Abstract
The cellular and molecular bases allowing tissue regeneration are not well understood. By performing gain- and loss-of-function experiments of specific members of the Wnt pathway during appendage regeneration, we demonstrate that this pathway is not only necessary for regeneration to occur, but it is also able to promote regeneration in axolotl, Xenopus, and zebrafish. Furthermore, we show that changes in the spatiotemporal distribution of β-catenin in the developing chick embryo elicit apical ectodermal ridge and limb regeneration in an organism previously thought not to regenerate. Our studies may provide valuable insights toward a better understanding of adult tissue regeneration.
Keywords: Wnt, regeneration, limb, apical ectodermal cap, p63
Regeneration is a complex biological process by which animals restore shape, structure, and function of body parts lost to injury or experimentally amputated. In this regard, regeneration of vertebrate appendages has been one of the most extensively studied model systems (Tsonis 2000; Brockes and Kumar 2002). Urodeles and zebrafish are among the vertebrate species that retain a significant limb regenerative ability during adulthood. In these species, limb regeneration proceeds by the formation of a growth zone or blastema at the end of the stump. For regeneration to occur, this event needs to be preceded by the formation of the apical ectodermal cap (AEC), the multilayered epithelia that covers the wound surface after amputation (Bryant et al. 2002; Poss et al. 2003). During the last decade, and in part with the help of the knowledge gathered during the embryogenesis of the vertebrate limb, some of the molecular and cellular processes involved in AEC and blastema formation have been unveiled (Capdevila and Izpisua Belmonte 2001; Poss et al. 2003). Members of the Wnt and bone morphogenetic protein (BMP) signaling pathways have been shown to be required in vertebrates for the formation of the apical ectodermal ridge (AER), a pseudostratified epithelia that, like the regenerating AEC, is required for the proliferation of mesenchymal cells, and therefore for normal limb development. Here we show that reduction in Wnt and BMP signaling during limb regeneration in axolotls, Xenopus laevis, and zebrafish induce alterations in the formation of the AEC that prevent normal fin/limb regeneration. More importantly, by performing gain of function experiments of the Wnt/β-catenin pathway during appendage regeneration, we demonstrate that this pathway promotes Xenopus and zebrafish limb/fin regeneration. The ability of this pathway to promote regeneration is not only restricted to normally regenerating organisms, since activation of Wnt signaling during limb development in the chick embryo enables regeneration of the AER. While obviously not identical processes, the similarities encountered in the molecular and cellular processes involved during limb embryogenesis and limb regeneration suggest a mechanism whereby variations in the concentration and/or spatiotemporal distribution of developmental regulators may allow regeneration to occur.
Results and Discussion
Even though the Wnt signaling pathway has been shown to have a role in the regenerating zebrafish caudal fin (Poss et al. 2000a), the urodele tail (Caubit et al. 1997), and hydra head (Bode 2003), a direct demonstration for any of the pathway components in altering or eliciting vertebrate tissue regeneration is still lacking. To test a direct participation of this pathway in vertebrate regeneration, we developed an adenovirus-mediated protocol (Supplementary Fig. S1; Materials and Methods) that allowed us to perform gain- and loss-of-function experiments of specific members of the Wnt pathway during axolotl, Xenopus, and zebrafish regeneration.
After amputation, axolotls have the ability to regenerate their entire appendages, thus making them one of the most studied model systems in tissue regeneration (Bryant et al. 2002; Tanaka 2003; Brockes and Kumar 2005). To determine the involvement of the Wnt pathway in this process, we microinjected an adenoviral vector engineered to express Axin1 (an intracellular inhibitor of the Wnt pathway), Ad-Axin1 (Kawakami et al. 2001) during forelimb regeneration. This treatment led to a decrease in the regeneration capacity of the infected animals and resulted in spike-like regenerated limbs lacking all of the digits (n = 5, 60%) (Fig. 1A,B). The alterations in the regeneration capacity after Wnt down-regulation were not restricted to the adult limb but were also observed in the embryo (data not shown) and larvae (Fig. 1C,D), since infection of amputated, developing limbs with an adenovirus, Ad-Dkk1, harboring another inhibitor of the Wnt pathway, Dkk1 (Glinka et al. 1998), showed similar regeneration defects as those observed in adult infected regenerating limbs (Supplementary Table S1).
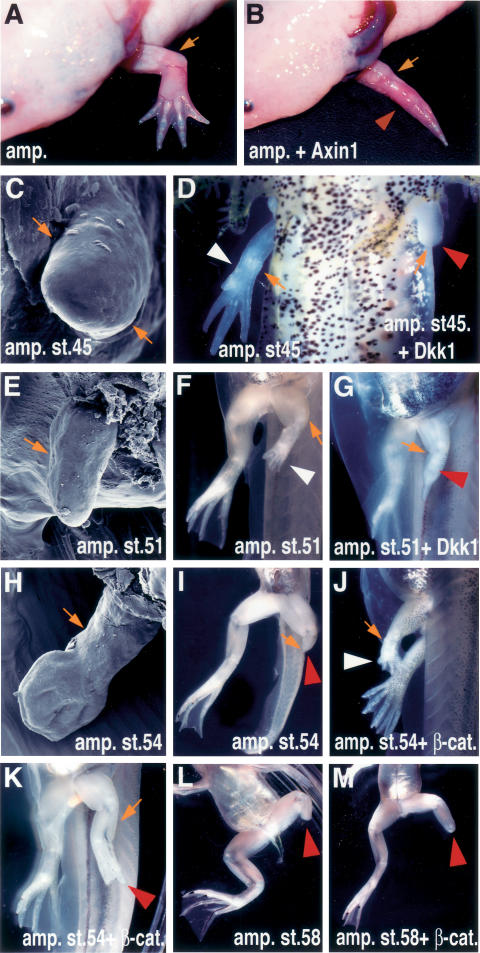
Figure 1.
Wnt/β-catenin signaling is required for limb regeneration in axolotls and Xenopus. (A–D) Gross morphology and scanning electron microscope (SEM) images of adult (A,B) and larvae (C,D) axolotls. (A) A fully regenerated wild-type adult axolotl forelimb develops 2 mo after amputation at the elbow level. (B) Microinjection of Ad-Axin1 after forelimb amputation prevented regeneration of the distal elements (red arrowhead). (C) SEM image of an axolotl larvae limb at the stage at which amputation was performed (stage 45). (D) Ventral view of axolotl larvae limbs 1 mo after amputation and virus injection. The right amputated limb regenerated normally (reader’s left side, white arrowhead), while the left amputated limb, injected with Ad-Dkk1, did not regenerate (reader’s right side, red arrowhead). (E–M) Gross morphology and SEM images of Xenopus larvae hindlimbs. (E) SEM image of the Xenopus larvae hindlimb at the amputated stage 51. (F) Ventral view of the control limb 21 d after amputation. The limb regenerated (white arrowhead) and formed the autopod. (G) Ventral view of the limb 21 d after amputation and injection of Ad-Dkk1. Dkk1 inhibited regeneration (red arrowhead), and prevented the formation of the more distal limb elements. (H) SEM image of Xenopus larvae hindlimb at the amputated stage (54). (I) Ventral view of the limb 21 d after amputation. The limb did not regenerate when amputated at stage 54 (red arrowhead). Lateral (J) and ventral (K) views of the limb 21 d after amputation and injection of Ad-CA-β-catenin. β-Catenin was able to regenerate the distal limb elements (white arrowhead, J), or partially restore regeneration of distal structures (red arrowhead, K). (L) Ventral view of a limb 21 d after amputation at the knee level at stage 58, showing lack of regeneration. (M) Ventral view of a limb 21 d after amputation and injection of Ad-CA-β-catenin at the knee level at stage 58, showing lack of regeneration. A small outgrowth is observed. In larvae shown in F–M, amputation with or without virus injection was done to the left limb while the right limb was left to develop normally. Orange arrows indicate the amputation level, and white and red arrowheads indicate regenerated and regeneration-defective limbs, respectively.
The fact that two different Wnt antagonists acting either extracellularly or intracellularly are able to perturb limb regeneration indicates the necessity but does not address the sufficiency of Wnt signaling for inducing regeneration in axolotls, animals that exhibit this capability throughout their life cycle. On the other hand, the amphibian Xenopus laevis, while an equally efficient regenerator as a tadpole, loses its regenerative capacity after metamorphosis. Complete limb regenerates are obtained only up to stages 50–51, after which the capacity to regenerate declines progressively until stage 58, when it is completely abolished (Dent 1962; Muneoka et al. 1986). With the objective of assessing the ability of Wnt signaling to promote regeneration, we microinjected an adenovirus, Ad-CA-β-catenin, containing a constitutively activated, form of β-catenin (Funayama et al. 1995) in amputated limbs at stages 53–54, when their regeneration potential is lost or greatly diminished. In most cases only partially regenerated limbs displaying large deviations from normal patterning were observed. In a few others, complete and well-patterned limbs or limbs with incomplete distal structures, although slightly delayed in their development when compared with their contralateral, nonamputated limbs, were obtained (Fig. 1H–K; Supplementary Table S2). On the other hand, Wnt signaling could not induce regeneration after the limbs have completely lost the ability to regenerate at stage 58 (Fig. 1L,M). As was the case for axolotls, down-regulation of Wnt activity in Xenopus also impaired regeneration at stages where the potential was still present (Fig. 1E–G; Supplementary Table S1). Overall, these results not only demonstrate the necessity of Wnt signaling in the early stages of axolotl and Xenopus limb regeneration, but also reflect on its ability to promote limb regeneration at specific stages. They also hint at the stage-dependent requirement of other factors, besides induction of the Wnt canonical pathway, to elicit regeneration in Xenopus.
While the axolotl and Xenopus have been at the forefront of regeneration research (Bryant et al. 2002; Maden and Hind 2003; Tanaka 2003; Slack et al. 2004; Brockes and Kumar 2005; Suzuki et al. 2005), the lack of molecular and genetic resources has hindered a rapid progression of this field. The zebrafish has proved to be an excellent model system to further enhance the cellular and molecular basis of tissue regeneration (Akimenko et al. 2003; Poss et al. 2003).
Induction of lef1 expression, a target of Wnt signaling, during zebrafish caudal fin regeneration was the first implication of Wnt signaling in zebrafish regeneration (Poss et al. 2000a). After amputation of the zebrafish caudal fin, microinjection of the fin rays with the Ad-Dkk1 virus resulted in an abrupt halt in the regeneration that normally would take place in an amputated, but not infected or Ad-eGFP-infected, caudal fin (Fig. 2A,B; data not shown). Similarly, down-regulation of the Wnt pathway in amputated pectoral fins led to a decrease in their regeneration capability (Fig. 2E,F; Supplementary Table S1). Thus, as in axolotls and Xenopus, blockade of the Wnt signaling pathway impairs the ability of different zebrafish appendages to regenerate. The variations in the ability to block regeneration by Ad-Dkk1 among the different species may reflect differences in adenovirus infectivity and/or differences in promoter efficiency to drive transgene expression in the animal model systems used. As indicated by the expression of different molecular markers, the observed regeneration defects were preceded by alterations in the formation of the basal epidermal layer, as well as by defects in proliferation in the underlying mesenchymal cells that form the blastema. lef1, a marker of the basal epidermal layer, as well as msxb, a marker of blastema formation, was down-regulated in Ad-Dkk1 infected fins (Fig. 2I–P). Signaling by fibroblast growth factors is key for successful fin regeneration (Poss et al. 2000b). Analysis of a target of Fgf signaling, mkp3 (Fig. 2Q–T), indicates a reduced activity of this pathway after Ad-Dkk1 infection.
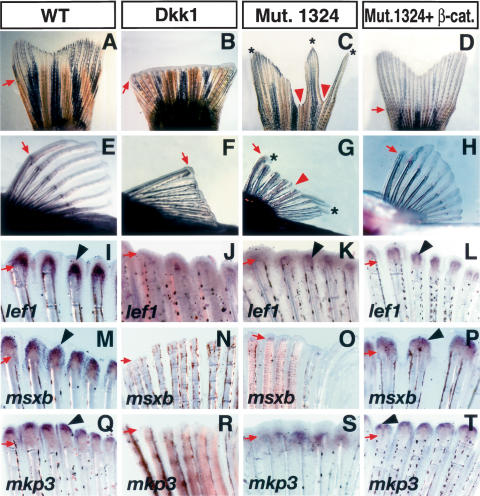
Figure 2.
Wnt/β-catenin is necessary and enhances fin regeneration in zebrafish. (A–H) Gross morphology of the caudal fin (A–D) and pectoral fin (E–H) of adult zebrafish 7–10 d after amputation with or without virus injection. The caudal fin (A) and pectoral fin (E) regenerated normally in wild-type zebrafish after amputation. Ad-Dkk1 injection after amputation inhibited regeneration in both the caudal fin (B) and pectoral fin (F), demonstrating the necessity of Wnt/β-catenin signaling for fin regeneration. The #1324 mutant fish caudal fin (C) and pectoral fin (G) showed defective regeneration, and only certain areas of the fin showed regeneration (asterisks). Injection of Ad-CA-β-catenin restored fin regeneration in the #1324 mutant. Both the caudal fin (D) and pectoral fin (H) regenerated similarly to that of wild-type fins. (I–T) In situ hybridization of lef1 (I–L), msxb (M–P), and mkp3 (Q–T) in pectoral fins 2 d after amputation with or without virus injection. In the wild-type fin, lef1 (I), msxb (M), and mkp3 (Q) are strongly expressed. In Ad-Dkk1 injected fins, expression of lef1 (J), msxb (N), and mkp3 (R) is barely detectable. In #1324 mutant fins, expression of lef1 (K), msxb (O), and mkp3 (S) is significantly down-regulated when compared with control fins. Injection of Ad-CA-β-catenin restored expression of lef1 (L), msxb (P), and mkp3 (T) in the #1324 mutant fin. Arrows indicate the amputation level. Red arrowheads and asterisks in C and G indicate defects in regeneration and regenerated tissue in the #1324 mutant. Arrowheads in I–T indicate gene expression.
Testing the ability of the Wnt pathway for enhancing regeneration was made possible by the amenability of the zebrafish to genetic manipulation. A small-scale mutagenesis screen was performed, and among the mutants obtained, one of them exhibited regeneration defects that were morphologically detectable in both caudal and pectoral fins. After fin amputation and microinjection of this mutant with Ad-CA-β-catenin virus the regeneration defects were partially rescued (Fig. 2C,D,G,H; Supplementary Table S2) as well as expression of lef1, msxb, and mkp3 (Fig. 2K,L,O,P,S,T), markers that were down-regulated in the mutant fish. Collectively, the zebrafish data support and enhance the notion that Wnt signaling is a key regulator in the regeneration ability displayed by different vertebrates.
After limb/fin amputation, the axolotl and zebrafish epidermal cells migrate to cover the wound surface; subsequently, they proliferate and form a multilayer AEC that is necessary for blastema formation and regeneration (Bryant et al. 2002; Poss et al. 2003). This process is altered upon blockade of Wnt signaling. While the early phase of epidermal migration appeared to be unaffected, the subsequent process that led to the formation of the AEC was altered (Fig. 3A–F). The Ad-Dkk1-infected epithelial lacked the characteristic cuboidal shape and smooth stratification characteristic of blastema formation and regeneration. The morphology of the epithelium resembled that of the one observed in zebrafish lacking fgf20, a gene essential for initiating regeneration (Whitehead et al. 2005).
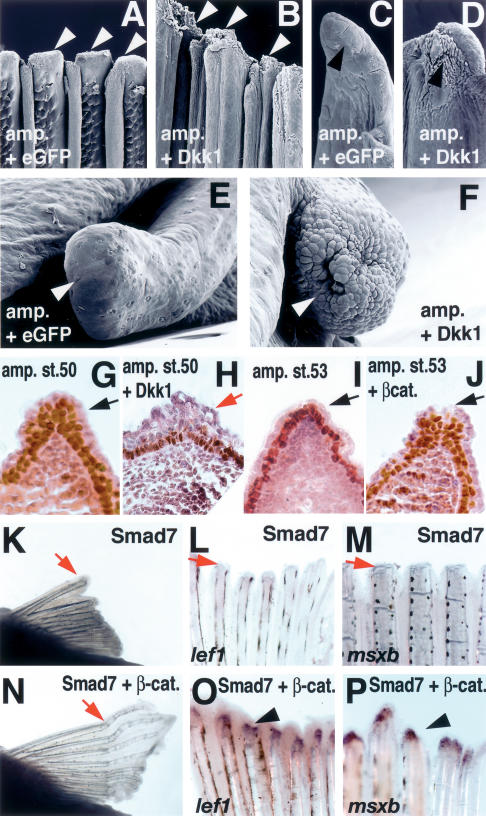
Figure 3.
Wnt and BMP signaling controls AEC formation during fin/limb regeneration. (A–F) SEM images of zebrafish pectoral fin (A–D) and axolotl larval limb (E,F) injected with Ad-eGFP (A,C,E) and Ad-Dkk1 (B,D,F). (A,C) Surface of the control pectoral fin with Ad-eGFP at 24 h post-amputation shows the characteristic smooth ectoderm (arrowheads). (B,D) Injection of Ad-Dkk1 caused thickening and alterations in the shape of ectodermal cells (arrowheads). C and D are close-ups of A and B, respectively, showing a single ray. (E) Axolotl larvae limb at 2 d post-amputation and Ad-eGFP-injected shows a smooth surface covering the amputation site (arrowhead). (F) Ad-Dkk1-injected larvae limb shows a rough and disorganized surface at the amputation site 2 d post-amputation (arrowhead). (G–J) Difference in ectoderm stratification during Xenopus hindlimb regeneration as revealed by immunoreactivity for p63 (brown color). Sections were counterstained with hematoxylin-eosin. (G) A Xenopus hindlimb, amputated at stage 50, formed stratified ectoderm 7 d after amputation (arrow). (H) Amputation of a stage 50 hindlimb, followed by injection of Ad-Dkk1, resulted in a single layer of ectoderm (red arrow). (I) Amputation at stage 53 resulted in the formation of a single layer of ectoderm after 15 d (red arrow). (J) Injection of Ad-CA-β-catenin to stage 53 amputated hindlimb induced ectoderm stratification after 15 d, similar to the one observed in G (arrow). (K–P) BMP/Smad signaling is necessary for pectoral fin regeneration and CA-β-catenin can restore BMP-inhibited regeneration. Gross morphology of pectoral fin (K,N) at 7 d post-amputation and expression of lef1 (L,O) and msxb (M,P) at 2 d post-amputation Ad-Smad7-injected (K–M) and coinjection of Ad-Smad7 + Ad-CA-β-catenin (N–P). Inhibition of BMP/Smad signaling by overexpression of Smad7 inhibited fin regeneration (K), lef1 (L), and msxb expression (M). Coinjection of Ad-CA-β-catenin restored the defects in regeneration caused by Smad7 (N) and lef1 (O) msxb expression (P).
To gain insights into the molecular mechanisms by which down-regulation of Wnt signaling might alter formation of the AEC, we analyzed the expression of several genes known to be involved in AEC formation during Xenopus limb regeneration. Of particular interest were the changes observed in p63 expression. p63 is required in higher vertebrates for the stratification and maintenance of the AER (Mills et al. 1999; Yang et al. 1999), a pseudostratified epithelia that, like the regenerating AEC, is required for the proliferation of underlying mesenchymal cells, and therefore for normal limb development (Capdevila and Izpisua Belmonte 2001; Tickle 2002). p63 protein distribution in stage 50–51 amputated Xenopus limbs was comparable to the pattern observed in stage 53–54 amputated and Ad-CA-β-catenin-injected limbs (Fig. 3G,J). On the contrary, the stratified p63 expression was absent in the distal part of limbs where regeneration potential was greatly diminished (Fig. 3I) or Wnt signaling was down-regulated (Fig. 3H). To ascertain possible alterations in ectodermal integrity and/or apoptosis, we examined immunoreactivity for E-cadherin and cleaved caspase3. No significant changes in the immunoreactivity of E-cadherin were observed (data not shown), while a slight increase in immunoreactivity for cleaved caspase3 was observed, suggesting that apoptosis might contribute, at least in part, to reduced regeneration by Dkk1 (data not shown). These observations indicate that the alterations in AEC formation and limb regeneration caused by changes of Wnt activity correlate with changes in the spatiotemporal distribution of p63.
p63 has been identified as an ectoderm-specific direct transcriptional target of BMP signaling (Bakkers et al. 2002), a pathway involved in AER formation (Ahn et al. 2001; Pizette et al. 2001). To investigate whether BMP signaling might also be involved in regeneration, we microinjected an adenovirus containing Smad7 (an inhibitory Smad that interferes with receptor-activated Smad signaling), Ad-Smad7, in amputated pectoral fins. Regenerative defects were observed in the resulting fins (Fig. 3K), defects that were preceded by the down-regulation in the ectoderm of the transcription factor downstream from Wnt, lef1 (Fig. 3L), and the down-regulation in the underlying mesenchyme of the BMP target gene, msxb (Fig. 3M). The morphological alterations in AEC formation, gene expression, and regeneration defects, were partially rescued upon coinjection of Smad7 and activated β-catenin adenoviruses (Fig. 3N–P; Supplementary Table S3). Overall, our results build on previous results (Poss et al. 2000a, 2003; Akimenko et al. 2003; Whitehead et al. 2005) and suggest that a primary action of Wnt and BMP signaling at the early stages of regeneration resides in the epidermal cell layer, whereby they regulate the formation of the AEC by controlling the expression of transcription factors, like p63 and lef1, and of signaling molecules and their intracellular effectors, like fgf/mkp3—factors that direct the early epithelial/mesenchymal interactions needed for blastema formation and subsequent regeneration.
The remarkable similarities in the molecular (i.e., the use of the Wnt and BMP pathways) and cellular (i.e., AEC and AER formation) processes involved in limb regeneration and embryogenesis, both within one and between different organisms (i.e., the use of the BMP/dpp and the Wnt/wg pathways during Drosophila regeneration) (Maves and Schubiger 2003), may not be coincidental. As such, and notwithstanding the possible existence of specific and distinct regulators for regeneration not present during development, differences in the deployment (concentration and spatiotemporal distribution) and in the fine-tuning of developmental regulators, might constitute a mechanism for allowing tissue homeostasis and regeneration.
We set up to test this notion by manipulating, in a nonregenerating organism, the chick embryo, two of the players shown here (p63 and β-catenin) to be involved in AEC formation during limb/fin regeneration. We overexpressed dNp63α in the pre-AER chick embryo ectoderm followed by removal of the AER 36–48 h after infection. Removal of the AER in the chick embryo leads to a halt in proliferation and truncation of the more distal limb elements (Fig. 4B,F,J). Overexpression of dNp63α was not able to rescue the defects of AER removal (Fig. 4C,G,K) and elicited a similar phenotype as the one observed in embryos where only the AER had been removed (Supplementary Table S4). Thus, while FGF bead implantation after AER removal rescues limb outgrowth but not AER formation (Niswander et al. 1993), p63, which is necessary for limb ectoderm stratification and AER formation (Mills et al. 1999; Yang et al. 1999), is not sufficient by itself to induce fgf8 expression and/or AER regeneration. These results contrasted with the capability of β-catenin to induce AER regeneration. Removal of the AER after overexpression of an activated form of β-catenin in the pre-AER chick embryo ectoderm was able to rescue both fgf8 expression and AER formation (Fig. 4D,H,L). Given the fact that BMP signaling and p63 expression are required for ectoderm stratification and AER induction but, contrary to β-catenin, p63 is not able to induce AER regeneration in our assay, the role of BMP/p63 pathway might lie in providing competence for ectoderm stratification, competence that is only elicited by cells exposed to Wnt/β-catenin activity at the distal limb ectoderm.
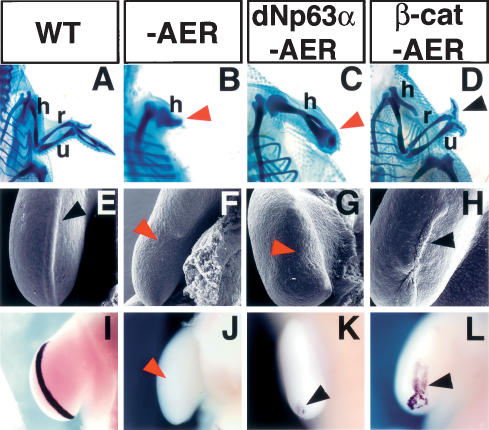
Figure 4.
β-Catenin induces AER and limb regeneration in chick embryos. (A–D) Cartilage staining of the chick forelimb at 10 d post-fertilization. (A) Wild-type chick limb showing the stylopod (humerus), zeugopod (radius and ulna), and autopod elements. (B,C) Removal of the AER at stage 20/21 prevented limb outgrowth and resulted in a truncated limb independent of dNp63α overexpression. (D) Ad-CA-β-catenin injection prior to AER removal restored limb development and induced the formation of all the stylopod, zeugopod elements, and two digits (black arrowhead). Red arrowheads in B and C indicate truncated limbs. (E–L) SEM images (E–H) and fgf8 expression (I–L) of the limb ectoderm 30 h after AER removal. The control limb shows the typical thickened AER ectoderm (black arrowhead, E) associated with fgf8 expression (I). Absence of distal thickened ectoderm after AER removal (red arrowhead, F) results in the down-regulation of fgf8 expression (red arrowhead, J). Overexpression of dNp63α did not rescue AER (red arrowhead, G) or fgf8 expression (residual expression is observed at the posterior of the developing limb bud) (black arrowhead, K). Infection of limb ectoderm cells with Ad-CA-β-catenin prior to AER removal regenerated the AER (black arrowhead, H) and elicited fgf8 expression (black arrowhead, L). (h) Humerus; (r) radius; (u) ulna.
The detailed mechanism by which Wnt overexpression in ectodermal cells adjacent to the amputation entails both AER regeneration in the chick, as well as AEC formation in the axolotl/zebrafish/Xenopus, remains to be elucidated. Perhaps, and more importantly, some of the results discussed here—specifically (1) the observations that changes in Wnt and BMP activities during limb/fin regeneration–limb development induced alterations in the formation of the AEC–AER that are related to spatiotemporal deregulation of p63, and (2) the accomplishment of AER regeneration and subsequent limb development in an embryo not previously shown to have this capability—support the notion that variations in the concentration and/or spatiotemporal distribution of molecules involved in tissue generation during embryogenesis may be the raw material upon which evolution has granted some animals the ability to regenerate. Understanding the mechanisms responsible for the deployment and fine-tuning of developmental regulators might constitute the basis for inducing tissue regeneration in adult nonregenerating animals.
Materials and methods
Animals
Wild-type AB zebrafish, adult axolotls, axolotl larvae at stage 45 and Xenopus larvae at stages 49–51, 53–54, or 58–59 were used for the study. The #1324 mutant fish line was established after N-ethyl-N-nitrosourea mutagenesis screening with standard protocols (Mullins et al. 1994; Solnica-Krezel et al. 1994), and F5 generation homozygous fish were used. This mutant fish exhibited normal embryonic development and fertility, and was kept under standard conditions. The identification of the mutation is currently ongoing, and will be published elsewhere. Details are described in the Supplemental Material.
Adenovirus
Adenoviruses carrying mouse Dkk1, Axin1, constitutively active β-catenin, Smad7, or eGFP were used for this study. Transgene expression was driven by the CAGGS or CMV promoter (Miyake et al. 1996). The viruses were purified by cesium chloride ultracentrifugation twice and dialyzed in PBS prior to injection.
During the experiments using Dkk1 and Axin1 adenoviruses, we noticed that some adult axolotls appeared to be ill. Presumably, this is due to some adenoviruses entering the blood circulation, and thus affecting other organs. Published data indicate the adverse effects of Ad-Dkk1 infection in adult mice livers. Due to the large axolotl limbs, we needed to inject larger volumes than in other animal model systems (axolotl larvae, Xenopus tadpoles, and zebrafish). Therefore, to reduce possible secondary effects of Ad-Dkk1, we used Ad-Axin1 in adult axolotl limbs with the expectation that Axin1 only affects infected cells, while we used Ad-Dkk1 in the other animal model systems.
In situ hybridization and immunohistochemistry
In situ hybridization was done as previously published (Kawakami et al. 2001; Raya et al. 2003). Immunoreactivity for p63 was detected with the standard ABC system on 10-μm paraffin sections with the 4A4 monoclonal antibody (Santa Cruz Biotechnology, sc-8431). Details are available upon request.
Electron microscopy
Samples were fixed with 2.5% glutaraldehyde in cacodylate buffer (0.2 M at pH 7.4) for 2 h at 4°C, washed with cacodylate buffer, and subsequently processed using standard procedures.
Chick manipulation
Chick embryos were injected with RCAS BP dNp63α at stage 9–10 or Ad-CA-β-catenin at stage 14–15. The AER was surgically removed by using a sharp tungsten needle at stage 20–21. The embryos were then allowed to develop for molecular and morphological analyses.
Acknowledgments
We thank all the members of the laboratory, especially Agustin Rojas and Isao Oishi for their help and advice in zebrafish regeneration, Ana Merino for her technical skills with the antibody stainings, Chris Kintner for discussions and advice, Randall Moon for sharing results before publication, May Schwarz for help in preparation of this manuscript, and the Microscopy Service from the Autonoma University of Barcelona. This work was supported by Fundacion Cellex, the G. Harold and Leila Y. Mathers Charitable Foundation, and the National Institutes of Health.
Footnotes
Supplemental material is available at http://www.genesdev.org.
Article published online ahead of print. Article and publication date are online at http://www.genesdev.org/cgi/doi/10.1101/gad.1475106
References
- Ahn, K., Mishina, Y., Hanks, M.C., Behringer, R.R., Crenshaw, E.B., III BMPR-IA signaling is required for the formation of the apical ectodermal ridge and dorsal–ventral patterning of the limb. Development. 2001;128:4449–4461. doi: 10.1242/dev.128.22.4449. [DOI] [PubMed] [Google Scholar]
- Akimenko, M.A., Mari-Beffa, M., Becerra, J., Geraudie, J. Old questions, new tools, and some answers to the mystery of fin regeneration. Dev. Dyn. 2003;226:190–201. doi: 10.1002/dvdy.10248. [DOI] [PubMed] [Google Scholar]
- Bakkers, J., Hild, M., Kramer, C., Furutani-Seiki, M., Hammerschmidt, M. Zebrafish ΔNp63 is a direct target of Bmp signaling and encodes a transcriptional repressor blocking neural specification in the ventral ectoderm. Dev. Cell. 2002;2:617–627. doi: 10.1016/s1534-5807(02)00163-6. [DOI] [PubMed] [Google Scholar]
- Bode, H.R. Head regeneration in Hydra. Dev. Dyn. 2003;226:225–236. doi: 10.1002/dvdy.10225. [DOI] [PubMed] [Google Scholar]
- Brockes, J.P., Kumar, A. Plasticity and reprogramming of differentiated cells in amphibian regeneration. Nat. Rev. Mol. Cell Biol. 2002;3:566–574. doi: 10.1038/nrm881. [DOI] [PubMed] [Google Scholar]
- Brockes, J.P., Kumar, A. Appendage regeneration in adult vertebrates and implications for regenerative medicine. Science. 2005;310:1919–1923. doi: 10.1126/science.1115200. [DOI] [PubMed] [Google Scholar]
- Bryant, S.V., Endo, T., Gardiner, D.M. Vertebrate limb regeneration and the origin of limb stem cells. Int. J. Dev. Biol. 2002;46:887–896. [PubMed] [Google Scholar]
- Capdevila, J., Izpisua Belmonte, J.C. Patterning mechanisms controlling vertebrate limb development. Annu. Rev. Cell Dev. Biol. 2001;17:87–132. doi: 10.1146/annurev.cellbio.17.1.87. [DOI] [PubMed] [Google Scholar]
- Caubit, X., Nicolas, S., Le Parco, Y. Possible roles for Wnt genes in growth and axial patterning during regeneration of the tail in urodele amphibians. Dev. Dyn. 1997;210:1–10. doi: 10.1002/(SICI)1097-0177(199709)210:1<1::AID-AJA1>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Dent, J.N. Limb regeneration in larvae and metamorphosing individuals of the South African clawed toad. J. Morphol. 1962;110:61–77. doi: 10.1002/jmor.1051100105. [DOI] [PubMed] [Google Scholar]
- Funayama, N., Fagotto, F., McCrea, P., Gumbiner, B.M. Embryonic axis induction by the armadillo repeat domain of β-catenin: Evidence for intracellular signaling. J. Cell Biol. 1995;128:959–968. doi: 10.1083/jcb.128.5.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinka, A., Wu, W., Delius, H., Monaghan, A.P., Blumenstock, C., Niehrs, C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357–362. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- Kawakami, Y., Capdevila, J., Buscher, D., Itoh, T., Rodriguez Esteban, C., Izpisua Belmonte, J.C. WNT signals control FGF-dependent limb initiation and AER induction in the chick embryo. Cell. 2001;104:891–900. doi: 10.1016/s0092-8674(01)00285-9. [DOI] [PubMed] [Google Scholar]
- Maden, M., Hind, M. Retinoic acid, a regeneration-inducing molecule. Dev. Dyn. 2003;226:237–244. doi: 10.1002/dvdy.10222. [DOI] [PubMed] [Google Scholar]
- Maves, L., Schubiger, G. Transdetermination in Drosophila imaginal discs: A model for understanding pluripotency and selector gene maintenance. Curr. Opin. Genet. Dev. 2003;13:472–479. doi: 10.1016/j.gde.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Mills, A.A., Zheng, B., Wang, X.J., Vogel, H., Roop, D.R., Bradley, A. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature. 1999;398:708–713. doi: 10.1038/19531. [DOI] [PubMed] [Google Scholar]
- Miyake, S., Makimura, M., Kanegae, Y., Harada, S., Sato, Y., Takamori, K., Tokuda, C., Saito, I. Efficient generation of recombinant adenoviruses using adenovirus DNA-terminal protein complex and a cosmid bearing the full-length virus genome. Proc. Natl. Acad. Sci. 1996;93:1320–1324. doi: 10.1073/pnas.93.3.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins, M.C., Hammerschmidt, M., Haffter, P., Nusslein-Volhard, C. Large-scale mutagenesis in the zebrafish: In search of genes controlling development in a vertebrate. Curr. Biol. 1994;4:189–202. doi: 10.1016/s0960-9822(00)00048-8. [DOI] [PubMed] [Google Scholar]
- Muneoka, K., Holler-Dinsmore, G., Bryant, S.V. Intrinsic control of regenerative loss in Xenopus laevis limbs. J. Exp. Zool. 1986;240:47–54. doi: 10.1002/jez.1402400107. [DOI] [PubMed] [Google Scholar]
- Niswander, L., Tickle, C., Vogel, A., Booth, I., Martin, G.R. FGF-4 replaces the apical ectodermal ridge and directs outgrowth and patterning of the limb. Cell. 1993;75:579–587. doi: 10.1016/0092-8674(93)90391-3. [DOI] [PubMed] [Google Scholar]
- Pizette, S., Abate-Shen, C., Niswander, L. BMP controls proximodistal outgrowth, via induction of the apical ectodermal ridge, and dorsoventral patterning in the vertebrate limb. Development. 2001;128:4463–4474. doi: 10.1242/dev.128.22.4463. [DOI] [PubMed] [Google Scholar]
- Poss, K.D., Shen, J., Keating, M.T. Induction of lef1 during zebrafish fin regeneration. Dev. Dyn. 2000a;219:282–286. doi: 10.1002/1097-0177(2000)9999:9999<::aid-dvdy1045>3.3.co;2-3. [DOI] [PubMed] [Google Scholar]
- Poss, K.D., Shen, J., Nechiporuk, A., McMahon, G., Thisse, B., Thisse, C., Keating, M.T. Roles for Fgf signaling during zebrafish fin regeneration. Dev. Biol. 2000b;222:347–358. doi: 10.1006/dbio.2000.9722. [DOI] [PubMed] [Google Scholar]
- Poss, K.D., Keating, M.T., Nechiporuk, A. Tales of regeneration in zebrafish. Dev. Dyn. 2003;226:202–210. doi: 10.1002/dvdy.10220. [DOI] [PubMed] [Google Scholar]
- Raya, A., Koth, C.M., Buscher, D., Kawakami, Y., Itoh, T., Raya, R.M., Sternik, G., Tsai, H.J., Rodriguez-Esteban, C., Izpisua-Belmonte, J.C. Activation of Notch signaling pathway precedes heart regeneration in zebrafish. Proc. Natl. Acad. Sci. 2003;100 (Suppl. 1):11889–11895. doi: 10.1073/pnas.1834204100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack, J.M., Beck, C.W., Gargioli, C., Christen, B. Cellular and molecular mechanisms of regeneration in Xenopus. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2004;359:745–751. doi: 10.1098/rstb.2004.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solnica-Krezel, L., Schier, A.F., Driever, W. Efficient recovery of ENU-induced mutations from the zebrafish germline. Genetics. 1994;136:1401–1420. doi: 10.1093/genetics/136.4.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki, M., Satoh, A., Ide, H., Tamura, K. Nerve-dependent and -independent events in blastema formation during Xenopus froglet limb regeneration. Dev. Biol. 2005;286:361–375. doi: 10.1016/j.ydbio.2005.08.021. [DOI] [PubMed] [Google Scholar]
- Tanaka, E.M. Cell differentiation and cell fate during urodele tail and limb regeneration. Curr. Opin. Genet. Dev. 2003;13:497–501. doi: 10.1016/j.gde.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Tickle, C. Molecular basis of vertebrate limb patterning. Am. J. Med. Genet. 2002;112:250–255. doi: 10.1002/ajmg.10774. [DOI] [PubMed] [Google Scholar]
- Tsonis, P.A. Regeneration in vertebrates. Dev. Biol. 2000;221:273–284. doi: 10.1006/dbio.2000.9667. [DOI] [PubMed] [Google Scholar]
- Whitehead, G.G., Makino, S., Lien, C.L., Keating, M.T. fgf20 is essential for initiating zebrafish fin regeneration. Science. 2005;310:1957–1960. doi: 10.1126/science.1117637. [DOI] [PubMed] [Google Scholar]
- Yang, A., Schweitzer, R., Sun, D., Kaghad, M., Walker, N., Bronson, R.T., Tabin, C., Sharpe, A., Caput, D., Crum, C., et al. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature. 1999;398:714–718. doi: 10.1038/19539. [DOI] [PubMed] [Google Scholar]