Abstract
Coelacanths are well-known sarcopterygian (lobe-finned) fishes, which together with lungfishes are the closest extant relatives of land vertebrates (tetrapods). Coelacanths have both living representatives and a rich fossil record, but lack fossils older than the late Middle Devonian (385–390 Myr ago), conflicting with current phylogenies implying coelacanths diverged from other sarcopterygians in the earliest Devonian (410–415 Myr ago). Here, we report the discovery of a new coelacanth from the Early Devonian of Australia (407–409 Myr ago), which fills in the approximately 20 Myr ‘ghost range’ between previous coelacanth records and the predicted origin of the group. This taxon is based on a single lower jaw bone, the dentary, which is deep and short in form and possesses a dentary sensory pore, otherwise seen in Carboniferous and younger taxa.
Keywords: Actinistia, Sarcopterygii, Early Devonian, ghost range
1. Introduction
Morphological and molecular sequence data clearly demonstrate that the living coelacanth Latimeria (Actinistia) is the closest relative of lungfishes and tetrapods (Cloutier & Ahlberg 1997; Brinkmann et al. 2004). However, the earliest known lungfishes and their relatives (Dipnomorpha) are Early Devonian in age (Lochkovian–Pragian, ca 410–415 Myr ago), as are the onychodonts, another fossil group of basal sarcopterygians (Chang 1982; Chang & Yu 1984; Zhu et al. 2001; Zhu & Yu 2004). Previously, the earliest known coelacanths, such as Miguashaia, Euporosteus and Gavinia (Jaekel 1927; Schultze 1973; Cloutier 1996; Long 1999; Forey et al. 2001), were Middle–Late Devonian (i.e. 375–390 Myr ago) in age, such that coelacanths had an unrecorded ‘ghost range’ of at least 20 Myr. The recent description of Holopterygius nudus as an aberrant late Middle Devonian coelacanth with eel-like morphology pin-points the beginning of coelcanths radiating into varying niches by this stage (Friedman & Coates 2005). The description below of a new coelacanth dentary from the Early Devonian of Australia (mid–Late Pragian, ca 407–409 Myr ago; Gradstein et al. 2005), reduces this ghost range considerably and brings the coelacanth fossil record closer to the estimated timing of divergence of the Sarcopterygii. This new dentary possesses a dentary sensory pore, which is otherwise a derived character among coelacanths (Forey 1998). Either a dentary sensory pore arose twice during coelacanth evolution (supported by the stratigraphic position of this material) or this new early coelacanth belongs to a more crownward taxon within the group, suggesting that the origins of the actinistian clade may be even older. The third possibility is that the dentary sensory pore is a primitive character, with the absence of the pore a derived character among Devonian taxa. However, following the most recent coelacanth phylogeny (Forey 1998), the presence of a dentary sensory pore would need to be re-acquired in Carboniferous and later taxa (three evolutionary steps versus two).
2. Material and methods
Specimens were recovered by acetic acid preparation of large rock samples and manual picking through the resulting residues. Scanning electron microscopy utilized a LEO 435 VP, with drawings made directly from these images.
3. Results
(a) Taxonomy
We classified our new coelacanth as follows: Osteichthyes Huxley, 1880; Sarcopterygii Romer, 1955; Actinistia Cope, 1871; Eoactinistia gen. nov.
Type species. Eoactinistia foreyi sp. nov., mid–Late Pragian, Fairy Formation, located near the town of Buchan, eastern Victoria, Australia (figure 1).
Figure 1.
Stratigraphic position of the Fairy Formation and location of fossil locality in eastern Victoria, Australia.
Holotype. NMV (Museum Victoria, Melbourne) 218301, left dentary.
Diagnosis. Differs from other actinistians in having a rectangular dentary approximately only twice as long as deep, with distinct groove along the posterior margin, forming complex interlocking overlap with angular. Also, combination of characters in the lower jaw: dentary short, teeth fused to dentary, dentary pore present.
Etymology. Eoactinistia, ‘dawn coelacanth’, foreyi, for Peter Forey (Natural History Museum, London), for his contributions to coelacanth studies.
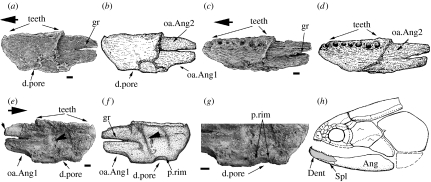
Description. The dentary of E. foreyi is complete, with unbroken margins anteriorly and posteriorly, except perhaps for the posterodorsal corner (figure 2e, small arrow). The dentary is short, as is characteristic for the Actinistia, being slightly more than twice as long as deep, giving the dentary a ‘squat’ appearance overall. By comparison, the dentary of other Devonian taxa such as Gavinia, Holopterygius and Miguashaia is narrower and more slender in appearance (Long 1999; Forey et al. 2001; Friedman & Coates 2005; figure 2h). Although it would be preferable to determine the length of the dentary by comparison with other associated bones of the jaw, the undamaged margins and relative proportions of the dentary of Eoactinistia suggest a short, rectangular element. Among other sarcopterygian fishes, the dentary is longer, comprising much of the length of the lower jaw, although a shorter dentary also characterizes the Dipnoi (lungfish). However, in lungfish, when teeth occur on the dentary, they often originate and are patterned in a manner similar to those on the prearticular. For example, in lungfish with a tooth-plated dentition, radiating rows of teeth are found on both prearticular and dentary (e.g. Chang & Yu 1984; Reisz & Smith 2001). In taxa such as Orlovichthys limnatus and Holodipterus longi, two single rows of teeth are present on the dentary, but are positioned at a 90° angle to one another (Pridmore et al. 1994; Krupina et al. 2001). Both these morphologies are very different from dentary teeth in other sarcopterygians, including coelacanths, which supports the assignment of Eoactinistia to this group.
Figure 2.
Eoactinistia foreyi, NMV 218301, dentary in (a, b) lateral, (c, d) dorsal and (e, f) medial or internal views, (g) close up view of dentary pore, internal dentary surface, (h) Gavinia syntrips, representing a phylogenetically basal coelacanth (Middle Devonian, Australia), to illustrate the relationship between the dentary (grey) and other bones of the jaw. Note absence of dentary pore (from Long 1999). Scale, 200 μm; Ang, angular bone; d.pore, dentary pore; Dent, dentary bone; gr, narrow groove; oa.Ang1, 2, overlap surfaces for angular; p.rim, rim surrounding dentary pore; Spl, splenial bone; teeth, teeth associated with dentary in marginal tooth row. Larger arrows indicate anterior. ((h) Courtesy of Western Australian museum.)
The dentary of Eoactinistia is divided posteriorly by a narrow groove (figure 2a–f, gr), comparable to the Carboniferous taxon Polyosteorhynchus (Lund & Lund 1985). The ornamentation comprises small, irregularly distributed ridges with small pores in the furrows. Internally the bone is smooth, with a low ridge running dorsoventrally, beginning near the posterior margin of the dentary pore (described below), and ending and defining, the end of the tooth row (figure 2e,f, arrowhead). Up to seven teeth form this tooth row, which are represented by rounded sockets and form a typical sarcopterygian row along the dentary, as in both species of Miguashaia, Gavinia and other basal coelacanth taxa such as Diplocercides (Forey 1998; figure 2a,c–e, teeth), rather than having teeth on isolated plates, the latter being the derived character state (Forey 1998).
In external view, the ventral margin is marked by a shallow indentation (figure 2a,b), matched in internal view by a narrow, scalloped area, distinct from the internal surface of the bone. This scalloped area forms a distinct rim around the indentation (figure 2f,g, p.rim). The combination of these features suggests the presence of a true opening along the dentary margin, which we suggest represents the dentary sensory pore, separating the dentary from the (unpreserved) splenial (figure 2a,b,e–g, d.pore). A dentary sensory pore is also absent in lungfish. As noted by Forey (1998), the dentary pore is not homologous to the infradentary foramina characterizing the lower jaws of several members of the Dipnomorpha, as this foramen separates the infradentary bones themselves (e.g. splenial, angular, surangular), whereas in coelacanths, the dentary pore separates the dentary from the splenial. Forey (1998) suggested that the dentary pore was derived within the coelacanths, also supporting identification of Eoactinistia as a coelacanth.
Posteriorly, the ventral margin of the dentary is strongly inflected, presenting a smooth surface and forming a curved contact with the angular bone (figure 2b,e,f, oa.Ang1).
Dorsal to this contact with the angular, the narrow groove separates the posterior region of the dentary into dorsal and ventral halves. The dorsal half is slightly depressed relative to the rest of the bone, representing a second overlap area for the angular (figure 2b,d, oa.Ang2). The end of the tooth row marks the beginning of this overlap surface (figure 2a, the end of the tooth row is continued ventrally by some type of overgrowth). Thus, we suggest the angular has a complex interlocking relationship with the dentary, possibly to increase strength of the bite.
(b) Geological age and stratigraphic distribution
The Fairy Formation (Warren & Talent 1967) has produced a diverse fauna, including plant, invertebrate and vertebrate remains, from a lens 33 m thick. The lens outcrops beneath 310–460 m of ignimbrites high in the Snowy River Volcanics. This unit is underlain with angular unconformity by the Cowombat Formation, the uppermost horizons of which are latest Silurian (Přidolí; Simpson & Talent 1995). Conodonts from the stratigraphically lowest unit of the overlying Buchan Group, the Buchan Caves Limestone (Talent 1956; Mawson et al. 1992), show that the Pragian–Emsian (pireneae Zone-dehiscens Zone) boundary falls approximately halfway up the Buchan Caves Limestone, implying a pre-Emsian age for E. foreyi and associated biota. The youngest age that could be allocated to the Fairy Formation is therefore mid–Late Pragian pireneae Zone. The thickness of Snowy River Volcanics accumulated prior to the Fairy Formation (thousands of metres versus 310–460 m; Orth et al. 1995) rules against the Fairy Formation being much older than mid–Late Pragian (ca 407–409 Myr ago).
4. Discussion
The Late Silurian–Early Devonian (ca 416–418 Myr ago; Gradstein et al. 2005) marks the origin of nearly all the known major groups within the osteichthyans (i.e. bony fishes, including ray-finned fishes, or actinopterygians, and sarcopterygians; Janvier 1996). Within sarcopterygians, taxa assigned to the crown-group Sarcopterygii, including groups such as the onychodonts (Zhu et al. 2001; Zhu & Yu 2004) and the Dipnomorpha (Chang 1982; Chang & Yu 1984) first appear, as well as phylogenetically more basal stem-group sarcopterygian taxa such as Psarolepis and Achoania (Zhu & Schultze 1997; Yu 1998; Zhu et al. 2001; Zhu & Yu 2002). Coelacanths (also part of the crown-group Sarcopterygii) such as Miguashaia, Holopterygius, Euporosteus, Gavinia and Diplocercides are all Middle (Givetian) to Late Devonian (Frasnian) in age. The Early Devonian (mid–Late Pragian) age of E. foreyi provides the first record of coelacanths prior to the Middle Devonian, and fills a gap of at least 20 Myr between these previously oldest known coelacanths and the nearest sarcopterygian relatives of the group. Interestingly, the dentary pore is absent from all other Devonian taxa, being shared by Eoactinistia and some more crownward coelacanths (fig. 9.7, node 6 in Forey 1998) of Carboniferous and younger age. Lack of material precludes a phylogenetic analysis of the Actinistia including Eoactinistia, though the resolution of Eoactinistia as a more crownward taxon would create a new ghost range within the group, extending prior to the Early Devonian and even closer to the origin of the Osteichthyes as a whole. Alternatively, a dentary pore evolved twice in the Actinistia. Either way, Eoactinistia presents new challenges to the interpretation of early coelacanth evolution.
Acknowledgments
Acid-leaching of limestone nodules was undertaken by K. Simpson, I. R. Stewart and M. J. Engelbretsen. A. H. M. VandenBerg provided maps and data of the Snowy River Volcanics. Z.J. thanks the Muséum National d'Histoire Naturelle for a visiting fellowship in 2004, where part of this work was completed.
References
- Brinkmann H, Venkatesh B, Brenner S, Meyer A. Nuclear protein-coding genes support lungfish and not the coelacanth as the closest living relatives of land vertebrates. Proc. Natl Acad. Sci. USA. 2004;101:4900–4905. doi: 10.1073/pnas.0400609101. doi:10.1073/pnas.0400609101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, M.-M. 1982 The braincase of Youngolepis, a Lower Devonian crossopterygian from Yunnan, south-eastern China. Ph.D. thesis, Stockholm: University of Uppsala.
- Chang M.-M, Yu X.-B. Structure and phylogenetic significance of Diabolichthys speratus gen. et sp. nov., a new dipnoan-like form from the Lower Devonian of eastern Yunnan, China. Proc. Linn. Soc. NSW. 1984;107:171–184. [Google Scholar]
- Cloutier R. The primitive actinistian Miguashaia bureaui Schultze (Sarcopterygii) In: Schultze H.-P, Cloutier R, editors. Devonian fishes and plants of Miguasha, Quebec, Canada. Dr. Friedrich Pfiel; München: 1996. pp. 227–247. [Google Scholar]
- Cloutier R, Ahlberg P.E. Morphology, characters, and the interrelationships of basal sarcopterygians. In: Stiassny L.J, Parenti L.R, Johnson G.D, editors. Interrelationship of fishes. Academic Press; San Diego, CA: 1997. pp. 445–479. [Google Scholar]
- Forey P.L. Chapman & Hall; London: 1998. History of coelacanth fishes. [Google Scholar]
- Forey P.L, Ahlberg P.E, Luksevics E, Zupins I. A new coelacanth from the Middle Devonian of Latvia. J. Vert. Paleo. 2001;20:243–252. [Google Scholar]
- Friedman M, Coates M.I. A newly recognised fossil coelacanth highlights the early morphological diversification of the clade. Proc. R. Soc. B. 2005;273:245–250. doi: 10.1098/rspb.2005.3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradstein F.M, Ogg J, Smith A. Cambridge University Press; Cambridge, UK: 2005. A geologic time scale 2004. [Google Scholar]
- Jaekel O. Der Kopf der Wirbeltiere. Ergeb. Anat. Entwicklung. 1927;27:815–827. [Google Scholar]
- Janvier P. Oxford Science Publications; Oxford: 1996. Early vertebrates. [Google Scholar]
- Krupina N.I, Reisz R.R, Scott D. The skull and tooth system of Orlovichthys limnatis, a Late Devonian dipnoan from Russia. Can. J. Earth Sci. 2001;38:1301–1311. doi:10.1139/cjes-38-9-1301 [Google Scholar]
- Long J.A. A new genus of fossil coelacanth (Osteichthyes: Coelacanthiformes) from the Middle Devonian of southeastern Australia. Rec. West. Aust. Mus. 1999;(Suppl. 57):37–54. [Google Scholar]
- Lund R.L, Lund W.L. Coelacanths from Bear Gulch limestone (Namurian) of Montana and the evolution of Coelacanthiformes. Bull. Carnegie Mus. Nat. Hist. 1985;25:1–74. [Google Scholar]
- Mawson R, Talent J.A, Brock G.A, Engelbretsen M.J. Conodont data in relation to sequences about the Pragian–Emsian boundary (Early Devonian) in southeastern Australia. Proc. R. Soc. Victoria. 1992;104:23–56. [Google Scholar]
- Orth K, VandenBerg A.H.M, Nott R.J, Simons B.A. Murrindal 1 : 100,000 map geological report. Geol. Surv. Victoria Rep. 1995;100:1–235. [Google Scholar]
- Pridmore P.A, Campbell K.S.W, Barwick R.E. Morphology and phylogenetic position of the holodipteran dipnoans of the Upper Devonian Gogo Formation of northwestern Australia. Phil. Trans. R. Soc. B. 1994;344:105–164. [Google Scholar]
- Reisz R.R, Smith M.M. Lungfish dental pattern conserved for 360 Myr. Nature. 2001;411:548. doi: 10.1038/35079187. doi:10.1038/35079187 [DOI] [PubMed] [Google Scholar]
- Schultze H.-P. Crossopterygier mit heterozerker Schwanzflose aus dem Oberdevon Kanadas, Nebst einer Beschreibung von Onychodontida-Resten aus dem Mittledevon Spaniens und dem Karbon der USA. Palaeontographica. 1973;143A:188–208. [Google Scholar]
- Simpson A.J, Talent J.A. Silurian conodonts from the headwaters of the Indi (upper Murray) and Buchan rivers, southeastern Australia, and their implications. Cour. Forsch-Inst. Senckenberg. 1995;182:79–215. [Google Scholar]
- Talent J.A. Devonian brachiopods and pelycopods from the Buchan Caves limestone, Victoria. Proc. R. Soc. Victoria. 1956;68:1–56. [Google Scholar]
- Warren J.W, Talent J.A. Devonian fish from Buchan, Victoria. Aust. NZ Assoc. Adv. Sci., Abstr., Sect., C. 1967:K1. [Google Scholar]
- Yu X.-B. A new porolepiform-like fish, Psarolepis romeri, gen. et sp. nov. (Sarcopterygii, Osteichthyes) from the Lower Devonian of Yunnan, China. J. Vert. Paleo. 1998;18:261–274. [Google Scholar]
- Zhu M, Schultze H.-P. The oldest sarcopterygian fish. Lethaia. 1997;30:293–304. [Google Scholar]
- Zhu M, Yu X.-B. A primitive fish close to the common ancestor of tetrapods and lungfish. Nature. 2002;418:767–770. doi: 10.1038/nature00871. doi:10.1038/nature00871 [DOI] [PubMed] [Google Scholar]
- Zhu M, Yu X.-B. Lower jaw character transitions among major sarcopterygian groups—a survey based on new material from Yunnan, China. In: Arratia G, Wilson M.V.H, Cloutier R, editors. Recent advances in the origin and early radiation of vertebrates. Dr. Friedrich Pfeil; München: 2004. pp. 271–286. [Google Scholar]
- Zhu M, Yu X.-B, Ahlberg P.E. A primitive sarcopterygian fish with an eyestalk. Nature. 2001;410:81–84. doi: 10.1038/35065078. doi:10.1038/35065078 [DOI] [PubMed] [Google Scholar]