sinistrality may be as important a selection pressure as mate selection, especially for long-lived species that are exposed to crab predation for a significant amount of time before reaching sexual maturity. It is not all about sex all of the time.
Acknowledgments
We thank W. Allmon, D. Briggs, G. Herbert, E. Hermsen, G. Vermeij and an anonymous reviewer for helpful discussions and comments on the manuscript. We thank R. Portell for access to fossil localities in Florida and the collections of the Florida Museum of Natural History. J.R.H.'s contributions to this paper were supported in part by the Geological Society of America and the Paleontological Research Institution.
Footnotes
Present address: Department of Geology, University of Kansas, Lawrence, KS 66045, USA.
Supplementary Material
References
- Alexander R.R, Dietl G.P. The fossil record of shell-breaking predation on marine bivalves and gastropods. In: Kelley P.H, Kowalewski M, Hanson T.A, editors. Predator–prey interactions in the fossil record. Kluwer Academic/Plenum Publishers; New York, NY: 2003. pp. 141–176. [Google Scholar]
- Arthur W. Intraspecific variation in developmental characters: the origin of evolutionary novelties. Am. Zool. 2000;40:811–818. [Google Scholar]
- Asami T, Cowie R.H, Ohbayashi K. Evolution of mirror images by sexually asymmetric mating behavior in hermaphroditic snails. Am. Nat. 1998;152:225–236. doi: 10.1086/286163. doi:10.1086/286163 [DOI] [PubMed] [Google Scholar]
- Brooks R, Bussière L.F, Jennions M.D, Hunt J. Sinister strategies succeed at the cricket World Cup. Proc. R. Soc. B. 2004;271(Suppl. 3):S64–S66. doi: 10.1098/rsbl.2003.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currey J.D, Kohn A.J. Fracture in the crossed-lamellar structure of Conus shells. J. Mater. Sci. 1976;11:1615–1623. doi:10.1007/BF00737517 [Google Scholar]
- Darwin C. John Murray; London, UK: 1871. The descent of man, and selection in relation to sex. [Google Scholar]
- Dietl G.P. Coevolution of a marine gastropod predator and its dangerous bivalve prey. Biol. J. Linn. Soc. 2003;80:409–436. doi:10.1046/j.1095-8312.2003.00255.x [Google Scholar]
- Faurie C, Raymond M. Handedness, homicide and negative frequency-dependent selection. Proc. R. Soc. B. 2005;272:25–28. doi: 10.1098/rspb.2004.2926. doi:10.1098/rspb.2004.2926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould S.J. WW Norton & Co; New York, NY: 2003. Triumph and tragedy in mudville: a lifelong passion for baseball. [Google Scholar]
- Gould S.J, Young N.D, Kasson B. The consequences of being different—sinistral coiling in Cerion. Evolution. 1985;39:1364–1379. doi: 10.1111/j.1558-5646.1985.tb05701.x. [DOI] [PubMed] [Google Scholar]
- Grouios G, Tsorbatzoudis H, Alexandris K, Barkoukis V. Do left-handed competitors have an innate superiority in sports? Percept. Mot. Skills. 2000;90:1273–1282. doi: 10.2466/pms.2000.90.3c.1273. [DOI] [PubMed] [Google Scholar]
- Kent B.W. Natural-history observations on the busyconine whelks Busycon contrarium (Conrad) and Busycotypus spiratum (Lamark) J. Mollus. Stud. 1983;49:37–42. [Google Scholar]
- Kraeuter J.N, Castagna M, Bisker R. Growth rate estimates for Busycon carica (Gmelin, 1791) in Virginia. J. Shellfish Res. 1989;8:219–225. [Google Scholar]
- Magalhaes H. An ecological study of snails of the genus Busycon at Beaufort, North Carolina. Ecol. Monogr. 1948;18:377–409. [Google Scholar]
- McManus I.C. Harvard University Press; Cambridge, MA: 2002. Right hand, left hand: the origins of asymmetry in brains, bodies, atoms and cultures. [Google Scholar]
- Ng P.K.L, Tan L.W.H. ‘Right handedness’ in heterochelous calappoid and xanthoid crabs—suggestion for a functional advantage. Crustaceana. 1985;49:98–100. [Google Scholar]
- Portell R.W, Agnew J.G. Pliocene and Pleistocene decapod crustaceans. Florida Fossil Invert. 2004;4:1–29. [Google Scholar]
- Shigemiya Y. Does the handedness of the pebble crab Eriphia smithii influence its attack success on two dextral snail species? J. Zool. 2003;260:259–265. doi:10.1017/S095283690300373X [Google Scholar]
- Shoup J.B. Shell opening by crabs of the genus Calappa. Science. 1968;160:887–888. doi: 10.1126/science.160.3830.887. [DOI] [PubMed] [Google Scholar]
- Thomas M.L.H, Himmelman J.H. Influence of predation on shell morphology of Buccinum undatum L. on Atlantic coast of Canada. J. Exp. Mar. Biol. Ecol. 1988;115:221–236. doi:10.1016/0022-0981(88)90156-6 [Google Scholar]
- Thompson D.W. The Macmillan Company; New York, NY: 1943. On growth and form. [Google Scholar]
- Ueshima R, Asami T. Single-gene speciation by left–right reversal—a land-snail species of polyphyletic origin results from chirality constraints on mating. Nature. 2003;425:679. doi: 10.1038/425679a. doi:10.1038/425679a [DOI] [PubMed] [Google Scholar]
- Vermeij G.J. Evolution and distribution of left-handed and planispiral coiling in snails. Nature. 1975;254:419–420. doi:10.1038/254419a0 [Google Scholar]
- Vermeij G.J. Unsuccessful predation and evolution. Am. Nat. 1982;120:701–720. doi:10.1086/284025 [Google Scholar]
- Vermeij G.J. The geography of evolutionary opportunity: hypothesis and two cases in gastropods. Integr. Comp. Biol. 2002;42:935–940. doi: 10.1093/icb/42.5.935. [DOI] [PubMed] [Google Scholar]
- Vermeij G.J. Princeton University Press; Princeton, NJ: 2004. Nature: an economic history. [Google Scholar]
- Zipser E, Vermeij G.J. Survival after nonlethal shell damage in the gastropod Conus sponsalis. Micronesica. 1980;16:229–234. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.