Abstract
Ligand-gated ion channels are a target for inhaled anesthetics and alcohols in the central nervous system. The inhibitory strychnine-sensitive glycine and γ-aminobutyric acid type A receptors are positively modulated by anesthetics and alcohols, and site-directed mutagenesis techniques have identified amino acid residues important for the action of volatile anesthetics and alcohols in these receptors. A key question is whether these amino acids are part of an alcohol/anesthetic-binding site. In the present study, we used an alkanethiol anesthetic to covalently label its binding site by mutating selected amino acids to cysteine. We demonstrated that the anesthetic propanethiol, or alternatively, propyl methanethiosulfonate, covalently binds to cysteine residues introduced into a specific second transmembrane site in glycine receptor and γ-aminobutyric acid type A receptor subunits and irreversibly enhances receptor function. Moreover, upon permanent occupation of the site by propyl disulfide, the usual ability of octanol, enflurane, and isoflurane to potentiate the function of the ion channels was lost. This approach provides strong evidence that the actions of anesthetics in these receptors are due to binding at a single site.
Despite their wide use, the mechanism of action of alcohols and general anesthetics remains controversial. In contrast to most other classes of drugs, which are either assumed or known to act on specific protein receptors, anesthetic action is often attributed to multiple nonspecific sites (1). Based on the relationship between the potencies of anesthetics and their lipid solubilities described by Meyer (2) and Overton (3), the lipid bilayer of neuronal membrane was long considered the primary target for general anesthesia. Even recent studies invoke this hypothesis (4), although other studies suggest proteins as the site of action of inhaled anesthetics and n-alcohols (5–7).
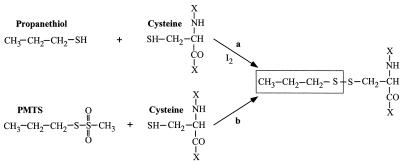
A traditional approach to distinguish between these two alternatives would apply radioligand-binding assays with alcohols and inhalational anesthetics. However, the low affinities and rapid kinetics of these compounds makes such studies unfeasible (8). In the present study, we used a new approach, using anesthetic alcohol analogs that form covalent bonds at their site of action, to show that binding at a single site produced irreversible anesthetic-like effects. This goal was accomplished with propanethiol and with propyl methanethiosulfonate (PMTS); both compounds can form propyl disulfide bonds with cysteine residues introduced at specific sites in brain proteins (Fig. 1).
Figure 1.
Reaction of propanethiol and PMTS with the sulfhydryl group of cysteine. Note that the side chain structure attached to the cysteine residue after the reaction is identical for both reagents.
Our candidate targets for the actions of the general anesthetics and n-alcohols were the main inhibitory receptors in spinal cord and brain: the strychnine-sensitive glycine receptor (GlyR) and the γ-aminobutyric acid type A (GABAA) receptor. Clinically relevant concentrations of volatile anesthetics and n-alcohols potentiate the action of glycine and GABA on these receptors (7, 9, 10, 11–14). Site-directed mutagenesis techniques defined regions in the second and the third transmembrane (TM2 and TM3) segments that mediate these effects (7). Serine-267 (S267) and alanine-288 (A288), in TM2 and TM3 of the α1 GlyR subunit, and the homologous residues at the equivalent positions in the α subunit of the GABAA receptor (S270 and A291), are essential for the modulation of these receptors by inhaled anesthetics and n-alcohols. Specific mutations of these residues can abolish or markedly reduce the effect of anesthetics or ethanol on these receptors without necessarily affecting receptor function (7) suggesting that alcohols and anesthetics may bind in a cavity located between TM2 and TM3 (15). Alternatively, anesthetics may bind elsewhere on the Gly/GABAA receptors, but S267 and/or A288 provide a transduction or gating site required for their action. To test the hypothesis that amino acids S267 and A288 in the GlyR (and the equivalent residues in the GABAA receptor) provide the binding site responsible for the action of alcohol and volatile anesthetics, we mutated either S267 or A288 to cysteine and then tested the ability of an unconventional anesthetic, propanethiol, to covalently bind to the thiol group of a cysteine residue introduced in the critical positions. Disulfide cross-linking by propanethiol was accomplished with oxidation by iodine. We reasoned that if either amino acid is the critical anesthetic-binding site, then propanethiol should irreversibly activate the mutant (S267C or A288C) receptors but reversibly activate wild-type receptor. In parallel experiments, we also used PMTS as a sulfhydryl-specific reagent. Alkyl methanethiosulfonates readily form disulfide bonds with cysteines but do not require the use of an oxidizing agent (ref. 16; Fig. 1).
Materials and Methods
Site-directed mutagenesis in GlyR α1 or GABA α2 subunits was performed on cDNA subcloned into pBK-CMV or pCIS2 vectors (Stratagene) by using the Quik-Change site-directed mutagenesis kit. Point mutations were verified by double-stranded DNA sequencing.
Xenopus laevis oocytes were isolated and injected with cDNAs encoding human wild-type or mutant α1 GlyR subunits (1 ng/30 nl), or wild-type or mutant α2β1 GABA receptor subunits combinations in a 1:1 ratio (1.5 ng/30 nl). Measurements were made in oocytes 1–4 days after injection (13). Either glycine (Bio-Rad) or GABA (Research Biochemicals) was dissolved in modified barth solution and applied for 30 s. Oocytes were perfused with a solution of either propanethiol (Aldrich), PMTS (Toronto Research Chemicals, Downsview, ON, Canada), anesthetics or octanol for 60 s to allow equilibration before a 30-s coapplication with either glycine or GABA. Control currents with glycine or GABA alone were determined before and after applications of these compounds. Propanethiol, isoflurane (Ohmeda, Liberty Corner, NJ) or enflurane (Apothecon, Princeton, NJ) were dissolved in modified barth solution. PMTS, octanol (Sigma), or alphaxalone (Glaxo) were first dissolved in dimethyl sulfoxide and then diluted in modified barth solution to a final dimethyl sulfoxide concentration not exceeding 0.05%. Experiments were performed by using glycine or GABA concentrations corresponding to the EC5–10 (25–40 μM for glycine and 0.5–1 μM for GABA in either wild-type or mutated receptors); i.e., they produced peak currents equal to 5–10% of the maximal current (obtained by application of 1 mM GABA or glycine). When iodine (Sigma) was used it was perfused for 60 s at a concentration of 0.5 mM. The excess iodine was washed out for 30 s, and the glycine response was redetermined (it is not possible to co-apply iodine and propanethiol because of formation of dipropane disulfide). Redetermination of the glycine response was necessary because in many oocytes expressing α1(S267C), iodine treatment potentiated the glycine response (no effect of iodine was found in α1 wild-type receptors). Immediately after control responses with glycine were reassessed, oocytes were perfused with 4 mM propanethiol to allow for disulfide bond formation and the response was redetermined by using a 30-s coapplication of glycine and propanethiol (concentration and conditions based on ref. 17). Bath concentrations of propanethiol, enflurane, isoflurane, and octanol were determined by gas chromatography (9, 13). The EC50 values and Hill coefficient for concentration-response curves were calculated by using GraphPad prism software (SanDiego, CA). Statistical analyses by Student's t test were performed to compare means and by two way ANOVA to compare populations.
Results
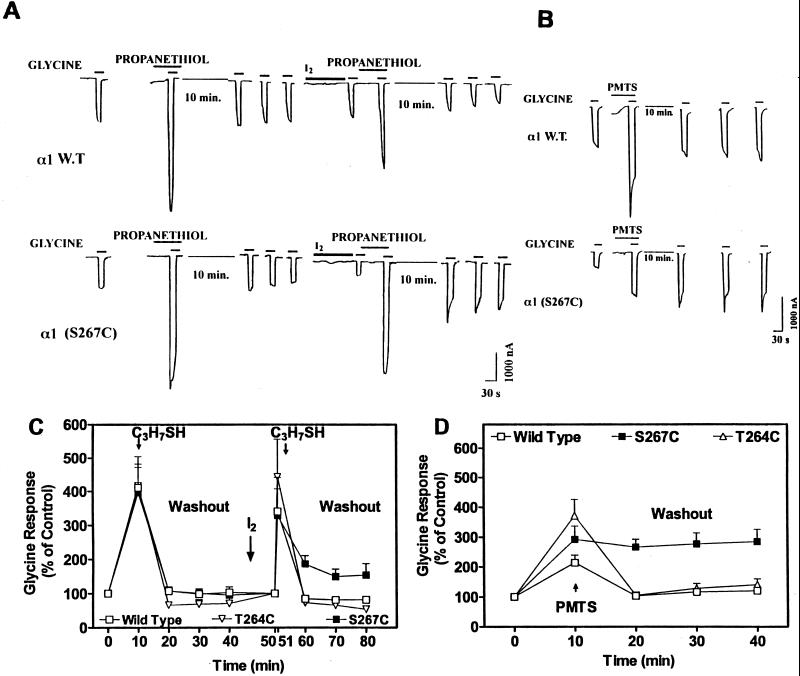
Propanethiol (4 mM) reversibly potentiated glycine action in both α1 wild-type and α1(S267C) mutant receptors (Fig. 2 A and C). Oxidation by iodine, which promotes disulfide bond formation (17), did not affect potentiation of the glycine response by propanethiol for either receptor. However, the removal of propanethiol (washout) reversed potentiation for the wild-type receptor, but only partially reversed in the mutant α1(S267C). We also tested the TM3 mutant α1(A288C) and the TM2 mutant α1(T264C); the latter mutation is one turn of the helix below S267. Propanethiol potentiated the glycine response in all of the mutants tested, but, in contrast to the results obtained with α1(S267C), washout produced complete reversal of potentiation with T264C and A288C (Fig. 2 and data not shown). The action of propanethiol on α1(S267C) receptor was partially reversible, suggesting that the sequential iodine-propanethiol treatment was not able to form covalent bonds at all S267C residues. To increase the rate and extent of disulfide bond formation, we used a sulfhydryl-specific reagent, PMTS that does not require iodine. PMTS (30 μM) produced reversible enhancement of the function of GlyR wild-type or the TM2 mutant α1(T264C) but irreversible enhancement of the mutant α1(S267C) (Fig. 2 B and D). Even after a 30 min washout, there was no evidence of any decrease in the action of PMTS on the mutant GlyR. Finally, PMTS did not affect the TM3 mutant α1(A288C) (data not shown).
Figure 2.
Effects of propanethiol or PMTS on α1 wild-type, α1(S267C), and α1(T264C) GlyR expressed in Xenopus oocytes. (A and B) Sample tracings of currents induced by application of an EC5 concentration of glycine alone or in the presence of propanethiol (4 mM) and iodine (0.5 mM) or PMTS (30 μM) in individual voltage-clamped oocytes. The time course of the washout of propanethiol (either before and after iodine application) or PMTS was measured every 10 min for a total of 30 min. (C) Propanethiol (4 mM) reversibly enhanced the glycine response in α1 wild-type (□), α1 (T264C) (▿), or α1 (S267C) (■) receptors before application with iodine (0.5 mM). After sequential application of iodine and propanethiol, the enhancement of the glycine response by propanethiol is reversible in wild-type and T264C but not in S267C receptors. Values are expressed as percentage of control. Control values determined at 0 time were set at 100% and were used to calculate data for 10–40 min; values determined at 50 min (immediately after application of I2) were set at 100% and used to calculate data for 51–80 min. Two-way ANOVA indicated significant differences between wild-type and S267C receptors in the GlyR responses after the application of iodine and propanethiol (P < 0.05). (D) PMTS (30 μM) enhanced the glycine response in all of the receptors and this was reversed to control level by washout in wild-type (□) and in T264C (▵) GlyR. In S267C receptors (■), the enhancement of the glycine response by PMTS was not reversible. Propanethiol or PMTS were perfused for 60 s before being coapplied with glycine for 30 s. The time course of the washout from propanethiol or PMTS was measured every 10 min for a total of 30 min. Two-way ANOVA indicated significant differences between wild-type and S267C receptors in the glycine responses after the application of PMTS (P < 0.001). For some points, error bars are smaller than symbols. Data are the mean ± SEM of five to nine oocytes.
Anesthetics enhance GlyR function by decreasing the glycine EC50 without changing the maximal effect (18), and we asked if PMTS produced the same action. Responses to glycine assessed in the mutant α1(S267C) were increased after application of PMTS (30 μM) due to a decrease in the glycine EC50 (from 217 ± 56 μM before treatment with PMTS to 108 ± 33 afterward; Hill coefficient was 1.2 ± 0.6 before PMTS treatment and 0.9 ± 0.2 afterward) with no change in the maximum currents induced by saturating (1 mM) concentration of glycine. This result is similar to the action of isoflurane and of propanethiol assessed on wild-type GlyR. Isoflurane (1.2 mM) and propanethiol (16 mM) decreased the EC50 for glycine by 55% and by 39% respectively; the Hill coefficient was 1.6 ± 0.05 before isoflurane treatment and 0.9 ± 0.06 afterward; 2.1 ± 0.2 before propanethiol and 2.3 ± 0.3 afterward. PMTS did not change the maximal response in α1(S267C) nor in α1 wild-type glycine receptors. Moreover, PMTS did not change the EC50 (125 ± 17 before PMTS treatment and 113 ± 20 after) of α1 wild-type glycine receptors.
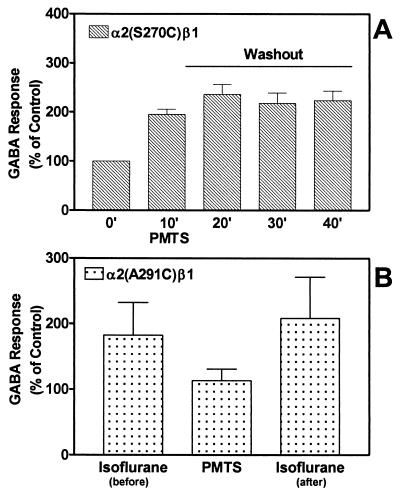
To determine whether the residue in the GABAA receptor α subunit that is homologous to S267 in GlyR α1 also was a binding site for inhaled anesthetics and n-alcohols, we compared receptors containing the GABA mutant α2(S270C) subunit coexpressed with the wild-type β1 subunit. Propanethiol (4 mM) reversibly enhanced receptor function in wild-type GABA receptors (data not shown), but PMTS (50 μM) irreversibly potentiated the function of the mutant α2(S270C)β1 (Fig. 3A), supporting the importance of the TM2 site. PMTS did not affect the wild-type α2β1 GABA receptors (data not shown) nor the TM3 mutant α2(S291C)β1 (Fig. 3B). To determine whether PMTS was bound to the mutant α2(S291C)β1, but was not affecting the function, the effect of isoflurane was tested before and after exposure of the receptor to PMTS. Isoflurane potentiated this receptor before and after application of PMTS, suggesting that PMTS did not bind to this mutant (Fig. 3B).
Figure 3.
Effects of PMTS on α2 (S270C)β1 and α2 (S291C)β1 GABAA receptors expressed in Xenopus oocytes. (A) In α2 (S270C)β1 GABAA receptors, PMTS (50 μM) irreversibly enhanced the GABA response. Washout from PMTS was measured every 10 min for a total of 30 min. (B) In α2 (S291C)β1 GABAA receptors, PMTS did not affect the GABA response. Isoflurane (0.6 mM) potentiated α2 (S291C)β1 GABA receptor function equally before and after application of PMTS. PMTS or isoflurane were perfused for 60 s before being coapplied with GABA for 30 s. Data are the mean ± SEM of five to six oocytes.
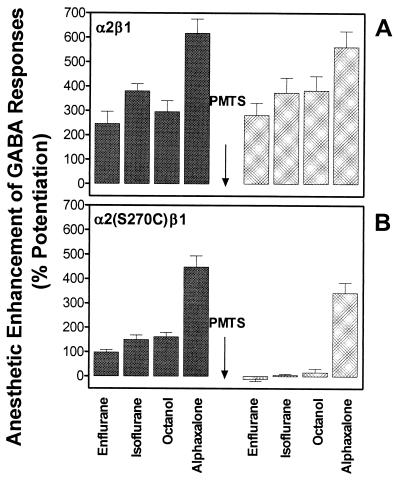
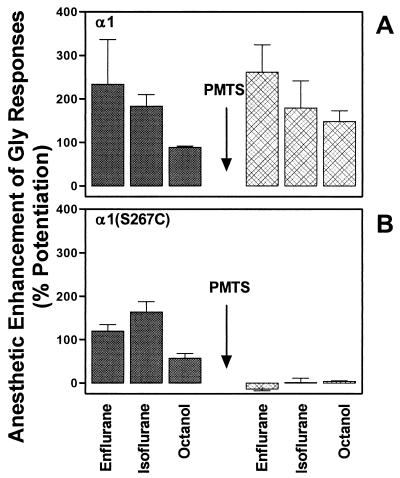
We next asked if n-alcohols and other volatile anesthetics act on the same site as propanethiol/PMTS. For wild-type α2β1 GABAA receptors, enflurane, isoflurane, octanol, and alphaxalone enhanced the GABA response to the same extent before and after application of PMTS (Fig. 4A). Irreversible occupation of the S270C site by PMTS in oocytes expressing GABA α2(S270C)β1 receptors prevented the potentiation of the GABA response by enflurane, isoflurane, and octanol (Fig. 4B). To test the specificity of the blocking action of PMTS, we used alphaxalone, an i.v. anesthetic that does not interact directly with the TM2 residue (19). Unlike the other anesthetics, alphaxalone still potentiated the GABA receptor function after PMTS treatment. This result demonstrates that the action of PMTS is specific and does not cause some gross alteration of receptor function. Results similar to those from the GABA receptor, were obtained in oocytes expressing GlyR α1 wild-type or α1(S267C) mutant receptors. In wild-type α1 GlyR, enflurane, isoflurane and octanol, enhanced the glycine response to the same extent before and after application of PMTS (Fig. 5A). In α1(S267C) GlyR, mutant enflurane, isoflurane and octanol, enhanced the glycine receptor function before application of PMTS. After application of PMTS this enhancement was abolished (Fig. 5B). Alphaxalone was not tested, as the strychnine-sensitive GlyR is insensitive to this anesthetic (10). As previously reported (18), volatile anesthetics were less effective in potentiating the agonist responses, in comparison to wild-type receptors, when cysteine are substituted for serine in the 267/270 position.
Figure 4.
Effects of anesthetics on α2β1 or α2(S270C)β1 GABAA receptors, expressed in Xenopus oocytes, before and after perfusion with PMTS. (A) In wild-type α2β1 receptors, enflurane (1.2 mM), isoflurane (0.6 mM), octanol (0.1 mM), or alphaxalone (1 μM) enhanced GABA responses before and after application of PMTS (50 μM). (B) In mutant α2(S270C)β1 receptors, application of PMTS abolished the effects of enflurane, isoflurane, or octanol but did not affect the enhancement by alphaxalone. PMTS was perfused for 60 s before being coapplied with GABA for 30 s. Anesthetics or octanol were tested again after 15 min of washout from PMTS. Data are expressed as a mean ± SEM of five to 12 oocytes.
Figure 5.
Effects of anesthetics on α1 or α1(S267C) GlyR, expressed in Xenopus oocytes, before and after perfusion with PMTS. (A) In α1 receptors, enflurane (1.2 mM), isoflurane (0.6 mM), or octanol (0.1 mM) enhanced glycine responses before and after application of PMTS (30 μM). (B) In mutant α1(S267C) receptors, application of PMTS abolished the effects of enflurane, isoflurane, or octanol. PMTS was perfused for 60 s before being coapplied with glycine for 30 s. Anesthetics or octanol were tested again after 15 min of washout from PMTS. Data are expressed as a mean ± SEM of three to four oocytes.
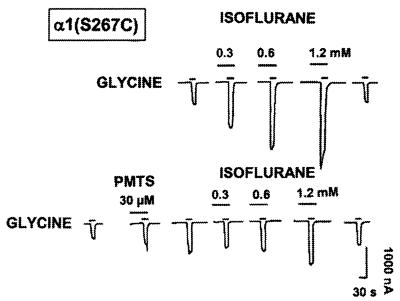
To test if the antagonism of isoflurane action was noncompetitive, as would be required for covalent occupation of a binding site, a concentration-response curve for isoflurane (0.3–1.2 mM) was obtained from the α1(S267C) GlyR. Before application of PMTS, isoflurane enhanced the function of the GlyR in a concentration-dependent mode. After application of PMTS, enhancement of the GlyR function by isoflurane was completely abolished at lower concentrations of isoflurane (0.3–0.6 mM) and dramatically reduced at a higher concentration (1.2 mM) (Fig. 6). It is possible that the small residual effect of isoflurane is due to incomplete labeling of the receptor by PMTS.
Figure 6.
Effects of isoflurane on α1(S267C) mutant GlyR expressed in Xenopus oocyte. Isoflurane (0.3–1.2 mM) potentiates in a concentration-dependent manner the function of α1(S267C) mutant receptor before application of PMTS (30 μM). Application of PMTS abolished or reduced the potentiation of the GlyR by isoflurane. Sample tracings of currents were induced by application of an EC10 concentration of glycine alone or glycine in the presence of isoflurane in an individual voltage-clamped oocyte. Tracing is from a single oocyte, and similar results were obtained from two other oocytes.
To visualize how propanethiol might bind to the receptor, we used a molecular model of TM2 and TM3 in the transmembrane domain of the α1 GlyR subunit. There is a consensus that TM2 is the pore-lining α helix in each of the five subunits that make up the pentameric ion channel of the ligand gated receptors (20, 21). We modeled the predicted transmembrane sequence of TM2 (residues A249–L274) (15, 22) as an α-R-helix with the biopolymer module of Discover 98 (Molecular Simulation, San Diego).
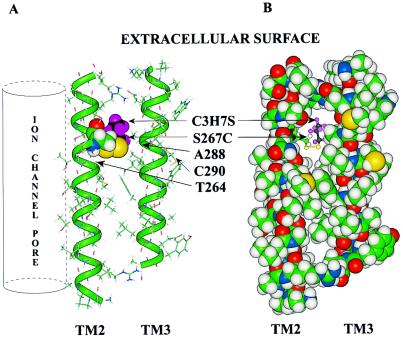
A nearly vertical line of threonine residues on TM2 clearly defines which side of the TM2 α helix faces the aqueous pore. Analogous threonine and serine residues were identified as pore-facing residues in the nicotinic acetylcholine receptor (20). S267C is on the side of TM2 directly opposite from the line of threonine residues that defines the aqueous pore, and it is likely that S267C faces the interior of the subunit and interacts with the neighboring TM3 (Fig. 7). Residues V280–A302 of TM3 were modeled as an α-R-helix and placed next to TM2 in an anti-parallel direction. The vertical alignment of TM2 and TM3 was fixed by placing residue S267 of TM2 on the same horizontal level as A288 of TM3. Our previous work suggested that residue A288 interacts with S267 (15). Therefore, TM3 was rotated about its vertical axis until A288 was opposite S267 and moved such that the distance between the axes of the anti-parallel helices was 11 Å (23). The backbone C and Cα carbons of the two α helices were tethered (all side chain atoms were free to move) with an initial quadratic force constant of 100 kcal/Å2. The structure was optimized with Discover 98 using the CFF91 potential function and a distance-dependent dielectric. The optimization was repeated with a force constant of 10 kcal/Å2 and finally with 1 kcal/Å2. S267 was replaced with a cysteine residue, propanethiol was bonded to S267C, and the propyl group was moved manually to avoid obvious steric overlaps with TM2 and TM3. The same tether of 100 kcal/Å2 was applied to all backbone C and Cα atoms and the structure was reoptimized (no tether was applied to propanethiol). The propanethiol group fits in the cavity between the transmembrane α helices (Fig. 7). Recent work questions whether TM3 is an α helix but places A288 within the membrane (24).
Figure 7.
Molecular model of TM2 and TM3 of the GlyR α1 subunit. TM2 is oriented so that the aqueous pore of the ion channel would be on the far left (vertical cylinder). This orientation places residue S267C on the interior of the subunit. The TM3 helix was aligned anti-parallel to TM2 so that S267 and A288 would be on the same horizontal plane. (A) The peptides are rendered as a stick structure, the backbones of the α helices are overlaid with a green tube, and both S267C and propanethiol are rendered with a space-filling (van der Waals) surface. The colors of atoms are: carbon, green; hydrogen, gray; oxygen, red; nitrogen, blue; and the propyl group of propanethiol is highlighted with black carbons and violet hydrogens. Those cysteine residues that did not form covalent bonds with PMTS or that formed them without any effect on potentiation of the effect of glycine are indicated (T264C, A288C, and C290). (B) The same molecular model of TM2 and TM3 is shown except that all residues in the two helices are rendered with a space-filling surface and the propanethiol is rendered as a ball-and-stick molecule. It can be seen that the propyl group occupies the cavity and may prevent the additional occupation of the cavity by octanol, isoflurane, or enflurane.
Discussion
Previous studies demonstrated that anesthetic and alcohol enhancement of the GlyR and GABAA receptor function requires specific amino acids in TM2 and TM3 of the α subunit. Two amino acid residues, S267 and A288 in the GlyR, S270 and A291 in the GABAA receptor, are essential for the effects of alcohol and anesthetics (7). However the crucial question that remains is whether these residues represent a specific binding site for alcohols and anesthetics. To answer this question, we examined the possibility that propanethiol, an anesthetic similar in structure to propanol (25), could react, in a irreversible way, with cysteines engineered at those critical positions. In addition, we tested the ability of a sulfhydryl-specific reagent, PMTS, to covalently bind to the mutated cysteines and alter permanently the receptor function.
Sequential treatment of the α1(S267C) GlyR by iodine and propanethiol produced potentiation of the receptor function that was only partially reversible by extended washing. In contrast, potentiation of the wild-type receptor was completely reversible by washing. These results suggest formation of a disulfide bond between propanethiol and the cysteine residue replacing S267, resulting in long lasting enhancement of GlyR channel function. The results obtained with propanethiol were confirmed by using PMTS. This sulfhydryl-specific reagent reacts with the SH group of a cysteine to provide the same residue as propanethiol (propyl disulfide); however, it reacts rapidly with no need for iodine or other oxidizing agents. PMTS was more efficient than propanethiol in providing a irreversible enhancement of the S267C mutant.
The importance of the TM2 segment as a possible binding site for alcohol and anesthetics, was confirmed in GABAA receptors. In α2(S270C)β1 GABAA receptors, PMTS appears to form covalent bonds with the cysteine residue in position 270. On the contrary, PMTS did not affect wild-type α2β1 receptors. A possible explanation for this lack of effect is that the much larger size and polarity of PMTS, as compared to propanethiol, prevents it from reaching the necessary anesthetic concentration at the active site of the wild-type receptors. However, the cumulative process of disulfide bond formation would cause a low concentration of PMTS at the S270C site to eventually occupy the cavity in all of the mutant receptors.
Neither propanethiol nor PMTS were able to irreversibly enhance the function of the TM3 mutants α1(A288C) or α2(A291C)β1 or prevent action of isoflurane on these receptors. The negative result with A288C and with A291C is surprising because mutagenesis studies suggested that this residue forms part of the anesthetic-binding site (7, 15, 26). Moreover, a recent study indicated that the negatively charged and much larger p-hydroxymercuribenzenesulfonate (pCMBS−) was able to covalently bind α1A291C in α1β1γ2 GABAA receptors, implying that this site might be accessible to PMTS, as it is to pCMBS− (27). However, our results suggest that propanethiol/PMTS did not form covalent bonds with A288C or A291C. Previous studies showed that introduction of amino acids larger than cysteine (e.g., isoleucine) at position A288 markedly enhance the action of glycine and reduce the action of volatile anesthetics (26). Thus, attachment of propyl sulfide to A288C should alter effects of both glycine and isoflurane, but this was not observed. Thus, the most likely explanation of our negative results is that propanethiol and PMTS do not have adequate access to A288C or A291C. It is interesting to note that a recent crystal structure of bromoform binding to firefly luciferase revealed specific orientation for two bromoform molecules. This finding is remarkable because bromoform is nearly spherical and suggests that propanethiol also may have a restricted orientation between TM2 and TM3, which precludes covalent bonds with A288C or A291C (28). Further studies with other sulfydryl reagents are in progress to explore the anesthetic binding site in the TM3 region.
Competition experiments performed before and after irreversible binding of PMTS to the cysteine mutations in the GABAA receptor suggest competition for a single binding site among PMTS and the inhaled anesthetics or octanol, but not between PMTS and alphaxalone. This is consistent with the proposal that inhaled anesthetics and n-alcohols bind to the same site, but alphaxalone binds to a different site (7, 19). Similar conclusions were reached for the GlyR.
The covalent labeling of substituted cysteines provides a powerful tool for studying structure–function relationship in proteins, including ligand-gated ion channels (20, 29). The present work combines this tool with the anesthetic action of alkanethiols, and their structural similarity to n-alcohols, to elucidate the location of the specific critical binding sites responsible for the capacity of anesthetics to enhance the action of glycine or GABA. Our results support the hypothesis that an amino acid in TM2 provide a specific site for anesthetic binding. However, it is of interest to consider alternative hypotheses. A prominent idea is that there are multiple anesthetic-binding sites on the protein, or even in the lipid phase. If this were true, one would expect the covalent labeling of a single (S267/270C) site by propanethiol to produce at most partial enhancement of receptor function and that subsequent application of other anesthetics would produce clear increases in function (due to action on the other sites). On the contrary, these are not the results we obtained. To reconcile the multiple sites hypothesis with our data requires two assumptions: i) the irreversible enhancement of glycine and GABA action produced by propanethiol + iodine or PMTS is unrelated to the reversible (anesthetic-like) enhancement of receptor function produced by propanethiol; and ii) covalent modification of S267C somehow blocks the actions exerted by anesthetics at other multiple sites (but does not block the action of alphaxalone). Although these possibilities cannot be completely ruled out, we feel the most parsimonious explanation for the present data and other mutational studies (7, 24, 30) is that binding of volatile anesthetics and n-alcohols in a protein cavity formed in part by a single amino acid is both necessary and sufficient for the enhancement of receptor function. This indicates that anesthetics act by a mechanism closer to that of traditional receptor-mediated pharmacology than was previous thought. This approach should have general applicability for defining anesthetic-binding sites on other brain proteins.
Acknowledgments
We thank Dr. W. Hubbell for help throughout this work; Drs. P. Schofield and H. Betz for supplying cDNAs; Dr. E. Eger and the University of California at San Francisco Anesthesia Research Foundation for supporting our collaborative project; V. Bleck and A. Gaudet for technical assistance; and Drs. C. Borghese, N. Harrison, and S. J. Mihic for many helpful discussions. This work was supported by the Texas Commission on Alcohol and Drug Abuse and National Institutes of Health grants to R.A.H. (AA06399 and GM47818) and to J.R.T. (GM47818).
Abbreviations
- GABAA
γ-aminobutyric acid type A
- GlyR
glycine receptor
- PMTS
propyl methanethiosulfonate
- TM2 and TM3
second and the third transmembrane
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.160128797.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.160128797
References
- 1.Kennedy S K, Longnecker D E. In: The Pharmacological Basis of Therapeutics. Moliniff P B, Ruddon R W, editors. New York: McGraw–Hill; 1996. pp. 304–305. [Google Scholar]
- 2.Meyer H H. Arch Exp Pathol Pharmakol. 1899;42:109–118. [Google Scholar]
- 3.Overton E. Studien uber die Narkose, Zugleich ein Beitrag zur Allgemeinen Pharmakologie. Jena, Germany: Gustav Fischer; 1901. pp. 1–195. [Google Scholar]
- 4.Cantor R S. Biochemistry. 1997;36:2339–2344. doi: 10.1021/bi9627323. [DOI] [PubMed] [Google Scholar]
- 5.Forman S A, Miller K W, Yellen G. Mol Pharmacol. 1995;48:574–581. [PubMed] [Google Scholar]
- 6.Franks N P, Lieb W R. Nature (London) 1994;367:607–614. doi: 10.1038/367607a0. [DOI] [PubMed] [Google Scholar]
- 7.Mihic S J, Ye Q, Wick M J, Koltchine V V, Krasowski M D, Finn S E, Mascia M P, Valenzuela C F, Hanson K K, Greenblatt E P, et al. Nature (London) 1997;389:385–389. doi: 10.1038/38738. [DOI] [PubMed] [Google Scholar]
- 8.Eckenhoff R G, Johansson J S. Pharmacol Rev. 1997;49:343–367. [PubMed] [Google Scholar]
- 9.Dildy-Mayfield J E, Mihic S J, Liu Y, Deitrich R A, Harris R A. Br J Pharmacol. 1996;118:378–384. doi: 10.1111/j.1476-5381.1996.tb15413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mascia M P, Machu T K, Harris R A. Br J Pharmacol. 1996;119:1331–1336. doi: 10.1111/j.1476-5381.1996.tb16042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Downie D L, Hall A C, Lieb W R, Franks N P. Br J Pharmacol. 1996;118:493–502. doi: 10.1111/j.1476-5381.1996.tb15430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harrison N L, Kugler J L, Jones M V, Greenblatt E P, Pritchett D B. Mol Pharmacol. 1993;44:628–632. [PubMed] [Google Scholar]
- 13.Lin L H, Chen L L, Zirrolli J A, Harris R A. J Pharmacol Exp Ther. 1992;263:569–578. [PubMed] [Google Scholar]
- 14.Mihic S J, McQuilkin S J, Eger E I, II, Ionescu P, Harris R A. Mol Pharmacol. 1994;46:851–857. [PubMed] [Google Scholar]
- 15.Wick M J, Mihic S J, Ueno S, Mascia M P, Trudell J R, Brozowsky S J, Ye Q, Harrison N L, Harris R A. Proc Natl Acad Sci USA. 1998;95:6504–6509. doi: 10.1073/pnas.95.11.6504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kenyon G L, Bruice T W. Methods Enzymol. 1977;47:407–430. doi: 10.1016/0076-6879(77)47042-3. [DOI] [PubMed] [Google Scholar]
- 17.Lee G F, Lebert M R, Lilly A A, Hazelbauer G L. Proc Natl Acad Sci USA. 1995;92:3391–3395. doi: 10.1073/pnas.92.8.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koltchine V V, Finn S E, Jenkins A, Nikolaeva N, Lin A, Harrison N L. Mol Pharmacol. 1999;56:1087–1093. doi: 10.1124/mol.56.5.1087. [DOI] [PubMed] [Google Scholar]
- 19.Rick C E, Ye Q, Finn S E, Harrison N L. NeuroReport. 1998;9:379–383. doi: 10.1097/00001756-199802160-00004. [DOI] [PubMed] [Google Scholar]
- 20.Akabas M H, Kaufmann C, Archdeacon P, Karlin A. Neuron. 1994;13:919–927. doi: 10.1016/0896-6273(94)90257-7. [DOI] [PubMed] [Google Scholar]
- 21.Unwin N. J Mol Biol. 1996;257:586–596. doi: 10.1006/jmbi.1996.0187. [DOI] [PubMed] [Google Scholar]
- 22.Rajendra S, Lynch J W, Schofield P. Pharmacol Ther. 1997;73:121–146. doi: 10.1016/s0163-7258(96)00163-5. [DOI] [PubMed] [Google Scholar]
- 23.Sankararamakrishnan R, Sansom M S. FEBS Lett. 1995;377:377–382. doi: 10.1016/0014-5793(95)01376-8. [DOI] [PubMed] [Google Scholar]
- 24.Leite J F, Amoscato A A, Cascio M. J Biol Chem. 2000;275:13683–13689. doi: 10.1074/jbc.275.18.13683. [DOI] [PubMed] [Google Scholar]
- 25.Zhang, Y., Trudell, J. R., Mascia. M. P., Laster, M. J., Gong, D., Harris, R. A. & Eger, E. I., II (2000) Anesth. Analg., in press. [DOI] [PubMed]
- 26.Yamakura T, Mihic S J, Harris R A. J Biol Chem. 1999;274:23006–23012. doi: 10.1074/jbc.274.33.23006. [DOI] [PubMed] [Google Scholar]
- 27.Williams D B, Akabas M H. Biophys J. 1999;77:2563–2574. doi: 10.1016/s0006-3495(99)77091-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franks N P, Jenkins A, Conti E, Lieb W R, Brick P. Biophys J. 1998;75:2205–2211. doi: 10.1016/S0006-3495(98)77664-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karlin A, Akabas M H. Methods Enzymol. 1998;293:123–144. doi: 10.1016/s0076-6879(98)93011-7. [DOI] [PubMed] [Google Scholar]
- 30.Ye Q, Koltchine V V, Mihic S J, Mascia M P, Wick M, Finn S E, Harrison N L, Harris R A. J Biol Chem. 1998;273:3314–3319. doi: 10.1074/jbc.273.6.3314. [DOI] [PubMed] [Google Scholar]