Abstract
The prenyl group on the G protein γ subunit is an important determinant of protein–protein interactions between the βγ dimer and its targets, such as α subunits, receptors, and effectors. In an effort to identify domains of the β subunit important for the activation of effectors, we have prepared two types of mutants, one set in the domain suggested to form a hydrophobic prenyl-binding pocket for the γ subunit's prenyl group (prenyl pocket mutants) and the other set in a domain between Gly306 and Gly319 in the β propeller, which undergoes a conformational change when the dimer binds to phosducin (conformational change mutants). Recombinant baculoviruses for each set of mutants were prepared, and the nine mutant β subunits were overexpressed with either the γ2 subunit (modified with geranylgeranyl) or the γ2-L71S subunit (γ2 with altered CAAX sequence and modified with farnesyl). The purified dimers were tested for their ability to couple Gαi1 to the A1 adenosine receptor and to activate phospholipase C-β or type II adenylyl cyclase. All dimers containing mutant β subunits were indistinguishable from wild-type β1γ2 or β1γ2-L71S in coupling the receptor to Gαi1. The prenyl pocket mutants expressed with γ2 were 10-fold less potent in activating phospholipase C-β and adenylyl cyclase than β1γ2 and had similar activities to β1γ2-L71S. The conformational change mutants caused a 15- to 23-fold decrease in EC50 values for activation of these two effectors. Overall, the results suggest that the sites in Gβ identified by these mutants are very important in the activation of effectors. Furthermore, the nature of the prenyl group on Gγ may play an important role in the conformational change leading to the activity of Gβγ on effectors.
The βγ subunit of heterotrimeric G proteins is a fundamental part of the signaling system used by G protein-coupled receptors (1). Gβγ is essential for the proper interaction of Gα with the receptor to form the high-affinity agonist-binding conformation of the receptor (2, 3) and to initiate GDP/GTP exchange on Gα (3, 4). Once released from Gα, Gβγ regulates over 12 effectors, including PLC-β, adenylyl cyclases, and ion channels (1, 5). Thus, understanding the domains in Gβγ that interact with these multiple effectors is necessary for understanding the function of this signaling molecule within the cell.
The activity of Gα is closely regulated by multiple mechanisms, including the receptor itself, Gβγ, and RGS proteins(1, 6). However, Gβγ has been assumed to be fully active once released from Gα (1, 5, 7), perhaps because structural studies initially suggested that the conformation of free Gβγ was the same as that in the α-βγ heterotrimer (8–10). Interestingly, one x-ray structure of Gβγ suggests that Gβ does undergo a conformational change in three regions when bound to at least one protein, phosducin (11). A more recent x-ray structure of the phosducin–β1γ1 complex solved with an intact Gγ containing the farnesyl group shows the farnesyl group inserts into a hydrophobic pocket in the β subunit, and a conformational change occurs in the amino acids between Gly306 and Gly319 near the C terminus (12).
All Gγ subunits are subject to posttranslational modification by the addition of the 15-carbon farnesyl group or the 20-carbon geranylgeranyl group to an invariant cysteine residue in the CAAX motif at their C terminus (13, 14). This lipid modification is important for anchoring Gβγ to the membrane and for determining functional interaction with other proteins (14, 15). We have found that switching the prenyl group on β1γ2 from geranylgeranyl to farnesyl causes a significant decrement in its ability to couple Gαi1 to A1 adenosine receptors (3) and to activate either PLC-β or adenylyl cyclase (16). These observations clearly indicate that the prenyl group on Gγ is an important determinant of interactions between βγ dimer and its targets.
To examine further the role of Gγ's prenyl group and the predicted conformational changes in Gβ on the ability of Gβγ to activate effectors, we investigated two types of Gβ1 mutants, one set in the hydrophobic prenyl-binding pocket (termed prenyl pocket mutants) and the other to minimize the potential effect of conformational changes in certain amino acids between Gly306 and Gly319 (termed conformational change mutants). The nine mutated β1 subunits were coexpressed with either the γ2 (modified with geranylgeranyl) or γ2-L71S (γ2 subunit modified with farnesyl) subunit and the purified dimers tested for their ability to support coupling of the A1 adenosine receptor to Gαi1 subunits or to activate PLC-β and adenylyl cyclase. Their activity was compared with that of wild-type β1γ2 and β1γ2-L71S. The results demonstrate that none of the dimers containing mutant β1 subunits affect the interaction between the A1 adenosine receptor and the α subunit. However, both the prenyl pocket mutants and the conformational change mutants decrease the activity of Gβγ on PLC-β and type II adenylyl cyclase, suggesting that these sites in Gβ are very important for activation of effectors.
Materials and Methods
Mutagenesis and Construction of Recombinant Baculoviruses.
The prenyl pocket mutants were made in the six amino acids predicted to interact with Gγ's prenyl group as observed in the phosducin-β1γ1 structure, Val315, Thr329, Ser331, Phe335, Lys337, and Trp339 (12). Each of these amino acids was mutated individually to Ala with the exception of Thr329, which was mutated to Lys. The point mutations were made in the cDNA encoding Gβ1 by using PCR-based site-directed mutagenesis. As an example of making the Val315 mutation (V315A), two independent PCRs were performed to switch GTC (Val) to GCC (Ala) at the position of 315 on Gβ1 by using the primers: sense I 5′-CCGCTCCAGAATTCAAGATGAGTG-3′ and antisense I 5′-AGGCAGCTGGCGCGGTTGTCA-3′ for one reaction and sense II 5′-TGACAACCGCGCCAGCTGCCT-3′ and antisense II 5′-CCAGGAAAGGATCCGCGTTAGTTC-3′ for the other reaction. The final PCR was performed by using the sense I primer and the antisense II primer to give an EcoRI restriction site at the 5′ position and a BamHI site at the 3′ position. The PCR product was subcloned into the pCNTR shuttle vector (5 Prime → 3 Prime), digested with XbaI, and the XbaI fragment was ligated into the pVL1393 baculovirus transfer vector (Invitrogen) (16). The five other mutations (T329K, S331A, F335A, K337A, and W339A) were generated by using the same method.
Two conformational change mutations were made at His311 and Arg314 as the side chains of these residues were observed to undergo a dramatic conformational change when free Gβγ (9) binds to phosducin (11, 12). These two amino acids were mutated to Ala (H311A and R314A). The point mutations were made in Gβ1 by using PCR as described above. A β subunit containing a mutant at Trp332 was also studied because this amino acid undergoes a significant conformational change in the complex between phosducin and β1γ1 (11). The recombinant baculovirus encoding the alanine mutation of Trp332 (W332A) was a kind gift from T. Kozasa (University of Illinois, Chicago). The pVL1393 transfer vectors encoding the eight mutant β1 subunits were sequenced to ensure fidelity. The recombinant baculoviruses encoding each mutant Gβ1, αs, αi1, γ2, and γ2-L71S subunits were produced as described (17–19).
Expression and Purification of Recombinant Gα and Gβγ.
Gα and Gβγ subunits were overexpressed in Sf9 insect cells and purified as described (17–19). The βγ dimers containing wild-type or mutated Gβ1 subunits with either γ2 (modified with geranylgeranyl) or γ2-L71S (farnesyl modification) subunits were purified on a DEAE column followed by affinity chromatography on a Gi1-α-agarose column (19). The purified βγ dimers were resolved on 12% polyacrylamide gels, stained with silver, and the concentration of the purified βγ dimers estimated by using ovalbumin standards (16, 17). The experiments shown in Results and Discussion were performed by using two different preparations of each βγ dimer.
Mass Spectrometrical Analysis of the Posttranslational Processing of Gγ.
Because analysis of many previous batches of Gβγ by using electrospray mass spectrometry has shown that the γ2 and γ2-L71S subunits are fully modified with the appropriate prenyl group (16, 20, 21), this set of 20 βγ dimers was analyzed by using matrix-assisted laser desorption ionization mass spectrometry. The deconvoluted mass spectra of all βγ dimers indicate that the γ2 and γ2-L71S proteins in the sample were composed of one molecular weight species with molecular masses of 7,751 Da or 7,683 Da, respectively. These results are consistent with full processing of Gγ2 by removal of three C-terminal amino acids (-AIL for γ2; -AIS for γ2-L71S), addition of either a geranylgeranyl for γ2 or a farnesyl for γ2-L71S and a carboxylmethyl group to the C-terminal cysteine, and the removal of the N-terminal methionine and acetylation of the resulting N-terminal alanine.
Expression of A1 Adenosine Receptors, PLC-β, and Type II Adenylyl Cyclase.
Sf9 cells were infected with a recombinant baculovirus encoding bovine A1 adenosine receptors, and membranes containing these receptors were prepared (2). Recombinant turkey PLC-β was purified as described (22). Sf9 insect cell membranes overexpressing recombinant, rat type II adenylyl cyclase were prepared as described (17). The experiments shown in Results and Discussion were performed by using a single preparation of each type of membrane and recombinant turkey PLC-β.
Measurement of the Activities of Wild-Type and Mutant βγ Dimers.
To measure the ability of each particular Gβγ to couple to receptors, Sf9 insect cell membranes expressing recombinant A1 adenosine receptors were reconstituted with G protein α and βγ subunits on ice for 30 min. Each reaction tube contained 20 fmol of receptor, 6 nM Gαi1, 0–30 nM of a Gβγ, and 50 nM GDP. After the reconstitution, the high-affinity agonist-binding conformation of the receptor was measured by using 0.3 nM of the agonist ligand [125I]-N6-(aminobenzyl)adenosine (3). The ability of each Gβγ dimer to stimulate PLC-β and type II adenylyl cyclase was measured as described (23). To determine whether the lower activity observed with the β1γ2-L71S dimer containing farnesyl could be increased by including geranylgeranyl in the assay medium, the synthetic lipid vesicles (23) used in the PLC-β assay were extruded in the presence of 10–50 μM geranylgeranyl or farnesyl alcohol (American Radiolabeled Chemicals, St. Louis). The vesicles were then passed over an AcA-34 column to remove unincorporated lipids (24). The vesicles enriched with geranylgeranyl or farnesyl were reconstituted with β1γ2, β1γ2-L71S, β1-S331Aγ2, or β1-K337Aγ2 and PLC assays performed as described (23).
Calculation and Data Expression.
Experiments presented under Results and Discussion are the average of three or more similar experiments. Data expressed as concentration-response curves were fit to sigmoid curves by using the fitting routines in the graphpad prism software (GraphPad Software, San Diego). Statistical differences between the fitted curves were determined by using all of the individual data points from multiple experiments to calculate the F statistic (25).
Results and Discussion
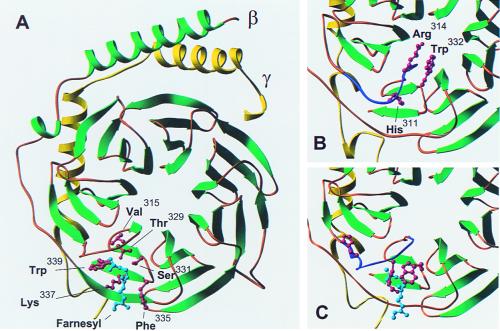
To examine the possibility that the prenyl group of Gγ participates in the activation of effectors by Gβγ, we have studied two types of Gβ mutants. On the basis of a crystal structure of the phosducin–β1γ1 complex that contained the farnesyl group, Loew et al. argued that the isoprenoid group folds into a hydrophobic pocket formed by blades 6 and 7 of the β propeller (12). Fig. 1A shows the structure of Gβγ from the βγ–phosducin complex reported by Loew et al. (12). The six amino acids forming contacts with the farnesyl group are highlighted in red. Therefore, our first strategy was to mutate these six amino acids to determine whether modifying this region had an effect on the function of the βγ dimer. Val315 appears to be capping the pocket-binding farnesyl (shown in cyan) and was mutated to Ala in an attempt to disrupt hydrogen bonding and remove a barrier at the end of the pocket. Thr329 makes a hydrogen bond with Trp339 and helps hold the alignment of the cavity. Thr329 was mutated to the larger Lys residue to force a bulky group to protrude into the prenyl-binding cavity and disrupt the hydrogen bond alignment with Trp339. This mutation was expected to force the prenyl group out of the pocket. Ser331, Phe335, Lys337, and Trp339 form the sides of the pocket and were mutated to Ala. These changes should make the cavity bigger and decrease the interactions holding the prenyl group in the pocket.
Figure 1.
Important features of the C-terminal region of Gβ. (A) The structure of Gβγ containing the farnesyl lipid derived from the β1γ1–phosducin complex as determined by Loew et al. (12). Gβ is shown in green and Gγ in yellow. The farnesyl group at the C terminus of Gγ is shown in cyan. The six amino acids predicted to interact with the farnesyl group based on the x-ray structure of the phosducin–β1γ1 complex are shown in red as ball-and-stick models. (B) The structure of free β1γ1 as determined by Sondek et al. (9). The molecule was placed in an identical orientation to the βγ structure shown in A by using the program O. Only the lower left corner of Gβγ is shown. The region of Gβ (Gly306–Gly319) that undergoes a conformational change when the dimer interacts with phosducin is shown in purple. Three amino acids that undergo dramatic conformational changes on formation of the phosducin–β1γ1 complex are shown as red ball-and-stick models. (C) The region of Gβγ shown in B from the βγ–phosducin complex determined by Loew et al. (12). The amino acids between Gly306 and Gly319 are shown in purple. The dramatic conformational changes in the side chains of His311, Arg314, and Trp332 are indicated by the red ball-and-stick models. The illustrations of Gβγ were generated with the program ribbons.
The structure of free Gβγ is nearly identical to that observed in the crystal structure of the heterotrimer (8, 10). Structures of the phosducin–βγ complex (11, 12) indicate that amino acids near the C-terminal end of Gβ undergo conformational changes when Gβγ binds to phosducin as compared with the structure of free Gβγ (9). One study of the phosducin–β1γ1 complex identified three local conformational changes in Gβ between residues 287–295, residues 308–318, and residues 329–338 (11). A second study highlighted the conformational changes in the region between Gly306 and Gly319 and identified the hydrophobic pocket that binds the farnesyl group (12). The conformational change between Gly306 and Gly319 is shown in purple in Fig. 1 B and C, respectively. Therefore, our second experimental strategy was to study certain amino acids that underwent dramatic conformational changes in the β1γ1–phosducin complex. Two amino acids in this region on Gβ1, His311, and Arg314 were chosen, and the conformational change mutants were made by mutating to Ala. Although not in this region, Trp332 [first studied as an α-βγ contact site (26, 27)] also undergoes a significant conformational change in the complex of phosducin–β1γ1 (11). Therefore, in addition to H311A and R314A, we also studied the alanine mutation of Trp332 (W332A) as a conformational change mutant. The positions of these three amino acids in free Gβγ are indicated as red ball-and-stick models in Fig. 1B, and the conformational changes induced by formation of the phosducin–βγ complex are shown in Fig. 1C.
The mutant Gβ1 were expressed with either the γ2 or the γ2-L71S subunit in baculovirus-infected Sf9 insect cells, and the resulting βγ dimers were purified by Gαi1-agarose affinity chromatography (19). Silver-stained SDS gels of the 20 βγ dimers used in this study showed the proteins to be highly pure; only two bands at about 36 and 7 kDa were visible, as described in our previous studies (16, 20).
Neither Type of Mutant Affects Receptor Coupling.
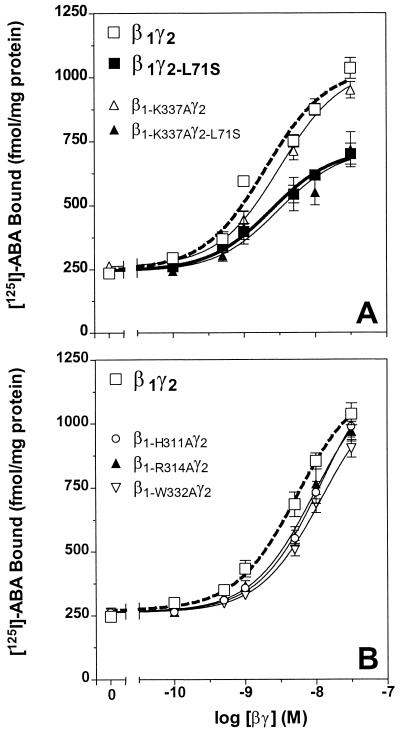
All 20 βγ dimers were tested for their ability to reestablish the high-affinity agonist-binding conformation of recombinant bovine A1 adenosine receptor expressed in Sf9 cell membranes. The data in Fig. 2A present the effect of β1-K337Aγ2 as representative of the activity of the prenyl pocket mutants. Each dimer containing a mutant β subunit was able to support high-affinity agonist binding at the bovine A1 adenosine receptor with a potency and efficacy equal to β1γ2 (see Fig. 2A and Table 1). As expected, the β1γ2-L71S dimer, in which the γ2 subunit was modified with farnesyl, was measurably less active than β1γ2 (3). Interestingly, the activity of β1-K337Aγ2-L71S was the same as that of β1γ2-L71S. All other βγ dimers containing mutant Gβ1 expressed with the γ2-L71S subunit were also equal in receptor coupling to the wild-type β1γ2-L71S (see Table 1). The data in Fig. 2B indicate that the activity of dimers containing any of the three conformational change mutants was equal to wild-type β1γ2. Therefore, neither type of mutant affects the ability of Gβγ to bind to the Gαi1 and support formation of the high-affinity ligand-binding state of A1 adenosine receptor. In addition, all of the nine βγ dimers containing altered β1 subunits were able to support the ability of an agonist-stimulated A1 adenosine receptor to initiate GDP/GTP exchange on Gαi1 (data not shown). These results are consistent with structural data showing that the C-terminal region of Gβ has no direct interaction with Gα (8, 10). Overall, these data suggest that when Gβγ interacts with Gα and the receptor, the prenyl group may extend away from Gβ and insert into the membrane. This possibility is consistent with data suggesting that the C-terminal end of Gγ and its prenyl group are important for the interaction with the receptor and the Gα subunit (3, 14).
Figure 2.
Comparison of the ability of wild-type βγ dimers and those containing prenyl pocket mutants or conformational change mutants to support the high-affinity agonist-binding state of the A1 adenosine receptor. (A) The activity of β1γ2 (open squares, thick dotted line) and β1γ2-L71S (closed squares, thick line) is compared with two representative prenyl pocket mutants, β1-K337Aγ2 and β1-K337Aγ2-L71S. (B) An analogous experiment performed with β1-H311Aγ2, β1-R314Aγ2, and β1-W332Aγ2 and compared to wild-type β1γ2 (open squares, thick dotted line). Overall, the EC50 values of wild-type and mutant βγ dimers containing the same γ subunit are not significantly different. Each data point is an average of three similar experiments performed in triplicate.
Table 1.
Comparison of the ability of the wild-type βγ dimers and those containing prenyl pocket mutants to support the high-affinity agonist-binding state of the A1 adenosine receptor and the activation of PLC-β or type II adenylyl cyclase
| Type of βγ dimer | Designation | Receptor coupling
|
PLC-β
|
AC-II
|
|||
|---|---|---|---|---|---|---|---|
| Kact, nM | Bmax, fmol/mg of protein | EC50, nM | Vmax, μmol/mg of PLC/min | EC50, nM | Vmax, nmol/mg of protein/min | ||
| Wild type | β1γ2 | 2.0 | 1,116.0 ± 43.0 | 3.0 | 3.26 ± 0.05 | 4.1 | 22.19 ± 0.65 |
| Prenyl pocket mutants with γ2 subunit modified with geranylgeranyl | β1-V315Aγ2 | 4.8 | 1,157.0 ± 91.3 | 31.0* | 2.81 ± 0.15† | 34.2* | 12.11 ± 1.96† |
| β1-T329Kγ2 | 4.2 | 1,120.9 ± 85.3 | 26.1* | 3.12 ± 0.10 | 35.2* | 12.39 ± 1.80† | |
| β1-S331Aγ2 | 4.7 | 1,108.4 ± 70.9 | 26.3* | 3.24 ± 0.09 | 46.2* | 22.01 ± 0.74 | |
| β1-F335Aγ2 | 4.2 | 1,030.9 ± 35.1 | 26.2* | 3.10 ± 0.15 | 33.4* | 16.50 ± 3.89† | |
| β1-K337Aγ2 | 3.2 | 1,036.7 ± 36.6 | 28.1* | 3.12 ± 0.08 | 35.9* | 21.02 ± 0.94 | |
| β1-W339Aγ2 | 4.5 | 1,059.9 ± 62.9 | 27.5* | 2.71 ± 0.20† | 35.2* | 16.48 ± 2.41† | |
| Wild type | β1γ2-L71S | 5.4 | 718.4 ± 30.7 | 30.3 | 3.08 ± 0.10 | 42.8 | 23.51 ± 0.88 |
| Prenyl pocket mutants with γ2-L71S subunit modified with farnesyl | β1-V315Aγ2-L71S | 5.6 | 741.2 ± 43.1 | 42.2‡ | 1.81 ± 0.22‡ | 40.2 | 9.05 ± 1.13‡ |
| β1-T329Kγ2-L71S | 9.4 | 834.9 ± 82.0 | 47.3‡ | 2.12 ± 0.18‡ | 45.3 | 7.22 ± 0.65‡ | |
| β1-S331Aγ2-L71S | 8.2 | 646.6 ± 40.4 | 49.5‡ | 2.01 ± 0.18‡ | 47.0‡ | 10.43 ± 3.04‡ | |
| β1-F335Aγ2-L71S | 5.3 | 685.6 ± 54.4 | 43.2‡ | 1.53 ± 0.11‡ | 52.4‡ | 14.28 ± 3.12‡ | |
| β1-K337Aγ2-L71S | 5.0 | 718.5 ± 57.7 | 39.5‡ | 1.81 ± 0.14‡ | 40.3 | 9.15 ± 0.61‡ | |
| β1-W339Aγ2-L71S | 6.3 | 692.7 ± 68.6 | 40.2‡ | 1.71 ± 0.20‡ | 41.9 | 12.98 ± 1.90‡ | |
The Kact, EC50, Bmax, or Vmax values of wild-type and mutant βγ dimers were determined by fitting each data set to sigmoid curves as described in Materials and Methods.
*Significant differences in responses to prenyl pocket mutants in comparison with corresponding wild-type β1γ2; P < 0.001.
Protein concentration of these mutants is too low to construct full concentration-response curves, so it is not possible for Vmax to be estimated with full accuracy.
Significant differences in responses to prenyl pocket mutants in comparison with corresponding wild-type β1γ2-L71S; P < 0.001.
Activity of Prenyl Pocket Mutants on Effectors.
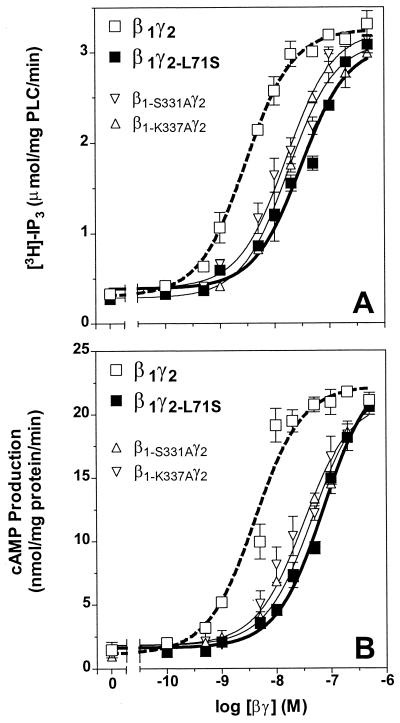
The full set of mutated βγ dimers was also tested for their ability to activate effectors. The data in Fig. 3A illustrate the ability of four representative βγ dimers to activate recombinant turkey PLC-β. As expected, β1γ2 activated PLC-β about 10-fold with an estimated EC50 value of 2.6 nM and was about 10-fold more potent at activating PLC-β than β1γ2-L71S (16). Note that βγ dimers containing β1-S331A or β1-K337A are significantly less potent than wild-type β1γ2. Interestingly, the EC50s of these mutants are not significantly different from that of β1γ2-L71S (see Table 1). Analogous experiments were performed with the other four dimers containing mutant β subunits and combined with either the γ2 or the γ2-L71S subunit. An analysis of the data from these experiments is presented in Table 1. Note especially that the potency of dimers containing any of six prenyl pocket mutants is similar to that of β1γ2-L71S and about 10-fold lower than that of β1γ2. Even if the Gγ subunit in the dimers was modified with geranylgeranyl, their ability to stimulate PLC-β was similar to that of β1γ2-L71S (Fig. 3A and Table 1). To determine whether the lower activity observed with the β1γ2-L71S dimer containing farnesyl could be increased by including geranylgeranyl in the assay medium, the vesicles (23) used in the PLC-β assay were extruded in the presence of 10–50 μM geranylgeranyl or farnesyl alcohol. These vesicles were reconstituted with 0, 2, 5, or 10 nM β1γ2, β1γ2-L71S, β1-S331Aγ2, or β1-K337Aγ2 and the activity of PLC-β measured. In the geranylgeranyl-enriched vesicles, the activity of the β1γ2 dimer is about the same as in the control vesicles; however, in this concentration range, the activity of the β1γ2-L71S dimer is increased about 2-fold to become equal to that of the β1γ2 dimer. Moreover, the activity of neither prenyl pocket mutant is increased in the geranylgeranyl-enriched vesicles, and the activity of the β1γ2-L71S dimer is not increased in farnesyl-enriched vesicles (n = 3; data not shown). Overall, these results and those in Table 1 strongly suggest that the geranylgeranyl lipid can bind in the prenyl-binding pocket of the β subunit and cause the most favorable conformation for activating effectors.
Figure 3.
Comparison of the ability of wild-type βγ dimers and those containing prenyl pocket mutants to activate PLC-β or type II adenylyl cyclase. (A) The activity of β1γ2 (open squares, thick dotted line) and two representative prenyl pocket mutants, β1-S331Aγ2 and β1-K337Aγ2, compared with that of β1γ2-L71S (closed squares, thick line). The indicated concentrations of the two representative prenyl pocket mutants were reconstituted with recombinant, turkey PLC-β in phospholipid vesicles containing [3H]phosphatidylinositol 4,5-bisphosphate, and PLC activity was measured as described in Materials and Methods. The difference between the effect of wild-type β1γ2 and the prenyl pocket mutants was statistically significant (P < 0.001; see Table 1). (B) The ability of the two representative prenyl pocket mutants, β1-S331Aγ2 and β1-K337Aγ2, to activate type II adenylyl cyclase as compared with the effect of wild-type β1γ2 (open squares, thick dotted line) and β1γ2-L71S (closed squares, thick line). The cyclase reaction was performed with the indicated concentrations of βγ dimers as described in Materials and Methods. The difference between the effect of wild-type β1γ2 and prenyl pocket mutant βγ dimers was statistically significant (P < 0.0001; see Table 1). Each data point is an average of three independent experiments, each performed in duplicate.
The data in Fig. 3B illustrate the effects of these four dimers on the activity of type II adenylyl cyclase. Note that the β1-S331Aγ2 and β1-K337Aγ2 dimers were about 10-fold less potent than wild-type β1γ2. Similar results were found with the four other mutant Gβ1 expressed and purified with γ2 (see Table 1). The six mutant β subunits expressed with γ2-L71S had even lower EC50s and estimated Vmax values in the cyclase assay (see Table 1).
Overall, the mutation of any of the six amino acids forming the postulated hydrophobic binding pocket for the prenyl group in Gβ decreases the potency of the dimer in both effector assays. Their maximal efficacies are not greatly changed. Interestingly, the loss of activity is very similar to that observed when the prenyl group on Gγ2 is switched from geranylgeranyl to farnesyl (γ2-L71S), and the lower activity of β1γ2-L71S can be increased by adding exogenous geranylgeranyl lipid in the PLC-β assay. Given that our previous experiments showed that the prenyl group on Gγ does not affect the partitioning of Gβγ into phospholipid vesicles (16, 24) or into Sf9 cell membranes (3), these results indicate that the nature of the prenyl group can affect the affinity of the interaction of the dimer with PLC-β or type II adenylyl cyclase. Thus, βγ dimers containing the geranylgeranyl group may assume somewhat different conformations when complexed with effectors as compared with βγ dimers containing the farnesyl group. This suggests the intriguing possibility that the interaction between the γ subunit's prenyl group and the β subunit may be important for inducing the active form of Gβγ.
Activity of Conformational Change Mutants on Effectors.
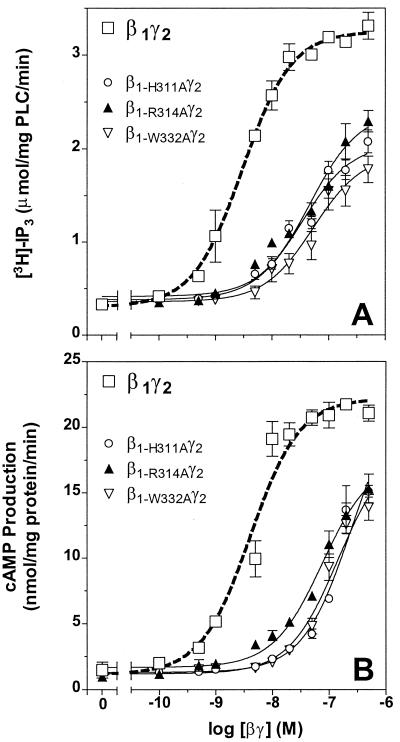
The data in Fig. 4A show that the ability of this set of mutant βγ dimers to activate PLC-β is much less than that of β1γ2. Analysis of the data shows that the EC50 for the activation of PLC-β is increased from 3.0 nM to about 50.0 nM, and the estimated Vmax is reduced about 40% (see Table 2). When tested in the type II adenylyl cyclase assay, the potency of these three mutant βγ dimers was about 25-fold less than β1γ2 (Fig. 4B). The estimated Vmax values were also slightly reduced (see Table 2). In both cases, the change is much larger than changes observed with the prenyl pocket mutants. Interestingly, any of the three mutants expressed with γ2-L71S is much less active at stimulating PLC-β or type II adenylyl cyclase than wild-type β1γ2-L71S (see Table 2). Therefore, alanine mutations of three amino acids whose conformation changes dramatically on binding phosducin result in a βγ dimer which has significantly reduced activity on either PLC-β or type II adenylyl cyclase. This observation indicates that rearrangement of the domain in Gβ between Gly306 and Gly319 (shown in purple in Fig. 1C) and Trp332 may be critical for forming the active conformation of Gβγ at these effectors. These findings provide a biochemical correlate to the observation that the structure of free Gβγ (9) is the same as that observed in the structure of the heterotrimer (8, 10) and the conformation changes when Gβγ is complexed with phosducin (11, 12). Overall, these results argue that Gβ undergoes a conformational change when it binds to effectors such as PLC-β and type II adenylyl cyclase, much as it does when it binds to phosducin. Thus, the domain of Gβ between Gly306 and Gly319 may be very important for the interaction with certain effectors.
Figure 4.
Comparison of the ability of the wild-type β1γ2 (open squares, dotted lines) and the conformational change mutants, β1-H311Aγ2, β1-R314Aγ2 and β1-W332Aγ2, to stimulate PLC-β (A) or type II adenylyl cyclase (B). In both assays, the difference between the effect of wild-type β1γ2 and the three conformational change mutants was significant (P < 0.0001; see Table 2). Each data point is an average of three independent experiments, each performed in duplicate.
Table 2.
Comparison of the ability of wild-type βγ dimers and those containing conformational change mutants to support the high-affinity agonist-binding state of the A1 adenosine receptor and the activation of PLC-β or type II adenylyl cyclase
| Type of βγ dimer | Designation | Receptor coupling
|
PLC-β
|
AC-II
|
|||
|---|---|---|---|---|---|---|---|
| Kact, nM | Bmax, fmol/mg of protein | EC50, nM | Vmax, μmol/mg of PLC/min | EC50, nM | Vmax, nmol/mg of protein/min | ||
| Wild type | β1γ2 | 2.0 | 1,116.0 ± 43.0 | 3.0 | 3.26 ± 0.05 | 4.1 | 22.19 ± 0.65 |
| Conformational change mutants with γ2 subunits modified with geranylgeranyl | β1-H311Aγ2 | 4.3 | 1,149.1 ± 75.7 | 45.3* | 2.06 ± 0.11* | 95.5* | 23.48 ± 1.89 |
| β1-R314Aγ2 | 5.2 | 1,180.4 ± 96.3 | 47.0* | 2.40 ± 0.12* | 76.8* | 17.56 ± 0.99* | |
| β1-W332Aγ2 | 4.6 | 1,142.9 ± 67.3 | 54.2* | 1.95 ± 0.15* | 95.3* | 18.55 ± 1.16* | |
| Wild type | β1γ2-L71S | 5.4 | 718.4 ± 30.7 | 30.3 | 3.08 ± 0.10 | 42.8 | 23.51 ± 0.88 |
| Conformational change mutants with γ2-L71S subunits modified with farnesyl | β1-H311Aγ2-L71S | 5.7 | 710.3 ± 27.0 | 49.7† | 0.91 ± 0.06† | 94.1† | 19.03 ± 6.15 |
| β1-R314Aγ2-L71S | 7.8 | 671.8 ± 35.6 | 41.3† | 1.08 ± 0.09† | 81.4† | 12.04 ± 1.89† | |
| β1-W332Aγ2-L71S | 7.6 | 728.3 ± 44.7 | 51.1† | 1.08 ± 0.12† | 98.3† | 7.65 ± 0.92† | |
The Kact, EC50, Bmax, or Vmax values of wild-type and mutant βγ dimers were determined by fitting each data set to sigmoid curves as described in Materials and Methods.
*Significant differences in responses to conformational change mutants in comparison with corresponding wild-type β1γ2; P < 0.0001.
†Significant differences in responses to conformational change mutants in comparison with corresponding wild-type β1γ2-L71S; P < 0.0001.
A number of studies have identified the C-terminal region of Gβ as a critical domain for the activation of effectors. Replacement of only four mammalian residues (Val327, Ala328, Phe335, and Asn340) in Gβ1 with those from Dictyostelium produced βγ dimers that were severely impaired in their ability to activate PLC-β2 (28). These residues overlap with the region of the hydrophobic prenyl-binding pocket in Gβ. In addition, mutant β subunits lacking the C-terminal two amino acids (Trp339 and Asn340) did not activate mitogen-activated protein kinase but were able to stimulate c-Jun N-terminal kinase/stress-activated protein kinase (29). Taylor et al. found that a photoaffinity-labeled peptide derived from the third intracellular loop of the α2-adrenergic receptor crosslinked to the C-terminal 60 amino acids of Gβ (4). Taken together, the results of these studies strongly indicate that the C-terminal region of Gβ is important for interaction with multiple effectors.
The crystal structure of the G protein heterotrimer has provided critical information for three recent mutagenesis studies. To examine the possibility that the βγ dimer uses a common surface to interact with both the α subunit and its downstream effectors, Ford et al. mutated 15 amino acids in the β1 subunit to alanine (26). These amino acids were located in the switch interfaces between the α and β subunits (Lys57, Tyr59, Ser98, Trp99, Met101, Leu117, Asn119, Thr143, Asp186, Asp228, and Trp332) and in the N-terminal interface (Leu55, Lys78, Ile80, and Lys89). The mutants were expressed with either the γ1 or the γ2 subunit and tested for their ability to interact with the Gt α subunit or to activate downstream targets such as the β-adrenergic receptor kinase, PLC-β2, type II adenylyl cyclase, or K+ and Ca2+ channels. The results of this study show that the regions of the βγ dimer that interact with effectors are in the domain covered by the α subunit in the heterotrimer. However, the different effectors were found to interact with distinct but partially overlapping domains on the β subunit. These results provide two important concepts for βγ signaling. First, they indicate that different domains of the βγ dimer may be used for activation of different effectors. Second, apparently all of these domains are covered by the α subunit in the heterotrimer. Thus, formation of the heterotrimer is able to regulate the activity of the βγ dimer on multiple downstream effectors.
Another study showed that four point mutations in the top surface of Gβ inhibited the ability of the dimers to activate PLC-β2, PLC-β3, and type II adenylyl cyclase (27). Interestingly, none of the mutations affected the ability of Gβγ to inhibit type I adenylyl cyclase. The two point mutations on the side of Gβ in blade 1 diminished the affinity for Gα but did not inhibit activation of effectors (27). In a more extensive study of the importance of the side surfaces of Gβ in activating effectors, Panchenko et al. mutated multiple amino acids in each of the seven blades of the β propeller and found that mutations in the amino acids of blades 2, 6, and 7 greatly inhibited the ability of Gβγ to activate PLC-β2 but did not alter the activity at type I or type II adenylyl cyclase (30). Moreover, Buck et al. showed that a peptide derived from amino acids 86–105 of Gβ1 could activate PLC-β2, and a peptide derived from 115–135 could block Gβγ activation of PLC-β2 (31). The latter region is on the outer surface of blade 2 of Gβ and overlaps one of the domains identified as important for activation of PLC-β2 by Panchenko et al. (30). Overall, the results of these studies suggest that the amino acids in both the top surface and the edges of the β torus are important for activating PLC-β, but that activation of type II adenylyl cyclase depends more on interaction with the top surface of Gβ, indicating that multiple regions of Gβγ are involved in the interaction with its targets.
Clearly, the region between Gly306 and Gly319 identified in the present work is another important region in Gβγ activity. One interesting aspect of the Ala mutations in the region between amino acids 306 and 319 in Gβ1 in this study is that they markedly inhibit activation of both PLC-β and type II adenylyl cyclase. These three conformational change mutants are located on the top surface of the β torus; thus, our results are in keeping with the general observations that the top surface of the β propeller is important for interaction with effectors. It is important to note that the amino acids represented by both the prenyl pocket mutants and the conformational change mutants are strictly conserved in 11 β subunits from a variety of species. The three amino acids that undergo a conformational change (His311, Arg314, and Trp332) are absolutely conserved in all five mammalian β subunits, yeast, Dictyostelium, Caenorhabditis elegans, Drosophila, and squid. Moreover, the glycines at positions 306 and 319 that provide the putative hinges for the conformational change in the structure are also strictly conserved across species (9). Thus, this domain in Gβ may be very important for the interaction of the dimer with multiple effectors. In addition, the residues that form the hydrophobic prenyl pocket are about 95% conserved in these six species. Therefore, both regions of Gβ studied in these experiments may be generally important for the activity of Gβγ at downstream effectors in multiple species.
Acknowledgments
We thank Drs. Ravi Iyengar (Mt. Sinai School of Medicine, New York) and Tohru Kozasa for the baculoviruses encoding type II adenylyl cyclase and the β1 subunit containing the W332A mutation, respectively. We thank Dr. T. K. Harden (University of North Carolina, Chapel Hill, NC) for purified recombinant turkey phospholipase C-β, the University of Virginia Diabetes Core Facility for cAMP Assays, and the Biomolecular Research Facility for DNA sequencing and mass spectrometric analysis. We also acknowledge Warren J. Clingan, III for excellent technical assistance and Qiang Zhao for help with the ribbons program. This work was supported by a fellowship from the Virginia Affiliate of the American Heart Association (to C.-S. M.) and by National Institutes of Health Grants CA40042 and DK-19952.
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Hamm H E. J Biol Chem. 1998;273:669–672. doi: 10.1074/jbc.273.2.669. [DOI] [PubMed] [Google Scholar]
- 2.Figler R A, Graber S G, Lindorfer M A, Yasuda H, Linden J, Garrison J C. Mol Pharmacol. 1996;50:1587–1595. [PubMed] [Google Scholar]
- 3.Yasuda H, Lindorfer M A, Woodfork K A, Fletcher J E, Garrison J C. J Biol Chem. 1996;271:18588–18595. doi: 10.1074/jbc.271.31.18588. [DOI] [PubMed] [Google Scholar]
- 4.Taylor J M, Jacob-Mosier G G, Lawton R G, VanDort M, Neubig R R. J Biol Chem. 1996;271:3336–3339. doi: 10.1074/jbc.271.7.3336. [DOI] [PubMed] [Google Scholar]
- 5.Clapham D E, Neer E J. Annu Rev Pharmacol Toxicol. 1997;37:167–203. doi: 10.1146/annurev.pharmtox.37.1.167. [DOI] [PubMed] [Google Scholar]
- 6.Berman D M, Gilman A G. J Biol Chem. 1998;273:1269–1272. doi: 10.1074/jbc.273.3.1269. [DOI] [PubMed] [Google Scholar]
- 7.Sprang S R. Annu Rev Biochem. 1997;66:639–678. doi: 10.1146/annurev.biochem.66.1.639. [DOI] [PubMed] [Google Scholar]
- 8.Wall M A, Coleman D E, Lee E, Iniguez-Lluhi J A, Posner B A, Gilman A G, Sprang S R. Cell. 1995;83:1047–1058. doi: 10.1016/0092-8674(95)90220-1. [DOI] [PubMed] [Google Scholar]
- 9.Sondek J, Bohm A, Lambright D G, Hamm H E, Sigler P B. Nature (London) 1996;379:369–374. doi: 10.1038/379369a0. [DOI] [PubMed] [Google Scholar]
- 10.Lambright D G, Sondek J, Bohm A, Skiba N P, Hamm H E, Sigler P B. Nature (London) 1996;379:311–319. doi: 10.1038/379311a0. [DOI] [PubMed] [Google Scholar]
- 11.Gaudet R, Bohm A, Sigler P B. Cell. 1996;87:577–588. doi: 10.1016/s0092-8674(00)81376-8. [DOI] [PubMed] [Google Scholar]
- 12.Loew A, Ho Y K, Blundell T, Bax B. Structure (London) 1998;6:1007–1019. doi: 10.1016/s0969-2126(98)00102-6. [DOI] [PubMed] [Google Scholar]
- 13.Casey P J. Curr Opin Cell Biol. 1994;6:219–225. doi: 10.1016/0955-0674(94)90139-2. [DOI] [PubMed] [Google Scholar]
- 14.Gautam N, Downes G B, Yan K, Kisselev O. Cell Signal. 1998;10:447–455. doi: 10.1016/s0898-6568(98)00006-0. [DOI] [PubMed] [Google Scholar]
- 15.Marshall C J. Science. 1993;259:1865–1866. doi: 10.1126/science.8456312. [DOI] [PubMed] [Google Scholar]
- 16.Myung C-S, Yasuda H, Liu W W, Harden T K, Garrison J C. J Biol Chem. 1999;274:16595–16603. doi: 10.1074/jbc.274.23.16595. [DOI] [PubMed] [Google Scholar]
- 17.Fletcher J E, Lindorfer M A, DeFilippo J M, Yasuda H, Guilmard M, Garrison J C. J Biol Chem. 1998;273:636–644. doi: 10.1074/jbc.273.1.636. [DOI] [PubMed] [Google Scholar]
- 18.Graber S G, Figler R A, Garrison J C. Methods Enzymol. 1994;237:212–226. doi: 10.1016/s0076-6879(94)37064-8. [DOI] [PubMed] [Google Scholar]
- 19.Graber S G, Lindorfer M A, Garrison J C. Methods Neurosci. 1996;29:207–226. [Google Scholar]
- 20.Lindorfer M A, Sherman N E, Woodfork K A, Fletcher J E, Hunt D F, Garrison J C. J Biol Chem. 1996;271:18582–18587. doi: 10.1074/jbc.271.31.18582. [DOI] [PubMed] [Google Scholar]
- 21.Lindorfer M A, Myung C-S, Savino Y, Yasuda H, Khazan R, Garrison J C. J Biol Chem. 1998;273:34429–34436. doi: 10.1074/jbc.273.51.34429. [DOI] [PubMed] [Google Scholar]
- 22.Waldo G L, Paterson A, Boyer J L, Nicholas R A, Harden T K. Biochem J. 1996;316:559–568. doi: 10.1042/bj3160559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yasuda H, Lindorfer M A, Myung C-S, Garrison J C. J Biol Chem. 1998;273:21958–21965. doi: 10.1074/jbc.273.34.21958. [DOI] [PubMed] [Google Scholar]
- 24.Myung C-S, Paterson A, Harden T K, Garrison J C. Anal Biochem. 1999;270:303–313. doi: 10.1006/abio.1999.4086. [DOI] [PubMed] [Google Scholar]
- 25.Motulsky H J, Ransnas L A. FASEB J. 1987;1:365–374. [PubMed] [Google Scholar]
- 26.Ford C E, Skiba N P, Bae H, Daaka Y, Reuveny E, Shekter L R, Rosal R, Weng G, Yang C S, Iyengar R, et al. Science. 1998;280:1271–1274. doi: 10.1126/science.280.5367.1271. [DOI] [PubMed] [Google Scholar]
- 27.Li Y, Sternweis P M, Charnecki S, Smith T F, Gilman A G, Neer E J, Kozasa T. J Biol Chem. 1998;273:16265–16272. doi: 10.1074/jbc.273.26.16265. [DOI] [PubMed] [Google Scholar]
- 28.Zhang S, Coso O A, Collins R, Gutkind J S, Simonds W F. J Biol Chem. 1996;271:20208–20212. doi: 10.1074/jbc.271.33.20208. [DOI] [PubMed] [Google Scholar]
- 29.Yamauchi J, Kaziro Y, Itoh H. J Biol Chem. 1997;272:7602–7607. doi: 10.1074/jbc.272.12.7602. [DOI] [PubMed] [Google Scholar]
- 30.Panchenko M P, Saxena K, Li Y, Charnecki S, Sternweis P M, Smith T F, Gilman A G, Kozasa T, Neer E J. J Biol Chem. 1998;273:28298–28304. doi: 10.1074/jbc.273.43.28298. [DOI] [PubMed] [Google Scholar]
- 31.Buck E, Li J, Chen Y, Weng G, Scarlata S, Iyengar R. Science. 1999;283:1332–1335. doi: 10.1126/science.283.5406.1332. [DOI] [PubMed] [Google Scholar]