Abstract
Glucocorticoids play a critical role in control of the cytokine response after immune challenge. Conversely, cytokines modulate glucocorticoid production by the hypothalamic–pituitary–adrenal axis. To define the potency and mechanism of interleukin-6 (IL-6) for augmentation of adrenal function, we exploited mice deficient in corticotropin-releasing hormone (CRH), IL-6, or both. Mice deficient in CRH action demonstrate severely impaired glucocorticoid production in response to psychological and metabolic challenge, but near normal responses to stressors that activate the immune system. In this paper, we demonstrate that IL-6 is essential for activation of the hypothalamic–pituitary–adrenal axis during immunological challenge in the absence of hypothalamic input from CRH. IL-6 receptors are present on pituitary corticotrophs and adrenocortical cells, consistent with the ability of IL-6 to bypass CRH in augmentation of adrenal function. Plasma corticosterone levels after bacterial lipopolysaccharide injection in mice deficient in CRH or IL-6 were significantly lower than in wild-type mice but significantly greater than in mice deficient in both CRH and IL-6. A second model of immune system activation using 2C11, an antibody to the T cell receptor, demonstrated a normal corticosterone response in mice deficient in CRH or IL-6, but a markedly decreased response in mice deficient in both CRH and IL-6. Surprisingly, the relative contribution of IL-6 for modulation of the adrenal response to stress is greater in female than in male mice. This gender-specific difference in IL-6 action in mice suggests the utility of further analysis of IL-6 in determining the female predominance seen in many human inflammatory/autoimmune diseases.
The nervous, endocrine, and immune systems interact to maintain physiological homeostasis during inflammation and stressors that induce systemic cytokine production (1, 2). Corticotropin-releasing hormone (CRH), synthesized in the hypothalamus, is the major secretagogue regulating pituitary adrenocorticotropin (ACTH) release and adrenal glucocorticoid production. CRH also modulates stress-induced autonomic, behavioral, and local inflammatory responses (3–6). The importance of the hypothalamic–pituitary–adrenal (HPA) axis and glucocorticoids in modifying the inflammatory and cytokine response is highlighted by studies in adrenalectomized animals (7–9). Adrenalectomized rodents demonstrate increased mortality after injection of bacterial lipopolysaccharide (LPS), interleukin (IL)-1, or tumor necrosis factor (TNF)-α. With glucocorticoid administration, adrenalectomized mice survive. LPS-injected, adrenalectomized mice also demonstrate higher plasma levels of IL-1 and TNF-α. Elevation of plasma glucocorticoids results in suppression of cytokines such as IL-1, IL-6, and TNF-α and in up-regulation of other cytokines, such as IL-4 and IL-10, and cytokine receptors (1, 2, 7, 8, 10). Thus, by regulation of cytokine production and action, the HPA axis contributes to modulation of the response to inflammation.
Initial studies in CRH-deficient knockout (KO) mice revealed markedly attenuated glucocorticoid production after restraint, ether, and fasting stressors as compared with CRH-intact mice (11, 12). In contrast, inflammatory stress induced by the seaweed polysaccharide, carrageenan, demonstrated that CRH KO male mice are able to significantly increase plasma corticosterone, although to a lesser degree than wild-type (WT) mice (13). Recent characterization of mice deficient in CRH receptor type 1 (Crhr1) (14, 15) has further highlighted the dichotomy in regulation of glucocorticoid production in response to inflammatory versus noninflammatory stressors. For example, plasma corticosterone levels in Crhr1 KO mice were significantly less than those of WT mice after forced-swim stress (15). After local inflammation caused by turpentine injection, however, Crhr1 KO mice significantly increased plasma corticosterone, approaching levels achieved in WT mice (16). The ability of inflammation to significantly augment serum glucocorticoid concentrations independent of CRH action on the pituitary Crhr1 confirms that immune system activation during the inflammatory response has the capacity to stimulate the HPA axis by CRH receptor-independent mechanisms.
Cytokines released during systemic and localized inflammation elicit a number of responses in the host, including fever, diarrhea, anorexia, and, in severe cases, shock (1, 2, 8, 17). Among those cytokines released, IL-6, IL-1, and TNF-α also demonstrate the ability to stimulate the HPA axis. Several reports on the effects of TNF-α on ACTH secretion have shown that the main actions of TNF-α are on the hypothalamus and depend on CRH (18, 19). Similarly, IL-1 stimulation of the HPA axis likely results from stimulation of CRH release (20–22). Further, as demonstrated in IL-1 KO mice, the initial rise in plasma corticosterone after induction of local inflammation (turpentine injection) is not affected by deficiency of IL-1 (23).
Plasma levels of IL-6 have been shown to rise in response to restraint, electrical foot shock, open-field exposure, systemic immune challenge (LPS), and local inflammation (6, 23, 24). Administration of exogenous IL-6 to rats stimulates ACTH and corticosterone production, and s.c. injection of IL-6 into human cancer patients has been shown to elevate plasma ACTH and cortisol levels (25–28). Induction of IL-6 receptor in the hypothalamic paraventricular nucleus in response to inflammation (29) suggests that at least part of the ability of IL-6 to augment pituitary and adrenal function occurs by a CRH-dependent mechanism, although CRH-independent actions of IL-6 on the pituitary and adrenal also may occur.
Because IL-6 is able to stimulate the HPA axis and perhaps ACTH release directly, we hypothesized that IL-6 is the molecule primarily responsible for augmenting corticotroph and adrenal function in the absence of CRH action on the Crhr1. To conclusively test this hypothesis, we generated mice deficient in both CRH and IL-6 (CRH KO/IL-6 KO) and analyzed their pituitary–adrenal axis responses to both stressors that predominantly activate the immune system and to noninflammatory stressors.
Materials and Methods
Animal Husbandry.
All mouse protocols were in accordance with National Institutes of Health guidelines and were approved by the Animal Care and Use Committee of Washington University School of Medicine, St. Louis. Mice were housed on a 12-h/12-h light/dark cycle with ad libitum access to rodent chow. All mice used were 8–16 weeks old and of a C57BL/6 × 129/Sv genetic background. IL-6 KO (30) mice were obtained from The Jackson Laboratory and were mated to CRH KO mice (31). Mice used as controls were the littermates of CRH KO, IL-6 KO, and CRH KO/IL-6 KO mice. Mice labeled as “WT” included those heterozygous or WT at the IL-6 locus and heterozygous or WT at the CRH locus. The doubly heterozygous mice demonstrated a corticosterone response to stress that did not differ from that in mice WT for both alleles (data not shown). Mice were singly housed 48 h before experiments. Blood was obtained by rapid retroorbital phlebotomy and collected in heparinized capillary tubes with total animal handling time <1 min. Plasma was separated by centrifugation and stored at −80°C until assay.
Hormone Assays.
Plasma concentration of corticosterone (ICN) or ACTH (Diasorin, Stillwater, MN) was determined by RIA as described previously (32). Plasma concentration of IL-6 (PharMingen) was measured by ELISA per the manufacturer's instructions.
In Vivo Assays.
IL-6 stimulation.
Recombinant mouse IL-6 (Peninsula Laboratories) was diluted in PBS with 1% BSA. Male mice were injected with 50 ng of IL-6 or vehicle at 08:00 h and blood was collected for plasma ACTH 30 min after injection. Noninjected mice also were bled at 08:00 h for basal ACTH measurement. Our pilot studies evaluating ACTH response at 15, 30, and 60 min demonstrated a peak response to IL-6 at 30 min after injection (data not shown). Each group consisted of three to four mice.
ACTH stimulation test.
Cortrosyn (Organon) was reconstituted in 1 ml of normal saline. The Cortrosyn solution was then diluted 1:250 in PBS, and 10 μg/kg was injected i.p. into each mouse at 08:00 h and blood was collected 60 min later. Each group consisted of five to six female mice.
LPS.
LPS from Escherichia coli serotype 0111:B4 (Sigma) was dissolved at 1 mg/ml in PBS and filter-sterilized. LPS (300 μg) or PBS was injected at 08:00 h, and blood was collected 3 h later. Each group consisted of four to five mice. No difference in plasma IL-6 concentration was found when comparing male and female mice of a given genotype, so genders were grouped for statistical analysis.
T cell receptor antibody (2C11).
No azide/low endotoxin 2C11 (100 μg; PharMingen) or PBS was injected at midnight and blood was collected at 08:00 h. Each group consisted of three to six male mice.
Restraint stress.
Restraint was performed in a 50-ml centrifuge tube with an opening at the cephalic end at 08:00 h for 30 min, after which blood was immediately collected. Each group consisted of three to five mice.
Immunohistochemistry.
The pituitaries from mice injected with LPS were fixed by immersion in 4% paraformaldehyde in PBS for 24 h at 4°C. Samples then were cryopreserved in 10% sucrose in PBS and embedded in OCT compound (Sakura Finetek, Torrance, CA). Ten-micrometer sections were thaw-mounted onto Superfrost-plus slides (Fisher Scientific) and incubated with antibodies specific for IL-6 receptor and ACTH. Rabbit anti-IL-6 receptor-α antibody (Santa Cruz Biotechnology) was used at a 1:5,000 dilution. Sheep anti-rabbit IgG-rhodamine secondary antibody (Boehringer Mannheim) was used at a 1:10 dilution. A guinea pig anti-ACTH (human) antibody was visualized with a fluorescein-conjugated goat secondary antibody (Peninsula Laboratories) according to the manufacturer's instructions.
Adrenal Histology.
Adrenals from female WT, CRH KO, IL-6 KO, and CRH KO/IL-6 KO mice were isolated and fixed in 4% paraformaldehyde in PBS for 24 h at 4°C. After embedding in paraffin, 10-μm sections were mounted onto Superfrost-plus slides. Adrenal sections were deparaffinized in xylene and rehydrated through graded ethanol washes, and then either stained with hematoxylin/eosin or subjected to in situ hybridization.
In Situ Hybridization.
In situ hybridization using an α-33P-UTP-labeled probe for IL-6 receptor was performed following a modification of the procedure of Simmons et al. (33). The radiolabeled probe was prepared by incubating 2 μg of EcoRI-linearized mouse IL-6 receptor template (Genome Systems; GenBank accession no. AA712092) with T3 RNA polymerase as previously described (34). Adrenal sections were hybridized to the IL-6 receptor probe in a humidified chamber at 56°C for 20 h. After washing and vacuum drying, slides were exposed to Hyperfilm β-max (Amersham Pharmacia) for 1 week at room temperature.
Statistical Methods.
All results are expressed as mean ± SEM. Statistical analysis was by ANOVA, with P ≤ 0.05 considered significant. Significant differences in plasma IL-6 after LPS were determined by Wilcoxon rank sum.
Results
IL-6 Activation of the HPA Axis and Localization of IL-6 Receptor to Corticotrophs and Adrenal Cortex.
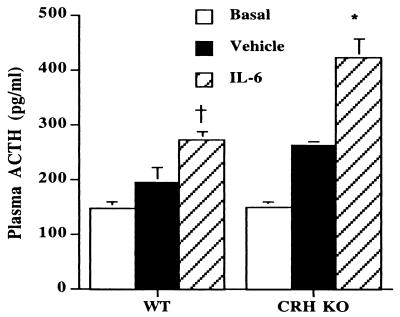
To determine whether IL-6 has the ability to stimulate ACTH secretion in a CRH-independent fashion, we injected IL-6 into WT and CRH KO mice and measured plasma ACTH levels (Fig. 1). At 30 min after injection of IL-6, plasma ACTH increased to 272 ± 14 pg/ml in WT mice, as compared with 193 ± 28 pg/ml for vehicle-injected WT mice (P < 0.05). CRH KO mice demonstrated an even further augmentation of ACTH release, increasing to 421 ± 36 pg/ml after IL-6 administration, in contrast to 261 ± 8 pg/ml after vehicle (P < 0.01 vs. both WT IL-6 and CRH KO vehicle). Despite the significant increase in ACTH, little change in plasma corticosterone was found in CRH KO mice after acute IL-6 injection (data not shown).
Figure 1.
Stimulation of plasma ACTH after IL-6 injection in WT and CRH KO mice. WT and CRH KO mice were injected with vehicle or IL-6 (n = 3–4). Noninjected mice that were bled at 08:00 for basal ACTH also are shown. *, P < 0.01 vs. WT IL-6, CRH KO vehicle, and CRH KO basal; †, P < 0.05 vs. WT vehicle and WT basal.
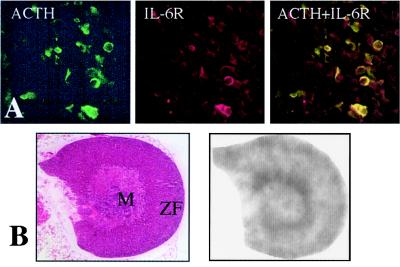
If IL-6 stimulates ACTH secretion directly, we reasoned that IL-6 receptor should be present on ACTH-producing cells in the anterior pituitary. Employing antibodies specific for ACTH and IL-6 receptor, we confirmed that most corticotrophs exhibit IL-6 receptor immunoreactivity (Fig. 2A). If IL-6 also has the capacity to stimulate the adrenal directly, IL-6 receptor should be expressed in the adrenal cortex. By using a riboprobe specific for mouse IL-6 receptor mRNA, we found IL-6 receptor expressed in the adrenal cortex of WT mice (Fig. 2B). Increased hybridization signal intensity occurs in the steroidogenic cells near the junction of the medulla and cortex, and in the mineralocorticoid-synthesizing adrenal glomerulosa cells, in accord with previous localization studies in the human adrenal (35). Similar results were seen with the IL-6 KO, CRH KO, and CRH KO/IL-6 KO mice (data not shown).
Figure 2.
Localization of IL-6 receptor in pituitary and adrenal. (A) IL-6 receptor colocalizes with ACTH to pituitary corticotrophs. Pituitaries from WT mice were stained with immunofluorescently labeled antibodies. Staining specific for ACTH (green) is shown (Left) or IL-6 receptor (IL-6R) (red) is shown (Middle). Colocalization of both ACTH and IL-6 receptor is indicated by yellow cells (Right). (B) IL-6 receptor is located in mouse adrenal cortex. (Left) A hematoxylin/eosin stained section of WT mouse adrenal is shown. (Right) Autoradiogram of an adjacent adrenal section subjected to in situ hybridization with a probe for mouse IL-6 receptor is shown. M, medulla; ZF, zona fasciculata.
Plasma Corticosterone After Acute Restraint Stress.
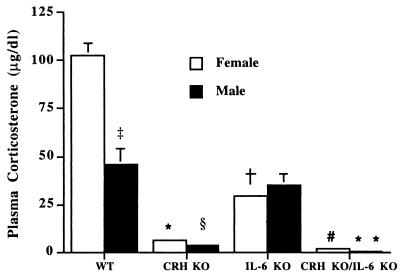
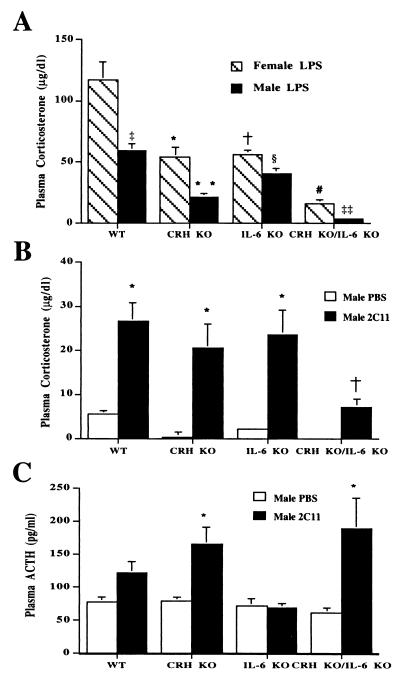
To determine whether IL-6 is important in stimulating the HPA axis during stressors not associated with immune cell activation, WT, CRH KO, IL-6 KO, and CRH KO/IL-6 KO mice were restrained for 30 min followed by measurement of plasma corticosterone (Fig. 3). After restraint stress, female and male WT mice increased plasma corticosterone to 103 ± 6 and 46 ± 8 μg/dl, respectively. In contrast, female and male CRH KO/IL-6 KO mice achieved plasma corticosterone concentrations of only 2.2 ± 1.1 and 0.8 ± 0.6 μg/dl, respectively (P < 0.001 vs. WT). CRH KO/IL-6 KO mice also significantly differed (P < 0.05) from corresponding CRH KO female and male mice, which elevated their plasma corticosterone to 6.3 ± 1.2 and 3.7 ± 0.6 μg/dl, respectively. Surprisingly, in female IL-6 KO mice, the plasma corticosterone level of 29.3 ± 0.7 μg/dl also significantly differed (P < 0.001) from female WT mice. In males, plasma corticosterone did not differ between IL-6 KO and WT mice.
Figure 3.
Plasma corticosterone response to restraint stress in female and male WT, CRH KO, IL-6 KO, and CRH KO/IL-6 KO mice (n = 3–5). Females: *, CRH KO, P < 0.001 vs. WT, P < 0.001 vs. IL-6 KO; †, IL-6 KO, P < 0.001 vs. WT; #, CRH KO/IL-6 KO, P < 0.001 vs. WT, P < 0.05 vs. CRH KO, P < 0.001 vs. IL-6 KO. Males: §, CRH KO, P < 0.01 vs. WT, P < 0.01 vs. IL-6 KO; **, CRH KO/IL-6 KO, P < 0.001 vs. WT, P < 0.05 vs. CRH KO, P < 0.001 vs. IL-6 KO. ‡, P < 0.01 WT males vs. WT females.
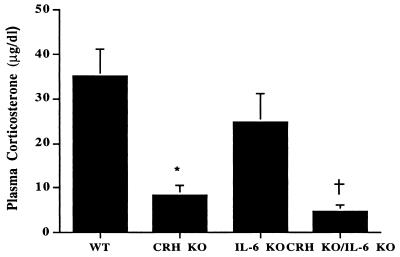
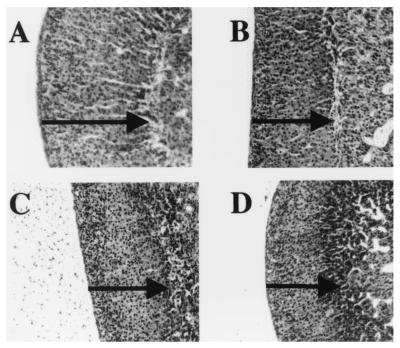
The differences observed in adrenal responses between CRH KO and CRH KO/IL-6 KO mice could result from the absence of acute IL-6 actions or long-term changes in adrenal function secondary to chronic IL-6 deficiency. To differentiate between these possibilities, we analyzed the response of the adrenal to administration of synthetic ACTH (Cortrosyn) in female mice of each genotype. Plasma corticosterone after ACTH injection did not significantly differ between CRH KO and CRH KO/IL-6 KO mice, which were each significantly less than WT or IL-6 KO mice (Fig. 4). Consistent with the ACTH-stimulation testing results, the size of the adrenal cortex as revealed by hematoxylin/eosin-stained sections also appeared similar in CRH KO and CRH KO/IL-6 KO mice (Fig. 5).
Figure 4.
Plasma corticosterone concentration after injection of synthetic ACTH into female WT, CRH KO, IL-6 KO, and CRH KO/IL-6 KO mice. *, CRH KO, P < 0.01 vs. WT, P = 0.05 vs. IL-6 KO, P = 0.18 vs. CRH KO/IL-6 KO. †, CRH KO/IL-6 KO, P < 0.01 vs. WT, P = 0.01 vs. IL-6 KO.
Figure 5.
Adrenal histology in mice with CRH and IL-6 deficiency. Hematoxylin/eosin-stained sections of adrenal from female WT (A), IL-6 KO (B), CRH KO (C) and CRH KO/IL-6 KO (D) mice are shown. The thickness of the adrenal cortex in each section is indicated by the solid line.
Plasma Corticosterone After LPS-Induced Immune Challenge.
To determine whether IL-6 is important for activation of the HPA axis after immune system stimulation, WT, CRH KO, IL-6 KO, and CRH KO/IL-6 KO mice were injected with LPS (Fig. 6A). The plasma corticosterone levels from female IL-6 KO mice and CRH KO mice were similar and half that of the injected WT female mice (IL-6 KO and CRH KO, 54.0 ± 7.8 and 56.0 ± 3.9 μg/dl, respectively, vs. WT 117 ± 15 μg/dl; P < 0.01). Plasma corticosterone levels from the female CRH KO/IL-6 KO mice were significantly lower than CRH or IL-6 KO mice (CRH KO/IL-6 KO, 16 ± 3.2 μg/dl vs. CRH KO or IL-6 KO; P < 0.01). Plasma corticosterone levels from male IL-6 KO mice were less than those in WT male mice and significantly higher than those in CRH KO male mice (IL-6 KO, 41 ± 4 μg/dl vs. WT, 59 ± 6.1 μg/dl; P = 0.05; CRH KO, 21.3 ± 3.2 μg/dl vs. IL-6 KO; P < 0.01). Plasma corticosterone levels in male CRH KO mice were significantly greater than those in CRH KO/IL-6 KO male mice (CRH KO/IL-6 KO, 3.8 ± 0.7 μg/dl; P < 0.01 vs. CRH KO).
Figure 6.
Plasma corticosterone and ACTH response to immune challenge. (A) Female and male WT, CRH KO, IL-6 KO, and CRH KO/IL-6 KO mice (n = 4–5) after injection with LPS. Females: *, CRH KO, P < 0.01 vs. WT; †, IL-6 KO, P < 0.01 vs. WT; #, CRH KO/IL-6 KO, P < 0.001 vs. WT, P < 0.01 vs. CRH KO, P < 0.001 vs. IL-6 KO. Males: **, CRH KO, P < 0.01 vs. WT, P < 0.01 vs. IL-6 KO; §, IL-6 KO, P = 0.05 vs. WT; ‡‡, CRH KO/IL-6 KO, P < 0.001 vs. WT, P < 0.001 vs. CRH KO, P < 0.001 vs. IL-6 KO; ‡, P = 0.01 WT females vs. WT males. (B) Male WT, CRH KO, IL-6 KO, and CRH KO/IL-6 KO mice (n = 3–6) after injection with PBS or 2C11. *, P < 0.01 vs. PBS of same genotype; †, P < 0.01 vs. PBS of same genotype and all other genotypes injected with 2C11. (C) Plasma ACTH after 2C11 or PBS injection in male WT, CRH KO, IL-6 KO, and CRH KO/IL-6 KO mice (n = 3–6). *, P < 0.05 vs. PBS of same genotype.
Plasma Corticosterone and ACTH After 2C11-Induced Immune Activation.
To further investigate the pituitary and adrenal function in the CRH KO/IL-6 KO mice, we used a second immune system stimulus with high-affinity engagement and activation of the T cell receptor by an antibody to its CD3ɛ chain (2C11). 2C11, in addition to promoting apoptosis of CD4+/CD8+ cells in the thymus, initiates a systemic cytokine response (36, 37). After injection of 2C11, a significant increase in plasma corticosterone was observed in all genotypes in comparison to vehicle-injected controls (Fig. 6B). The 2C11-stimulated plasma corticosterone levels did not differ between WT mice, CRH KO mice, or IL-6 KO mice (WT, 27 ± 4.2 μg/dl; CRH KO, 21 ± 5.6 μg/dl; IL-6 KO, 24 ± 5.4 μg/dl). In contrast, the plasma corticosterone concentration of CRH KO/IL-6 KO mice after 2C11 was 7.2 ± 1.7 μg/dl, significantly lower than that of WT, IL-6 KO, and CRH KO mice (P < 0.01 vs. all other groups). After injection of 2C11, there was also a significant rise in plasma ACTH in CRH KO and CRH KO/IL-6 KO mice as compared with PBS-injected controls of the same genotype (Fig. 6C), but not in the WT or IL-6 KO mice. The failure of CRH-intact mice to significantly augment plasma ACTH likely reflects more efficient glucocorticoid secretion with rapid glucocorticoid-mediated feedback at the level of the pituitary in light of the ACTH stimulation results (Fig. 4) and previous circadian studies (38).
Plasma IL-6 After Acute Restraint and Immune Activation.
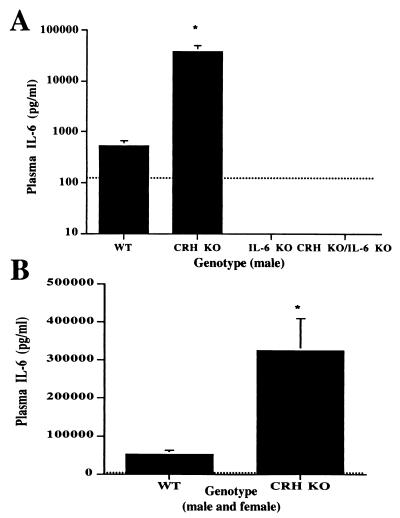
The significant reduction in plasma corticosterone in CRH KO/IL-6 KO mice as compared with CRH KO mice suggests that IL-6 is the primary activator of the HPA axis during stimulation of the immune system in the context of CRH deficiency. In CRH KO mice, activation of the HPA axis by IL-6 during immune system activation may be achieved by an exaggerated increase in IL-6 relative to WT mice because of impaired glucocorticoid secretion. In accord with this hypothesis, plasma IL-6 levels after 2C11 injection in CRH KO mice were 50- to 100-fold higher than in WT mice (Fig. 7A) (P < 0.01). Similarly, plasma IL-6 after injection of LPS was 5- to 10-fold higher in the CRH KO mice as compared with WT mice (Fig. 7B) (P < 0.01). Plasma IL-6 levels did not differ between male and female mice and were below the lower limit of detection for all groups after PBS injection or restraint stress (data not shown). Further confirming assay specificity, plasma IL-6 levels from the IL-6 KO and CRH KO/IL-6 KO mice were below the lower limits of detection after LPS or 2C11 injection.
Figure 7.
Plasma IL-6 levels after immune system stimulation in CRH KO and WT mice. The dotted line on each graph indicates the limit of assay sensitivity. All PBS-injected mice of each genotype (n = 3) were below the lower limit of detection. (A) Plasma IL-6 after 2C11 in male mice (n = 3–4). *, P < 0.01 vs. WT. (B) Plasma IL-6 from male and female mice after LPS injection (n = 6–9). *, P < 0.01 vs. WT.
Discussion
In this report, we demonstrate that IL-6 plays a critical role in augmentation of adrenal function in response to immune system activation, which becomes most apparent in the context of CRH deficiency. The i.p. administration of IL-6 results in a significant rise of plasma ACTH in both WT and CRH KO mice. IL-6-injected CRH KO mice exhibit a poor corticosterone response to the elevated ACTH, consistent with previous ACTH stimulation data in CRH KO mice (38). This impaired glucocorticoid feedback caused by adrenal atrophy likely results in the significantly higher plasma ACTH levels in CRH KO mice after IL-6 administration. To address whether IL-6 is capable of directly stimulating corticotrophs to release ACTH, we used immunofluorescence to colocalize ACTH and IL-6 receptor in the pituitary. Our demonstration of IL-6 receptors on corticotrophs suggests that one important site for IL-6 action on the HPA axis is on the corticotroph. Additionally, the ability of IL-6 to directly promote corticosterone release at the level of the adrenal is suggested by the localization of IL-6 receptors to adrenocortical cells and the markedly impaired corticosterone response to 2C11 injection in CRH KO/IL-6 KO mice in the context of plasma ACTH levels that did not differ from CRH KO mice. These findings do not exclude a role for IL-6 in activation of CRH release in WT mice or augmentation of secretion of other hypothalamic ACTH secretagogues such as vasopressin.
To investigate the contribution of IL-6 in stimulation of the HPA axis in the absence of CRH, CRH KO/IL-6 KO mice were exposed to restraint stress or injected with LPS or 2C11. Surprisingly, IL-6 plays an important role in stimulating the HPA axis during restraint stress. This effect is not simply the result of exaggerated adrenal atrophy caused by the combination of chronic CRH and IL-6 deficiency, because the response of the adrenal to exogenous ACTH and the overall histologic appearance of the adrenal did not differ between CRH KO and combined CRH KO/IL-6 KO mice. The different responses of the male and female mice suggest that IL-6, in the presence of CRH, is important in stimulating the HPA axis during this acute psychological stress in female but not in male mice. We found that IL-6 also plays a significant role in stimulating the HPA axis during systemic immune cell stimulation elicited by LPS in the absence of CRH. In contrast to Fattori et al. (39), our data show that IL-6 KO mice do not have a normal rise in plasma corticosterone after LPS injection. This finding may be explained by the fact that we used a larger dose of LPS and measured corticosterone later after injection. At the larger dose of LPS we used, IL-6 makes a significant contribution to stimulating the HPA axis, even in the presence of CRH. In addition, IL-6 in the presence of CRH seems to make a larger contribution stimulating the HPA axis in female mice during both restraint stress and systemic immune activation as compared with male mice. Further evidence that in the absence of CRH, IL-6 accounts for the major portion of HPA axis stimulation during a systemic immune response is seen in the greatly reduced corticosterone rise after 2C11 injection in CRH KO/IL-6 KO mice. CRH KO mice have a robust corticosterone response, although slightly reduced as compared with that of WT mice, to immune system activation at the expense of generating markedly increased levels of IL-6. It is likely that prolonged corticotroph stimulation by sustained, high levels of IL-6 are required to allow adequate recovery of previously atrophic adrenals as suggested by the failure of acute administration of IL-6 to significantly increase plasma corticosterone in CRH KO mice.
Female mice, in general, achieve higher corticosterone levels than do male mice in response to stress (6). CRH KO mice also demonstrate this sexual dichotomy in response to stress, indicating a mechanism independent of CRH (12, 38). In addition, female IL-6 KO mice show an impaired corticosterone response to both restraint and LPS-induced stress, whereas male IL-6 KO mice achieve plasma corticosterone levels that are similar to WT. This difference demonstrates a role for IL-6 in the dimorphic adrenal responses between sexes. Many autoimmune diseases have been found to exhibit a significant sexual dimorphism, including autoimmune thyroiditis, systemic lupus erythematous, and rheumatoid arthritis. In addition, autoimmune diseases change in severity during puberty, menses, pregnancy, menopause, and times of stress (40). These autoimmune diseases are associated with dysregulated production of IL-6 in humans (41–43) and impaired activation of the HPA axis in both humans (44) and animal models (1). Taken together with our data, which demonstrate an important, gender-specific role for IL-6 in modulation of the HPA axis and increased IL-6 production with impaired HPA function, further support is provided for the reciprocal interaction of endocrine and immune systems in the pathogenesis of these disorders.
Acknowledgments
We thank Dr. J. A. Majzoub for manuscript review and members of the Muglia laboratory for helpful discussions during the course of these studies. This work was supported by the Howard Hughes Medical Institutes, a Burroughs Wellcome Fund Career Development Award, and National Institutes of Health Grant DK02270 to L.J.M.
Abbreviations
- ACTH
adrenocorticotropin
- CRH
corticotropin-releasing hormone
- Crhr1
corticotropin-releasing hormone receptor type 1
- HPA
hypothalamic–pituitary–adrenal
- TNF
tumor necrosis factor
- LPS
lipopolysaccharide
- KO
knockout
- WT
wild type
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Sternberg E M. J Clin Invest. 1997;100:2641–2647. doi: 10.1172/JCI119807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chrousos G P. N Engl J Med. 1995;332:1351–1362. doi: 10.1056/NEJM199505183322008. [DOI] [PubMed] [Google Scholar]
- 3.Chrousos G P, Gold P W. J Am Med Assoc. 1992;267:1244–1252. [PubMed] [Google Scholar]
- 4.Givalois L, Dornand J, Mekaouche M, Solier M D, Bristow A F, Ixart G, Siaud P, Assenmacher I, Barbanel G. Am J Physiol. 1994;267:R164–R170. doi: 10.1152/ajpregu.1994.267.1.R164. [DOI] [PubMed] [Google Scholar]
- 5.Karalis K, Muglia L J, Bae D, Hilderbrand H, Majzoub J A. J Neuroimmunol. 1997;72:131–136. doi: 10.1016/s0165-5728(96)00178-6. [DOI] [PubMed] [Google Scholar]
- 6.Wilder R L. Annu Rev Immunol. 1995;13:307–338. doi: 10.1146/annurev.iy.13.040195.001515. [DOI] [PubMed] [Google Scholar]
- 7.Bertini R, Bianchi M, Ghezzi P. J Exp Med. 1988;167:1708–1712. doi: 10.1084/jem.167.5.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kapcala L P, Chautard T, Eskay R L. Ann N Y Acad Sci. 1995;771:419–437. doi: 10.1111/j.1749-6632.1995.tb44699.x. [DOI] [PubMed] [Google Scholar]
- 9.Butler L D, Layman N K, Riedl P E, Cain R L, Shellhaas J, Evans G F, Zuckerman S H. J Neuroimmunol. 1989;24:143–153. doi: 10.1016/0165-5728(89)90108-2. [DOI] [PubMed] [Google Scholar]
- 10.Munck A, Naray-Fejes-Toth A. Mol Cell Endocrinol. 1992;90:C1–C4. doi: 10.1016/0303-7207(92)90091-j. [DOI] [PubMed] [Google Scholar]
- 11.Jacobson L, Muglia L, Weninger S, Pacak K, Majzoub J. Neuroendocrinology. 2000;71:79–87. doi: 10.1159/000054524. [DOI] [PubMed] [Google Scholar]
- 12.Muglia L, Jacobson L, Dikkes P, Majzoub J. Nature (London) 1995;373:427–432. doi: 10.1038/373427a0. [DOI] [PubMed] [Google Scholar]
- 13.Karalis K P, Kontopoulos E, Muglia L J, Majzoub J A. Proc Natl Acad Sci USA. 1999;96:7093–7097. doi: 10.1073/pnas.96.12.7093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith G W, Aubry J M, Dellu F, Contarino A, Bilezikjian L M, Gold L H, Chen R, Marchuk Y, Hauser C, Bentley C A, et al. Neuron. 1998;20:1093–1102. doi: 10.1016/s0896-6273(00)80491-2. [DOI] [PubMed] [Google Scholar]
- 15.Timpl P, Spanagel R, Sillaber I, Kresse A, Reul J M, Stalla G K, Blanquet V, Steckler T, Holsboer F, Wurst W. Nat Gen. 1998;19:162–166. doi: 10.1038/520. [DOI] [PubMed] [Google Scholar]
- 16.Turnbull A V, Smith G W, Lee S, Vale W W, Lee K F, Rivier C. Endocrinology. 1999;140:1013–1017. doi: 10.1210/endo.140.2.6675. [DOI] [PubMed] [Google Scholar]
- 17.Cerami A. Clin Immunol Immunopathol. 1992;62:S3–S10. doi: 10.1016/0090-1229(92)90035-m. [DOI] [PubMed] [Google Scholar]
- 18.Bernardini R, Kamilaris T C, Calogero A E, Johnson E O, Gomez M T, Gold P W, Chrousos G P. Endocrinology. 1990;126:2876–2881. doi: 10.1210/endo-126-6-2876. [DOI] [PubMed] [Google Scholar]
- 19.Watanobe H, Takebe K. Neuropeptides. 1992;22:81–84. doi: 10.1016/0143-4179(92)90058-5. [DOI] [PubMed] [Google Scholar]
- 20.Turnbull A V, Rivier C L. Physiol Rev. 1999;79:1–71. doi: 10.1152/physrev.1999.79.1.1. [DOI] [PubMed] [Google Scholar]
- 21.Sapolsky R, Rivier C, Yamamoto G, Plotsky P, Vale W. Science. 1987;238:522–524. doi: 10.1126/science.2821621. [DOI] [PubMed] [Google Scholar]
- 22.Berkenbosch F, de Goeij D E, Rey A D, Besedovsky H O. Neuroendocrinology. 1989;50:570–576. doi: 10.1159/000125283. [DOI] [PubMed] [Google Scholar]
- 23.Horai R, Asano M, Sudo K, Kanuka H, Suzuki M, Nishihara M, Takahashi M, Iwakura Y. J Exp Med. 1998;187:1463–1475. doi: 10.1084/jem.187.9.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barton B E. Clin Immunol Immunopathol. 1997;85:16–20. doi: 10.1006/clin.1997.4420. [DOI] [PubMed] [Google Scholar]
- 25.Naitoh Y, Fukata J, Tominaga T, Nakai Y, Tamai S, Mori K, Imura H. Biochem Biophys Res Commun. 1988;155:1459–1463. doi: 10.1016/s0006-291x(88)81305-6. [DOI] [PubMed] [Google Scholar]
- 26.Lyson K, McCann S M. Neuroendocrinology. 1991;54:262–266. doi: 10.1159/000125884. [DOI] [PubMed] [Google Scholar]
- 27.Mastorakos G, Chrousos G P, Weber J S. J Clin Endocrinol Metab. 1993;77:1690–1694. doi: 10.1210/jcem.77.6.8263159. [DOI] [PubMed] [Google Scholar]
- 28.Lenczowski M J, Van Dam A M, Poole S, Larrick J W, Tilders F J. Am J Physiol. 1997;273:R1870–R1877. doi: 10.1152/ajpregu.1997.273.6.R1870. [DOI] [PubMed] [Google Scholar]
- 29.Vallieres L, Rivest S. Endocrinology. 1999;140:3890–3903. doi: 10.1210/endo.140.9.6983. [DOI] [PubMed] [Google Scholar]
- 30.Kopf M, Baumann H, Freer G, Freudenberg M, Lamers M, Kishimoto T, Zinkernagel R, Bluethmann H, Kohler G. Nature (London) 1994;368:339–342. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- 31.Muglia L J, Jenkins N A, Gilbert D J, Copeland N G, Majzoub J A. J Clin Invest. 1994;93:2066–2072. doi: 10.1172/JCI117201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jacobson L, Zurakowski D, Majzoub J A. Endocrinology. 1997;138:1048–1057. doi: 10.1210/endo.138.3.5011. [DOI] [PubMed] [Google Scholar]
- 33.Simmons D M, Arriza J L, Swanson L W. J Histotechnol. 1989;12:169–181. [Google Scholar]
- 34.Muglia L M, Schaefer M L, Vogt S K, Gurtner G, Imamura A, Muglia L J. J Neurosci. 1999;19:2051–2058. doi: 10.1523/JNEUROSCI.19-06-02051.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Path G, Bornstein S R, Ehrhart-Bornstein M, Scherbaum W A. J Clin Endocrinol Metab. 1997;82:2343–2349. doi: 10.1210/jcem.82.7.4072. [DOI] [PubMed] [Google Scholar]
- 36.Donckier V, Flament V, Gerard C, Abramowicz D, Vandenabeele P, Wissing M, Delvaux A, Fiers W, Leo O, Velu T, et al. Transplantation. 1994;57:1436–1439. [PubMed] [Google Scholar]
- 37.Alegre M L, Vandenabeele P, Depierreux M, Florquin S, Deschodt-Lanckman M, Flamand V, Moser M, Leo O, Urbain J, Fiers W, et al. J Immunol. 1991;146:1184–1191. [PubMed] [Google Scholar]
- 38.Muglia L J, Jacobson L, Weninger S C, Luedke C E, Bae D S, Jeong K-H, Majzoub J A. J Clin Invest. 1997;99:2923–2929. doi: 10.1172/JCI119487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fattori E, Cappelletti M, Costa P, Sellitto C, Cantoni L, Carelli M, Faggioni R, Fantuzzi G, Ghezzi P, Poli V. J Exp Med. 1994;180:1243–1250. doi: 10.1084/jem.180.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whitacre C C, Reingold S C, O'Looney P A. Science. 1999;283:1277–1278. doi: 10.1126/science.283.5406.1277. [DOI] [PubMed] [Google Scholar]
- 41.al-Janadi M, Raziuddin S. J Rheumatol. 1993;20:1885–1891. [PubMed] [Google Scholar]
- 42.Fishman D, Faulds G, Jeffery R, Mohamed-Ali V, Yudkin J S, Humphries S, Woo P. J Clin Invest. 1998;102:1369–1376. doi: 10.1172/JCI2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Papanicolaou D A, Wilder R L, Manolagas S C, Chrousos G P. Ann Intern Med. 1998;128:127–137. doi: 10.7326/0003-4819-128-2-199801150-00009. [DOI] [PubMed] [Google Scholar]
- 44.Cutolo M, Foppiani L, Prete C, Ballarino P, Sulli A, Villagio B, Seriolo B, Giusti M, Accardo S. J Rheumatol. 1999;26:282–288. [PubMed] [Google Scholar]