Abstract
Selective brain cooling (SBC) is defined as the lowering of brain temperature below arterial blood temperature. Artiodactyls employ a carotid rete, an anatomical heat exchanger, to cool arterial blood shortly before it enters the brain. The survival advantage of this anatomy traditionally is believed to be a protection of brain tissue from heat injury, especially during exercise. Perissodactyls such as horses do not possess a carotid rete, and it has been proposed that their guttural pouches serve the heat-exchange function of the carotid rete by cooling the blood that traverses them, thus protecting the brain from heat injury. We have tested this proposal by measuring brain and carotid artery temperature simultaneously in free-living horses. We found that despite evidence of cranial cooling, brain temperature increased by about 2.5 °C during exercise, and consistently exceeded carotid temperature by 0.2–0.5 °C. We conclude that cerebral blood flow removes heat from the brain by convection, but since SBC does not occur in horses, the guttural pouches are not surrogate carotid retes.
Keywords: guttural pouches, selective brain cooling, horses
1. Introduction
Guttural pouches are enigmatic, air-filled, mucus-secreting diverticula of the Eustachian tubes. In domestic horses, their volume varies but typically is about 300–500 ml. The pouch wall is traversed by the internal carotid artery. The pouches are of veterinary importance in domestic horses (Equus caballus) because fungal infections can result in erosion of the carotid artery and precipitate fatal haemorrhages (Newton et al. 1997; Lepage et al. 2004). The physiological function, if any, of guttural pouches is unknown, but recently it has been proposed that they ‘…might function during selective brain-cooling (SBC) to maintain blood carried by the internal carotid artery (ICA) at a temperature below core temperature during hyperthermia…’ (Baptiste et al. 2000).
Heating the brain, or any other tissue, will ultimately have a direct destructive effect on the cells. Although evidence exists to the contrary, brain tissue is often regarded as being more vulnerable to the effects of high temperature than is other tissue. Thus, the prevention of heat injury to brain cells is the usual explanation given for the survival advantage of SBC. SBC is defined as ‘the lowering of brain temperature, either locally or as a whole, below arterial blood temperature’ (IUPS Thermal Commission 2001). SBC is achieved best in artiodactyls, which have a carotid rete. The carotid rete functions as a heat exchanger and cools carotid arterial blood shortly before it enters the brain. Horses do not have a carotid rete, develop high body temperatures during exercise and yet show no noticeable clinical or neurological consequences.
One reason for this lack of effect is that brain tissue is no more vulnerable to heat damage than are other tissues (Mitchell et al. 2002). However, it also has been argued that horses prevent thermal damage during exercise by employing SBC (McConaghy et al. 1995; Baptiste 1998; Baptiste et al. 2000). If so, and in the absence of a carotid rete, SBC must occur through other means. McConaghy et al. (1995) presented data showing brain temperature transiently cooler than blood temperature at the onset of exercise when body temperatures were rising rapidly, which may have been a consequence of thermal inertia: differences in temperature at different body sites appear for passive physical rather than physiological reasons. Baptiste et al. (2000) did not measure brain temperature in their horses, so their conclusion was speculative.
We report an attempt to resolve the uncertainty, by simultaneous measurement of brain and carotid artery blood temperature during exercise and at rest in free-living domestic horses.
2. Methods
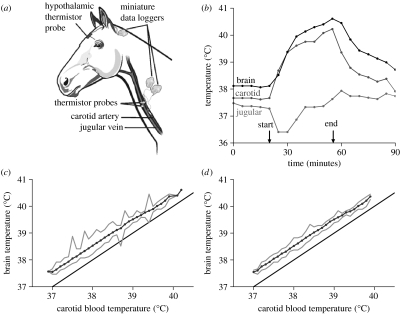
Three horses were anaesthetised with 1–2% halothane (Fluothane, Zeneca) in oxygen, administered via a face mask. Using aseptic techniques, we implanted miniature data logger/thermistor assemblies subcutaneously in the neck. A thermistor sealed in a blind-ended and thin-walled polytetrafluoroethylene (PTFE) tube (outer diameter (o.d.) 0.9 mm) was inserted 100 mm into the common carotid artery towards the heart to record the temperature of arterial blood before it reached the guttural pouches, and another was inserted 100 mm into the jugular vein towards the head to record the temperature of venous blood returning from the head. After placement, the thermistors were situated about midway along the length of the neck (figure 1a). A third thermistor in a cellulose acetate guide tube (o.d. 3.2 mm) was pushed through a small hole drilled in the skull, to a depth of 50 mm to lie near the hypothalamus (figure 1a).
Figure 1.
(a) Sites on horses at which temperatures were measured with implanted miniature thermometric loggers. (b) Blood and brain temperatures recorded at 5 min intervals in a free-living horse, at 32 °C globe temperature. Except for a short transient at the beginning of exercise, brain temperature exceeded carotid blood temperature before, during and after exercise. The difference between jugular venous blood temperature and carotid arterial blood temperature widened during exercise. (c), (d) Plots of mean, maximum and minimum hypothalamic temperature recorded at each 0.1 °C class of carotid blood temperature, and the line of identity, showing all data collected in horses at rest (lower end) and exercise (higher end), over 11 days ((c) 3168 data points and (d) excluding the 315 data points for which carotid blood temperature changed by 0.1 °C or more in the preceding 5 min). Except during the occasional transients, hypothalamic temperature always exceeded carotid blood temperature.
The miniature loggers (50×45×20 mm, Stowaway XT1, Onset Computer Corporation, Pocasset, MA, USA) were covered in inert wax (Elvax, Mini Mitter, Sunriver, OR, USA) and weighed 40 g. The loggers had a storage capacity of 32 kb and a resolution of 0.04 °C. Scan interval was 5 min. The thermistors were ruggedized glass-coated bead thermistors with insulated extension leads (bead diameter 0.3 mm; ABOE3-BR11KA103 N, Thermometrics, Edison, NJ, USA). The loggers and their thermistors were calibrated against a high-accuracy quartz thermometer (Quat 100, Heraeus, Hanau, Germany) in an insulated water bath, and proved to have an accuracy of one sampling step of the logger (0.04 °C).
After surgery, the animals were released into a 172 ha enclosure at the Lichtenburg Game Breeding Centre (26°07′ S 26°10′ E) 220 km west of Johannesburg, South Africa. They had permanent access to grass and water. After a three week period to allow for recovery from surgery, over a 12 day study period, the horses were chased to produce half hour long episodes of running at an average speed of 18 km h−1. Integrated data on air temperature, solar radiation and wind speed were obtained from a 150 mm diameter black globe using a weather station (MCS, Cape Town, South Africa) with instruments at a height of 1 m above ground level. At the end of the study period, the horses were recaptured, the instruments removed and the animals returned to the breeding herd.
3. Results
Mean black globe temperature over the study period was 21.2±1.7 °C, with a 24 h maximum of 40.7±2.2 °C and a minimum of 9.9±2.6 °C. Mean peak solar radiation was approximately 800 W m−2 at solar noon, with maxima exceeding 1000 W m−2.
In all three horses, the data collected in the study period showed that mean brain temperature exceeded mean carotid artery temperature by 0.2–0.5 °C (figure 1c) at all times, except for brief periods soon after the start of exercise when temperatures were changing rapidly (figure 1b). When the data points relating to rapid transients were removed, brain temperature was higher than carotid artery temperature by 0.5 °C over the full range of temperatures exhibited by the horses (figure 1d). During the sustained hyperthermia of exercise, brain temperature was higher than blood temperature by the same amount. During the cool-down phase after exercise, brain temperature also exceeded carotid artery temperature. Substantial superficial cranial cooling occurred during exercise, presumably by skin and nasal mucosa evaporation, as evidenced by the increased gap between jugular vein and carotid artery temperature (figure 1b).
4. Discussion
The heat generated by brain tissue metabolism is removed by cerebral blood flow, in all animals, by convection. In our horses, this mechanism is evident as the constant temperature gradient between brain and arterial blood temperatures. In artiodactyls, an additional mechanism of enhancing convective heat loss exists, namely cooling of arterial blood destined for the brain by the carotid rete. Cooled cerebral arterial blood establishes a larger temperature gradient between brain and blood, and heat can be removed at a rate faster than it is being produced. As a result, brain temperature can become lower than the temperature of arterial (carotid) blood that supplies it. Although horses do not have a carotid rete, Baptiste et al. (2000) and McConaghy et al. (1995) have concluded that another mechanism exists to produce SBC, and Baptiste et al. (2000) have implicated the guttural pouches as the source of cooling.
Our data do not support those conclusions. However they do support those from an earlier study (Fuller et al. 2000) on free-living zebras (Equus burchelli), in which zebras were exposed to environmental temperatures similar to those that the horses experienced, and to exercise. Their brain temperature, like that of the domestic horses, was found to be 0.2–0.4 °C above carotid artery temperature, and during exercise their brain temperature reached the same maximum temperature of 40.5 °C found in the domestic horses. In other words, in neither free-living domestic horses nor zebras, have we been able to find any evidence of SBC during hyperthermia, although we did find that during rapid body heating, brain temperature transiently fell below blood temperature; this phenomenon was also observed by McConaghy et al. (1995). In both species, brain temperature was measured near the hypothalamus, and increases in body temperature did not produce an immediate increase in hypothalamic temperature. This delay does not constitute SBC. In the domestic horses, it was clear from the fall in jugular blood temperature that substantial cranial cooling occurred during exercise, but this cooling did not prevent brain temperature from increasing. This situation is the same as that in humans, who, like horses, do not have a carotid rete. In exercising humans, cranial cooling does not prevent brain temperature from increasing, and brain temperature consistently exceeds that of arterial blood (Nybo et al. 2002).
Baptiste et al. (2000) argued that as a result of ventilation of the guttural pouches, the temperature of the pouches is lowered, and that the passage of arterial blood over them cools the blood destined for the brain, leading to SBC. They came to this conclusion without measuring brain temperature, although an analysis of the potential cooling effect of guttural pouches shows that their proposal is unlikely. At least 230 litres of air, at unattainably high ventilation rates, must enter the pouches each minute (Maloney et al. 2002), and even then, carotid blood would be in contact with the guttural pouches for only a few seconds, an insufficient time to lower blood temperature. Also, if cooling of blood destined for the brain by the guttural pouches were crucial to survival, then obliteration of the branch of the carotid arteries that crosses them would have clear consequences. However, occlusion of the arteries, the preferred treatment for preventing fatal haemorrhages following pouch infections, has no known neurological sequelae (Lepage et al. 2004).
The speed at which our horses ran was on average 18 km h−1, slightly higher than the average achieved by horses used to deliver mail via the Overland Pony Express, but well within the Express's calculated range of speeds (16 km h−1; range 10–22 km h−1; Minetti 2003). The speed of the Pony Express horses was chosen so as not to exhaust the horses; therefore our horses were not subjected to maximum exercise. However, it is not the speed of running but the brain temperature reached that triggers SBC (Kuhnen & Jessen 1994). Both Baptiste et al. (2000) and McConaghy et al. (1995) reported the onset of apparent SBC at body temperatures of near 38 °C, well below the 40.5 °C that our horses reached. So our horses certainly reached high enough body temperatures to exhibit SBC, if indeed it does occur in horses.
We conclude that SBC does not occur during exercise or at rest in domestic horses, and probably not in any equid. Horses in their natural state seem to respond to increasing brain temperatures and prevent any consequences for the brain and other tissues by initiating sweating and ceasing exercise if they can. Attractive as the idea was, our data do not support the proposal that guttural pouches produce SBC.
Acknowledgments
Experiments were approved by the Animals Ethics Screening Committee of the University of the Witwatersrand (clearance number 2002/27/5). We thank the South African National Zoological Gardens for access to their Lichtenburg Game Breeding Centre, Mr. Andre Matthee for his management of the horses and help with procedures, and the South African National Research Foundation for financial support.
References
- Baptiste K.E. A preliminary study on the role of the equine guttural pouches in selective brain cooling. Vet. J. 1998;155:115–117. doi: 10.1016/s1090-0233(98)80009-9. doi:10.1016/S1090-0233(98)80009-9 [DOI] [PubMed] [Google Scholar]
- Baptiste K.E, Naylor J.M, Bailey J, Barbers E.M, Post K, Thornhill J. A function for guttural pouches in the horse. Nature. 2000;403:382–383. doi: 10.1038/35000284. doi:10.1038/35000284 [DOI] [PubMed] [Google Scholar]
- Fuller A, Maloney S.K, Kamerman P.R, Mitchell G, Mitchell D. Absence of brain cooling in free-ranging zebras in their natural habitat. Exp. Physiol. 2000;85:209–217. doi:10.1017/S0958067000019540 [PubMed] [Google Scholar]
- IUPS Thermal Commission Glossary of terms for thermal physiology: third edition. Jpn. J. Physiol. 2001;51:245–280. [Google Scholar]
- Kuhnen G, Jessen C. Thermal signals in control of selective brain cooling. Am. J. Physiol. 1994;267:R355–R359. doi: 10.1152/ajpregu.1994.267.2.R355. [DOI] [PubMed] [Google Scholar]
- Lepage O.M, Perron M.-F, Cadore J.-L. The mystery of fungal infection in the guttural pouches. Vet. J. 2004;168:60–64. doi: 10.1016/S1090-0233(03)00108-4. doi:10.1016/S1090-0233(03)00108-4 [DOI] [PubMed] [Google Scholar]
- Maloney S.K, Fuller A, Mitchell G, Mitchell D. On the guttural pouch and selective brain cooling in equids. S. Afr. J. Sci. 2002;98:189–191. [Google Scholar]
- McConaghy F.F, Hales J.R.S, Rose R.J, Hodgson D.R. Selective brain cooling in the horse during exercise and environmental heat stress. J. Appl. Physiol. 1995;79:1849–1854. doi: 10.1152/jappl.1995.79.6.1849. [DOI] [PubMed] [Google Scholar]
- Minetti A.E. Efficiency of equine express postal systems. Nature. 2003;426:785–786. doi: 10.1038/426785a. doi:10.1038/426785a [DOI] [PubMed] [Google Scholar]
- Mitchell D, Maloney S.K, Jessen C, Laburn H.P, Kamerman P.R, Mitchell G, Fuller A. Adaptive heterothermy and selective brain cooling in arid-zone mammals. Comp. Biochem. Physiol. B. 2002;131:571–585. doi: 10.1016/s1096-4959(02)00012-x. doi:10.1016/S1096-4959(02)00012-X [DOI] [PubMed] [Google Scholar]
- Newton J.R, Wood J.L.N, Dunn K.A, De Brauwere M.N, Chanter N. Naturally occurring and persistent infection of the guttural pouches of horses with Streptococcus equi. Vet. Rec. 1997;140:84–90. doi: 10.1136/vr.140.4.84. [DOI] [PubMed] [Google Scholar]
- Nybo L, Secher N.H, Nielsen B. Inadequate heat release from the human brain during prolonged exercise with hyperthermia. J. Physiol. 2002;545:697–704. doi: 10.1113/jphysiol.2002.030023. doi:10.1113/jphysiol.2002.030023 [DOI] [PMC free article] [PubMed] [Google Scholar]