Abstract
cis-Jasmone, or (Z)-jasmone, is well known as a component of plant volatiles, and its release can be induced by damage, for example during insect herbivory. Using the olfactory system of the lettuce aphid to investigate volatiles from plants avoided by this insect, (Z)-jasmone was found to be electrophysiologically active and also to be repellent in laboratory choice tests. In field studies, repellency from traps was demonstrated for the damson-hop aphid, and with cereal aphids numbers were reduced in plots of winter wheat treated with (Z)-jasmone. In contrast, attractant activity was found in laboratory and wind tunnel tests for insects acting antagonistically to aphids, namely the seven-spot ladybird and an aphid parasitoid. When applied in the vapor phase to intact bean plants, (Z)-jasmone induced the production of volatile compounds, including the monoterpene (E)-β-ocimene, which affect plant defense, for example by stimulating the activity of parasitic insects. These plants were more attractive to the aphid parasitoid in the wind tunnel when tested 48 h after exposure to (Z)-jasmone had ceased. This possible signaling role of (Z)-jasmone is qualitatively different from that of the biosynthetically related methyl jasmonate and gives a long-lasting effect after removal of the stimulus. Differential display was used to compare mRNA populations in bean leaves exposed to the vapor of (Z)-jasmone and methyl jasmonate. One differentially displayed fragment was cloned and shown by Northern blotting to be up-regulated in leaf tissue by (Z)-jasmone. This sequence was identified by homology as being derived from a gene encoding an α-tubulin isoform.
Plants have evolved a variety of mechanisms to withstand the damage and stresses caused by pathogens and herbivorous animals, as well as by many abiotic factors. One such mechanism involves the emission of volatile compounds, either constitutively or as a result of biotic infestation or physical damage, which can affect pathogen development and the behavior of insect herbivores searching for a food source. A recent review by Agrawal and Karban (1) compares the benefits to the plant of constitutive gene expression and induced defense strategies. Constitutively produced plant volatiles play a role in attracting pollinators and seed-dispersing animals; in addition, they can repel a wide range of potential herbivores and attract a smaller number of pest species that have evolved to take advantage of these chemicals in finding food. Plant volatiles that are induced on damage to repel insect attack also may act as an indirect plant defense mechanism by attracting other insects that prey on or parasitize the herbivores. Such compounds also may act as signals between plants, whereby defense mechanisms are induced in undamaged plants in response to volatiles produced by neighboring infested plants, and specific volatiles, methyl salicylate and methyl jasmonate, have been implicated (2–5). Compounds containing six carbon atoms, e.g., (E)-2-hexenal, which are rapidly emitted from damaged or wounded plant tissue, also have recently been shown to induce the expression of defense-related genes in intact plants (6).
The investigation of insect interactions with plant volatiles is now greatly facilitated by using sophisticated electrophysiological techniques, in particular gas chromatography (GC) coupled directly to neuronal or single-cell recording (SCR) from the olfactory organs of insects. Methyl salicylate, which was found to repel aphids (Homoptera: Aphididae) such as the black bean aphid, Aphis fabae, and cereal aphids including the grain aphid, Sitobion avenae, and also to inhibit attraction to their host plants (7, 8), originally was discovered to be an aphid signal by SCR on the antenna of the bird-cherry-oat aphid, Rhopalosiphum padi (7). More than 30 species of insects, both plant feeders and their natural enemies, from five orders subsequently have been found, by SCR and by recording from the whole antenna (electroantennography, or EAG), to possess olfactory receptors for this compound (C.M.W., unpublished work). Subsequently, Raskin's group (3) showed that methyl salicylate may act as an airborne plant signal mediating plant pathogen resistance.
Following the successful use of the techniques outlined above, we began the investigation of other plant/aphid interactions, selecting plants that were nonhosts for certain aphid species or morphs. Ribes nigrum, the black currant (Saxifragaceae), which with other Ribes species is the winter (primary) host of the lettuce aphid, Nasonovia ribis-nigri, was studied as part of the investigation. This plant is attractive to the morphs of N. ribis-nigri produced in the autumn but is avoided by, and indeed repellent to, the spring and summer morphs, which are attracted to the summer (secondary) hosts such as lettuce, Lactuca sativa (Asteraceae). In this paper, we describe how cis-jasmone, or (Z)-jasmone, a component of flower volatiles also known to be induced on damage to plant vegetative tissue (9, 10), was located in the volatile profile of R. nigrum by coupled GC-EAG on the summer morph of N. ribis-nigri. (Z)-Jasmone was singled out from other electrophysiologically active compounds in the extract because of its clear biosynthetic association to the stress-induced jasmonic acid or octadecanoid pathway. The compound was shown to be repellent to this aphid in laboratory tests and the generality of its repellent activity for aphids was demonstrated in field trials with other species. In addition, the effects of (Z)-jasmone on two natural control agents of aphids, a predator, the seven-spot ladybird, Coccinella septempunctata (Coleoptera: Coccinellidae), and the aphid parasitoid Aphidius ervi (Hymenoptera: Braconidae), were determined. Finally, the bean plant Vicia faba (Fabaceae) was exposed to (Z)-jasmone in the vapor phase to investigate whether this compound affects insect behavior by altering the release of volatiles and gene expression by the plant.
Materials and Methods
Identification of Active Components in the Volatile Profile of R. nigrum.
Microwave-assisted distillation of plant volatiles.
Volatiles from leaves of the black currant, R. nigrum (cv. Ben Sarek), were extracted by using microwave-assisted distillation, based on the method of Craveiro et al. (11). Plant material (30–40 g fresh weight) was heated in a 500-ml florentine flask for approximately 1 min in an 800-W microwave oven until the plant cells ruptured. The volatile materials were picked up in a stream of nitrogen (60 ml⋅min−1), carried through polytetrafluoroethylene tubing (3 mm internal diameter) and trapped in a flask containing cooled hexane. All connections between tubing and flasks were sealed with polytetrafluoroethylene tape. Dehydrated magnesium sulfate was added to remove water and the extract was filtered. Extraction was confined to the leaves because the stems tended to pyrolyse, producing additional compounds not originally present in the plant. Using a rotary evaporator, extracts were concentrated to the equivalent of 25 g, fresh weight, of plant material per ml.
Electrophysiology: EAG.
Recordings from whole antennae of alate virginoparous N. ribis-nigri were made by using Ag/AgCl glass electrodes filled with saline solution [composition as described elsewhere (12) but without the glucose]. The head of the aphid was excised and mounted on the indifferent electrode. The tip of the recording electrode was removed so that its inside diameter was just wide enough to accept the terminal process of the antenna. The signals were passed through a high impedance amplifier (UN-03b, Syntech, Hilversum, The Netherlands) and displayed on a chart recorder.
Coupled GC-EAG.
The coupled GC-electrophysiology system, in which the effluent from the GC capillary column is delivered simultaneously to the antennal preparation and the GC detector, has been described (13). Separation of the volatile sample was achieved on an AI 93 GC (AI, Cambridge, U.K.) equipped with a cold on-column injector and a flame ionization detector (FID). The column (30 m × 0.53 mm i.d., HP-1) was maintained at 40°C for 2 min and then programmed at 10°C min−1 to 250°C. The carrier gas was hydrogen. The outputs from the EAG amplifier and the FID were monitored simultaneously on a chart recorder.
Electrophysiology: SCR dose–response analysis.
Recordings from a cell associated with the olfactory receptors on the proximal primary rhinarium of an alate virginoparous N. ribis-nigri were made by using tungsten microelectrodes. The indifferent electrode was placed in the first or second antennal segment, and the recording electrode then was brought into contact with the rhinarium until impulses were recorded. The signals were passed through a high impedance amplifier (UN-06, Syntech), and data storage and processing were carried out with a PC-based interface and software package (Syntech). The stimulus was delivered into a purified airstream (1 liter⋅min-1) flowing continuously over the preparation. The delivery system, using a filter paper strip in a disposable Pasteur pipette cartridge, has been described (14). The impulse frequency was determined as the number of impulses elicited during the first 1 sec after stimulus application. (Z)-Jasmone and methyl jasmonate were diluted in purified hexane (0.1, 1, and 10 μg in 10 μl), and the solutions were presented twice to the preparation in ascending order of concentration, at intervals of 2, 5, and 10 min, respectively for the three concentrations.
GC analysis.
GC analysis was carried out by using a Hewlett–Packard 5890 GC equipped with a temperature programmable on-column injector and flame ionization detector. This was fitted with two columns of different polarities, 50 m × 0.32 mm i.d. HP-1 (nonpolar) and 30 m × 0.32 mm i.d. SPB-35 (polar), with hydrogen as the carrier gas. The oven was maintained at 40°C for 1 min then programmed at 10°C min-1 to 250°C.
GC-mass spectrometry (MS).
A Hewlett–Packard 5890 GC was connected to a VG Autospec mass spectrometer (Fisons, Manchester, U.K.). Ionization was by electron impact at 70 eV, 230°C, and the GC, using the HP-1 column, was maintained at 30°C for 5 min then programmed at 5°C min-1 to 180°C. Detection limits for (Z)-jasmone in the entrainment samples were 40 pg⋅h-1 for GC and 400 pg⋅h-1 for GC-MS. Compounds tentatively identified by GC-MS were confirmed by coinjection of authentic samples on GC using polar and nonpolar columns (see above). Authentic samples were obtained from commercial sources, except for (E)-β-ocimene and (E,E)-α-farnesene, which were synthesized by the methods of Chou et al. (15), and (E)-4,8-dimethyl-1,3,7-nonatriene, which was prepared by using the method reported by Boland and Gaebler (16).
Behavioral Studies.
Olfactometry: aphids.
Behavioral assays were done in a Perspex olfactometer similar to that described by Pettersson (17), with a weak air stream directed toward the center from each of four side arms. The test compound (1 μg) in hexane (10 μl) was placed on filter paper (Whatman no. 1) at the end of one of the side arms, with hexane alone used as a control in the other arms. One alate virginopara of N. ribis-nigri was placed in the center of the arena and its position was noted at 1-min intervals for 10 min. The apparatus, maintained at 24°C, was lit from above by fluorescent tubing and rotated by 90o every 2.5 min to avoid any directional bias. The experiment was replicated six times. The results were analyzed by Student's t test, comparing the cumulative number of observations, and the mean time spent, in the treatment arm and the three control arms over the 10-min test period.
Field studies: hop aphids.
Traps were made from 14-cm diameter plastic Petri dishes that had been painted on the outside with yellow (BS 381C: 1980 Color 309 Canary Yellow) and then with matte black so that the yellow coloring was visible only from the inside. Each trap was filled with an aqueous solution of a nonionic detergent (0.2%) and a sachet releasing (Z)-jasmone (2.05 μg⋅day-1⋅trap-1) was suspended 4 cm above the center. Empty sachets were used as controls. The traps were set on canes 0.6 m above ground and 2.5 m apart in six separate randomized blocks arranged down the center of a 10-m wide alley separating 1-ha plots of dwarf hops, Humulus lupulus (cv. First Gold). The traps operated during July 8–20, 1996 and were emptied and the treatments were rerandomized daily. Numbers of spring migrants of the damson-hop aphid, Phorodon humuli, in the daily trap catches were counted and the data were subjected to ANOVA.
Field studies: cereal aphids.
Plots (6 m × 6 m) of winter wheat, Triticum aestivum (cv. Consort), were arranged in a 5 × 5 quasi-complete Latin square design. The five (Z)-jasmone plots were sprayed on May 5 and June 11, 1999 by using a hand-held hydraulic device, at a rate of 50 g (Z)-jasmone ha-1 solubilized in 200 liters⋅ha-1 of water by means of a nonionic surfactant (Ethylan BV, Akcros Chemicals, Manchester, U.K., 0.1%). Because the surfactant showed no significant effects on cereal aphid settling behavior in field simulation experiments, control plots were untreated. [Field simulation tests: single leaves of cereal seedlings, either sprayed with aqueous Ethylan BV (0.1%) or an unsprayed control (nonchoice test), or pairs of leaves, one sprayed with aqueous Ethylan BV (0.1%) and an unsprayed control (choice test), were presented to groups of five adult S. avenae (n = 10) and assessed after 24 h. Percentage aphids settled: nonchoice test, surfactant 70, control 68; choice test, surfactant 34, control 36; no significant differences by t test]. Cereal aphids and parasitized aphids were counted on eight occasions between early May and mid-July. At each count, five randomly selected tillers were inspected at 12 separate sites on two diagonal transects, totaling 60 tillers for each plot. Transformed data [y = log (y+1)] were subjected to ANOVA and the sums of squares of the treatments were partitioned to test for significant differences.
Olfactometry: ladybirds.
Apparatus and methodology were similar to those used for aphids. The test compound (0.5 μg) in hexane was applied in a 0.5 μl microcap (Drummond Scientific, Broomall, PA) at the end of one of the side arms, and each arm was supplied with moist filter paper to minimize differences in relative humidity. One adult C. septempunctata was introduced into the center of the arena and its position was noted at 2-min intervals for 20 min. The experiment was replicated eight times and results were analyzed as above.
Wind tunnel studies: aphid parasitoids.
Naïve female A. ervi were flown in a wind tunnel. Individual parasitoids were released 25 cm downwind (single-choice test) or 40 cm downwind (dual-choice test) of the target, which was either a plant (18, 19) or a synthetic compound (10 μg in 10 μl hexane) placed on a 2 × 1 cm strip of filter paper (Whatman no. 1) surrounded by a ring of green crepe paper. The proportions of parasitoids responding with an oriented flight to the synthetic chemicals were calculated each day on 3 separate days. These values then were subjected to a logit transformation to normalize the data before being analyzed by ANOVA followed by Tukey post hoc tests. The number of parasitoids orienting upwind to the single plant target were recorded and subjected to a χ2 contingency test (Pearson method on genstat) (20) to determine whether an orienting response was linked to the type of plant treatment. The numbers orienting to each plant in the dual-choice test were analyzed by a χ2 test to determine whether one plant was more attractive than the other.
Effects of (Z)-Jasmone Vapor on V. faba.
Induction studies and air entrainment.
Bean plants, V. faba (cv The Sutton), were grown in a glasshouse with supplementary lighting to give a 16-h day at a temperature of 20°C until the 2- to 4-leaf stage, when the roots were rinsed free of soil and the plants were transplanted into baked glass jars containing washed sand, with three plants per jar. These were left for 1–2 days to allow the plants to recover from transplantation. A set of nine plants then was sealed in a 25-liter glass tank with either (Z)-jasmone or methyl jasmonate (2.5 mg) applied to a piece of filter paper (Whatman no. 1) placed on the floor of the tank. Control plants were sealed in a separate identical tank with no treatment. After 24 h, during which time all of the test chemical had passed into the aerial phase, treated plants were removed and placed in a 10-liter glass entrainment vessel and the volatiles produced were collected in glass tubes containing Porapak Q (50 mg) over 48-h periods for 192 h. Volatiles from control (untreated) plants were collected similarly. The samples of volatiles were eluted by using freshly distilled diethyl ether (500 μl) and then concentrated to 100 μl for analysis by GC and GC-MS. The whole experiment was replicated four times.
Differential display.
Bean plants were grown under the same conditions and treated in the same way as those used for induction studies. After being removed from the tanks, plants were placed in the glasshouse for 96 h. Plant material was harvested and then flash-frozen in liquid nitrogen and stored at −80°C until used. Total RNA was isolated from leaf or stem tissues of control and treated plants by using the Qiagen (Chatsworth, CA) plant mini-RNA extraction kit, according to the supplier's instructions. RNA samples from leaf and stem tissue of control or treated plants were pooled at a concentration of 1 mg⋅ml-1. These pooled RNA samples then served as a template for reverse transcription using the Amersham Pharmacia RT system, primed with oligo(dT) primers containing single selective nucleotides (e.g., T14 A, T14 C). Differential display then was carried out on these single-stranded cDNA templates by using a panel of random oligonucleotides according to previously described methods (21). Displayed fragments of interest were recovered from polyacrylamide gels by elution and reamplification, and subsequently cloned and sequenced according to standard methods (22). Northern blotting of RNA isolated from control and treated plants was carried out according to previously described methods (23).
Results
Identification of Active Components in the Volatile Profile of R. nigrum.
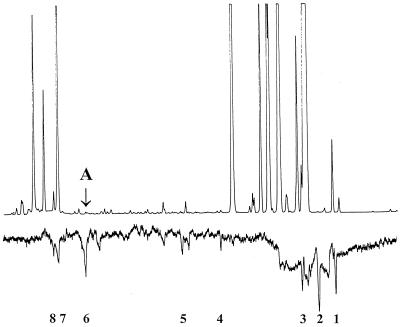
GC-EAG analysis using the summer morph of N. ribis-nigri with the volatile components of its winter host, R. nigrum, obtained by microwave-assisted distillation, showed that a number of flame ionization detector peaks, mostly from ubiquitous plant components, were associated with electrophysiological activity (Fig. 1). The strong EAG response to the very small peak at A was found to arise from (Z)-jasmone, i.e., cis-jasmone or (Z)-2-(pent-2-en-1-yl)-3-methylcyclopent-2-en-1-one, tentatively identified by GC-MS, where the spectrum was identical to that in the National Institute of Standards and Technology database (24), and identity was confirmed by cochromatography with authentic compound.
Figure 1.
Coupled GC-EAG with summer morph of the lettuce aphid, N. ribis-nigri. (Upper) GC of volatiles from black currant, R. nigrum. (Lower) Simultaneous EAG response of N. ribis-nigri. Compounds eliciting major EAG responses (in order of elution) are: 1, 5-methylfurfural; 2, 1-octen-3-ol; 3, β-pinene; 4, chrysanthenone; 5, methyl salicylate; 6, (Z)-jasmone; 7, caryophyllene; 8, (E)-β-farnesene. A = peak from (Z)-jasmone.
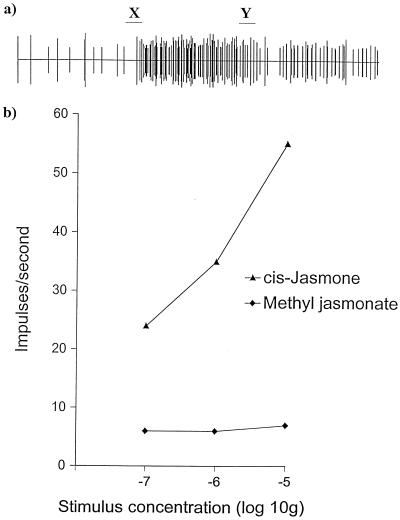
SCR investigations with N. ribis-nigri revealed that the (Z)-jasmone was detected by olfactory cells in the proximal primary rhinarium, i.e., on the fifth antennal segment (Fig. 2a). Dose–response data demonstrated that, although this cell was extremely sensitive to (Z)-jasmone, there was no response to methyl jasmonate at the levels tested (Fig. 2b).
Figure 2.
SCR with summer morph of the lettuce aphid, N. ribis-nigri. (a) Response of an olfactory cell in the proximal primary rhinarium (fifth antennal segment) to (Z)-jasmone (1 μg). Stimulus applied over 2 sec; beginning and end of stimulus period are marked as X and Y, respectively. (b) Dose–response of a single olfactory cell to (Z)-jasmone and methyl jasmonate. Each point is the mean of two stimulations on the same preparation.
Behavioral Studies with Aphids.
Experiments in the four-arm olfactometer showed that (Z)-jasmone was significantly repellent to the summer morph of N. ribis-nigri [mean number observations in: treated arm 2.0 ± 0.58, each control arm, 4.3 ± 0.58; mean time (min) spent in: treated arm 0.5 ± 0.16, each control arm 2.1 ± 0.36; P < 0.05]. In field trapping experiments, repellency also was demonstrated with spring migrants of the damson-hop aphid, P. humuli, where catches in visually attractive (i.e., yellow) water traps were reduced by 34% (total number aphids caught: treated 122, control 184; P < 0.05) through the slow release of (Z)-jasmone. In field trials on winter wheat, aphid numbers were consistently lower on the plots sprayed with (Z)-jasmone [mean number on 60 tillers at aphid population peak, June 24, 1999: treated 41.01, control 66.82; P < 0.05 (transformed means: treated 1.623, control 1.831; SE 0.0625)]. In this experiment, the predominant aphid species was Metopolophium dirhodum, the rose-grain aphid, with low numbers of S. avenae and R. padi also present. Very few parasitized aphids were found in either the treated or untreated plots.
Behavioral Studies with Aphid Predators and Parasitoids.
Experiments in the four-arm olfactometer showed that (Z)-jasmone was significantly attractive to the seven-spot ladybird, C. septempunctata (mean number observations in: treated arm 4.1 ± 1.55, each control arm 2.4 ± 0.69; P < 0.005). For the aphid parasitoid A. ervi in the single-choice wind tunnel test, (Z)-jasmone, as well as (E)-β-ocimene and (E,E)-α-farnesene, which were identified as enhanced volatiles from (Z)-jasmone treated V. faba (see following section), proved to be attractive to naïve females (Table 1). V. faba plants that had been exposed to (Z)-jasmone for 24 h but had then been left in the glasshouse for 48–96 h, when (Z)-jasmone was no longer detectable by air entrainment and GC, were significantly more attractive to A. ervi than untreated plants in the single-choice test (% parasitoids showing oriented flight: treated plant 44, control plant 20; χ2 = 6.62; P < 0.01; 50 parasitoids tested for each). The dual-choice wind tunnel test, in which individual A. ervi were offered a choice between treated and untreated plants, demonstrated that more than three times as many parasitoids oriented toward the (Z)-jasmone-treated plant compared with the control (% parasitoids orienting to: treated plant 32, control plant 10; χ2 = 5.762; P < 0.05; 50 parasitoids tested).
Table 1.
Responses of the aphid parasitoid Aphidius ervi in the wind tunnel to synthetic compounds (10 μg) released from filter paper (single-choice test)
| Stimulus | No. parasitoids tested | Mean % showing oriented flight* |
|---|---|---|
| (Z)-Jasmone | 64 | 52.22 ± 2.22a |
| (E,E)-α-Farnesene | 70 | 60.44 ± 5.27a |
| (E)-β-Ocimene | 63 | 46.25 ± 4.41a |
| Hexane control | 45 | 26.70 ± 0.00b |
Tests on 3 consecutive days. ANOVA analysis F = 16.19, P < 0.01. Values followed by a different letter are significantly different at P < 0.05 (Tukey multiple comparison test).
Effects of (Z)-Jasmone Vapor on V. faba.
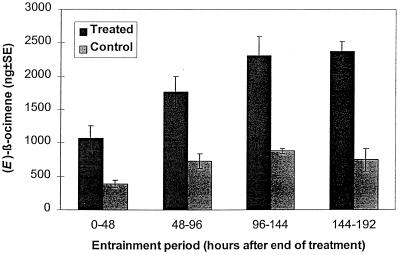
Plants that had been exposed to (Z)-jasmone in the vapor phase subsequently released significantly enhanced levels of (E)-β-ocimene, compared with control plants, over the four entrainment periods, i.e., 0–192 h (Fig. 3). The (Z)-jasmone itself was undetectable after 48 h. There was also, from some replicates, enhancement of levels of (E,E)-α-farnesene, β-caryophyllene, and (E)-4,8-dimethyl-1,3,7-nonatriene, although this was not consistent. In contrast, plants exposed to the vapor of methyl jasmonate showed increased levels of (E)-β-ocimene only during the first entrainment period, i.e., 0–48 h, and thereafter, levels released were similar to those from the control plants.
Figure 3.
Levels of (E)-β-ocimene produced by bean plants, V. faba, during 48-h entrainments after 24 h exposure to (Z)-jasmone (100 μg⋅liter-1 in air).
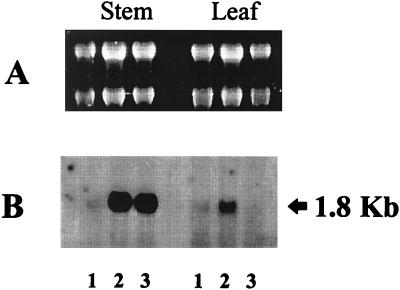
To determine whether (Z)-jasmone was capable of inducing alterations in plant gene expression, the sensitive technique of differential display was carried out on RNA extracted from plants that had been exposed to air, methyl jasmonate, or (Z)-jasmone. A number of the resulting PCR products were observed to show alterations in their abundance in the presence of (Z)-jasmone. To confirm this observation, bands of interest were recovered by excision from the dried gel and reamplified with the appropriate pair of oligonucleotide primers. The resulting PCR products were cloned and sequenced to confirm the homogeneous nature of the amplified product. These sequences then were used to probe RNA isolated from control or treated V. faba plants. As can be seen from Fig. 4, one particular sequence (D251, designated thus according to its size of 251 bp) was shown to be up-regulated in leaf tissue only in the presence of (Z)-jasmone. Interestingly, when this same (cloned) sequence was used to probe RNA isolated from V. faba stem tissues, it was up-regulated to a similar level in plants that had been treated with either (Z)-jasmone or methyl jasmonate. Although the nature of the differential display technique results in short gene-specific probes (containing mainly 3′ untranslated regions of transcribed sequences), sequencing of the D251 probe indicated that part of the sequence was highly similar to the C-terminal coding sequences of α-tubulin isoforms (Fig. 5). However, this homology does not extend beyond the coding sequence, implying that D251 is encoded by a novel, variant form of α-tubulin.
Figure 4.
Differential expression of a gene-specific sequence (D251) in V. faba plants. Total RNA was isolated from stem or leaf tissue of plants exposed to air, methyl jasmonate, or (Z)-jasmone. Ten micrograms RNA per sample was loaded and separated on a formaldehyde gel (A). The samples then were transferred to a nylon membrane and probed with the D251 sequence (B). Lane 1 = air treatment (control); lane 2 = (Z)-jasmone treatment; lane 3 = methyl jasmonate treatment. In the case of RNA isolated from leaf tissue, only (Z)-jasmone treatment results in up-regulation.
Figure 5.
Sequence comparison between the deduced amino acid sequence of the coding sequence of D251 and α-tubulin isoforms present on GenBank. Sequences shown are A. thaliana α-tubulin genes A1, A2, A4, A5, A6 and P. sativum α-tubulin A1.
Discussion
Jasmonic acid and its methyl ester are known to induce various pathways associated with the biosynthesis of secondary metabolites in plants (25); indeed, the ester, being a volatile compound, can act as an airborne signal for such processes (2, 5, 26–29). Other products from the lipoxygenase pathway of plants, from which jasmonic acid is derived, e.g., (E)-2-hexenal, are potent semiochemicals for aphids and other herbivorous insects. However, the roles described in this study have not previously been demonstrated for (Z)-jasmone, although its release from plants can be induced by damage, including that caused during feeding by lepidopterous larvae (9, 10). (Z)-Jasmone is a very volatile product of further catabolism of jasmonic acid; in fact, in elegant work on the metabolism of jasmonic acid, the question was raised as to whether (Z)-jasmone is a biologically inactive sink for the jasmonic acid pathway induced during plant defense (30). As a noninduced flower volatile, (Z)-jasmone is well known as an attractant of pollinating insects, although it also has been shown to attract the phytophagous Japanese beetle, Popillia japonica (Scarabaeidae), in field trapping trials (31).
By acting as a repellent to the morphs of N. ribis-nigri produced in early summer, (Z)-jasmone appears to play a role in the migration of the aphid from its winter host, R. nigrum. However, the widespread occurrence of this compound as a plant stress-related volatile may confer a more general role as an aphid repellent, as seen with the reduced attraction of P. humuli spring migrants to yellow water traps, (Z)-jasmone being undetectable in Prunus species that comprise the winter hosts of this aphid. Similar repellency to cereal aphids was demonstrated in the field, but in this case with aphids that do not usually alternate between summer and winter hosts. The question of whether the (Z)-jasmone is affecting the aphids directly or is modifying the cereal plant metabolism cannot as yet be answered unequivocally. The attractiveness of (Z)-jasmone to aphid antagonists such as the seven-spot ladybird, C. septempunctata, and the parasitoid A. ervi would be expected, because stress-related compounds, and often those arising from overcrowded colonies of herbivores and thereby acting as repellents, present valuable cues with which to locate prey on host plants. Thus, at the second trophic level, there is repellency by (Z)-jasmone, probably related either to inappropriateness of the host taxonomy or more generally through stress-induced mechanisms. However, at the higher trophic level, i.e., the third, there can be utilization of (Z)-jasmone as a signal indicating the presence of herbivorous hosts.
As proposed in the original hypothesis initiating this study, plant stress-related compounds such as methyl salicylate, having roles as semiochemicals for insects at various trophic levels, can be anticipated to have direct effects on plants. Therefore, although such activity had not previously been observed for (Z)-jasmone, its newly discovered role as a stress-related insect semiochemical suggested that this compound also may influence release of semiochemicals from intact plants. This was demonstrated when V. faba plants, exposed to the vapor of (Z)-jasmone and tested when the compound itself was no longer detectable, proved to be more attractive to the parasitoid A. ervi than control plants. V. faba was chosen for this experiment because it serves as a food plant for the pea aphid, Acyrthosiphon pisum, the preferred host of A. ervi. Increased levels of (E,E)-α-farnesene, β-caryophyllene, (E)-4,8-dimethyl-1,3,7-nonatriene, and particularly (E)-β-ocimene were induced, all of these compounds having been implicated in increased parasitoid foraging on insect damaged plants (32, 33). The nonatriene also can be produced innately by plants imitating damage for defense against herbivores and which are thus attractive to parasitoids (34).
Because (Z)-jasmone is biosynthetically related to jasmonic acid, the activity of the volatile methyl jasmonate was investigated with V. faba under the same conditions and at the same concentration as (Z)-jasmone. In contrast to treatment with (Z)-jasmone, which resulted in significantly increased levels of (E)-β-ocimene production over a period of at least 8 days, exposure to methyl jasmonate had only a short-term effect, which demonstrates that the two compounds, as airborne signals, have quite different properties. It is also noted that, whereas jasmonic acid and methyl jasmonate stimulate production of secondary metabolites such as paclitaxel (Taxol) in cell suspension cultures of the yew Taxus media (Taxaceae), (Z)-jasmone has no significant effect (35).
Using differential display and confirmatory Northern blotting, we have shown that methyl jasmonate and (Z)-jasmone have apparently distinct effects on plant gene expression. In this study, the differentially displayed PCR product D251 was cloned and used to probe Northern blots from leaf or stem tissues of V. faba plants treated with air, methyl jasmonate, or (Z)-jasmone. This clearly showed (Fig. 4) that, whereas the D251 sequence was up-regulated by treatment with vapors of both compounds in stem tissue, only (Z)-jasmone brought about an increase in the steady-state transcript level of this sequence in leaf tissue. Thus, the two compounds have distinct effects on plant gene expression and the response to these signaling compounds may be tissue-specific. We have identified the D251 sequence as being derived from a gene encoding an α-tubulin isoform, based on the clear homology between the coding sequence contained in D251 and the α-tubulins (Fig. 5). This homology does not extend into the 3′ untranslated region of D251, when compared with known α-tubulin gene sequences, and is the likely reason for the D251-specific nature of the hybridization observed in Fig. 4. The precise reason for the tissue-specific up-regulation by (Z)-jasmone of an α-tubulin isoform remains to be determined, although tubulins are considered to play an essential role in plant cell division and morphology (36). Studies on the α-tubulin A1 gene of Pisum sativum (Fabaceae) did not reveal any tissue-specific expression patterns, although the authors speculate that other α-tubulin isoforms might be expected to display differential expression patterns (37). This has previously been observed with the pollen-specific expression of an Arabidopsis thaliana (Brassicaeae) α-tubulin A1 gene (38).
Conclusions
We propose that rather than (Z)-jasmone being considered as another lipoxygenase-derived volatile and a possible sink for jasmonic acid, it should be viewed as a potential airborne plant signal relating to other aspects of plant signaling, although we have not yet demonstrated a role as a signal acting between plants. It also should be noted that (Z)-jasmone is more volatile than methyl jasmonate and, as such, could be a more effective signal compound. We have demonstrated that, far from being biologically inactive, (Z)-jasmone has activity at all three trophic levels investigated in this study.
Considerable advances have been made by the identification of volicitin [α-N-(17-hydroxylinolenoyl)-l-glutamine] as an elicitor, produced during feeding by caterpillars, that causes generation of parasitoid foraging stimuli such as (E)-4,8-dimethyl-1,3,7-nonatriene (39). The work reported here identifies a compound significantly inducing production of these types of compounds as an airborne signal. (Z)-Jasmone, an extremely benign component of perfumes and floral volatiles (40), eventually may find use in inducing gene expression for proteins with functions beyond the secondary metabolism involved in insect/plant interactions, because (Z)-jasmone responsive promoters could be useful tools in the molecular genetic engineering of novel traits in plants.
Acknowledgments
We acknowledge the help given by A. Todd of the Institute of Arable Crops Research-Rothamsted with the statistical analysis of some of the results. The Institute of Arable Crops Research receives grant-aided support from the Biotechnology and Biological Sciences Research Council of the United Kingdom. C.A.M.C. was funded by the Ministry of Agriculture Fisheries and Food. E.G. was funded by a National Research Council (Consiglio Nazionale delle Ricerche) Fellowship, and investigations in Sweden were supported by the Foundation for Strategic Environmental Research.
Abbreviations
- SCR
single-cell recording
- EAG
electroantennography
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.160241697.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.160241697
References
- 1.Agrawal A A, Karban R. In: The Ecology and Evolution of Inducible Defenses. Tollrian R, Harvell C D, editors. Princeton: Princeton Univ. Press; 1999. pp. 45–61. [Google Scholar]
- 2.Farmer E E, Ryan C A. Proc Natl Acad Sci USA. 1990;87:7713–7716. doi: 10.1073/pnas.87.19.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shulaev V, Silverman P, Raskin I. Nature (London) 1997;385:718–721. [Google Scholar]
- 4.Boland W, Hopke J, Piel J. In: Natural Product Analysis. Schreier P, Herderich M, Humpf H U, Schwab W, editors. Wiesbaden: Vieweg; 1998. pp. 255–269. [Google Scholar]
- 5.Thaler J S, Stout M J, Karban R, Duffey S S. J Chem Ecol. 1996;22:1767–1781. doi: 10.1007/BF02028503. [DOI] [PubMed] [Google Scholar]
- 6.Bate N J, Rothstein S J. Plant J. 1998;16:561–569. doi: 10.1046/j.1365-313x.1998.00324.x. [DOI] [PubMed] [Google Scholar]
- 7.Pettersson J, Pickett J A, Pye B J, Quiroz A, Smart L E, Wadhams L J, Woodcock C M. J Chem Ecol. 1994;20:2565–2574. doi: 10.1007/BF02036192. [DOI] [PubMed] [Google Scholar]
- 8.Hardie J, Isaacs R, Pickett J A, Wadhams L J, Woodcock C M. J Chem Ecol. 1994;20:2847–2855. doi: 10.1007/BF02098393. [DOI] [PubMed] [Google Scholar]
- 9.Loughrin J H, Manukian A, Heath R R, Tumlinson J H. J Chem Ecol. 1995;21:1217–1227. doi: 10.1007/BF02228321. [DOI] [PubMed] [Google Scholar]
- 10.Pare P W, Tumlinson J H. Plant Physiol. 1997;114:1161–1167. doi: 10.1104/pp.114.4.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Craveiro A A, Matos F J A, Alencar J W, Plumel M M. Flavour Fragrance J. 1989;4:43–44. [Google Scholar]
- 12.Maddrell S H P. J Exp Biol. 1969;51:71–97. [Google Scholar]
- 13.Wadhams L J. In: Chromatography and Isolation of Insect Hormones and Pheromones. McCaffery A R, Wilson I D, editors. New York: Plenum; 1990. pp. 289–298. [Google Scholar]
- 14.Wadhams L J, Angst M E, Blight M M. J Chem Ecol. 1982;8:477–492. doi: 10.1007/BF00987796. [DOI] [PubMed] [Google Scholar]
- 15.Chou T S, Tso H H, Chang L J. J Chem Soc Chem Commun. 1984;20:1323–1324. [Google Scholar]
- 16.Boland W, Gaebler A. Helv Chim Acta. 1989;72:247–253. [Google Scholar]
- 17.Pettersson J. Entomol Scand. 1970;1:63–73. [Google Scholar]
- 18.Du Y-J, Poppy G M, Powell W. J Chem Ecol. 1996;16:381–396. doi: 10.1007/BF02272400. [DOI] [PubMed] [Google Scholar]
- 19.Du Y-J, Poppy G M, Wadhams L J. J Insect Behav. 1997;10:509–522. [Google Scholar]
- 20.Genstat 5 Committee. genstat 5 Reference Manual, Release 3. Oxford: Clarenden; 1993. [Google Scholar]
- 21.Liang P, Pardee A B. Science. 1992;257:967–970. doi: 10.1126/science.1354393. [DOI] [PubMed] [Google Scholar]
- 22.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 23.Sayanova O, Smith M A, Lapinskas P, Dobson G, Christie W W, Stobart A K, Shewry P R, Napier J A. Proc Natl Acad Sci USA. 1997;94:4211–4216. doi: 10.1073/pnas.94.8.4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Institute of Standards and Technology. Standard Reference Data Base (Version 3.0.1) Gaithersburg, MD: National Institute of Standards and Technology; 1990. [Google Scholar]
- 25.Blechert S, Brodschelm W, Holder S, Kammerer L, Kutchan T M, Mueller M J, Xia Z-Q, Zenk M H. Proc Natl Acad Sci USA. 1995;92:4099–4105. doi: 10.1073/pnas.92.10.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farmer E E, Johnson R R, Ryan C A. Plant Physiol. 1992;98:995–1002. doi: 10.1104/pp.98.3.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doughty K J, Kiddle G A, Pye B J, Wallsgrove R M, Pickett J A. Phytochemistry. 1995;38:347–350. [Google Scholar]
- 28.Farmer E E. Science. 1997;276:912–913. [Google Scholar]
- 29.Wasternack C, Parthier B. Trends Plant Sci. 1997;2:302–307. [Google Scholar]
- 30.Koch T, Bandemer K, Boland W. Helv Chim Acta. 1997;80:838–850. [Google Scholar]
- 31.Loughrin J H, Potter D A, Hamilton-Kemp T R. Environ Entomol. 1998;27:395–400. [Google Scholar]
- 32.Turlings T C J, Loughrin J H, McCall P J, Rose U S R, Lewis W J, Tumlinson J H. Proc Natl Acad Sci USA. 1995;92:4169–4174. doi: 10.1073/pnas.92.10.4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pare P W, Tumlinson J H. Nature (London) 1997;385:30–31. [Google Scholar]
- 34.Khan Z R, Ampong-Nyarko K, Chiliswa P, Hassanali A, Kimani S, Lwande W, Overholt W A, Pickett J A, Smart L E, Wadhams L J, Woodcock C M. Nature (London) 1997;388:631–632. [Google Scholar]
- 35.Yukimune Y, Tabata H, Higashi Y, Hara Y. Nat Biotechnol. 1996;14:1129–1132. doi: 10.1038/nbt0996-1129. [DOI] [PubMed] [Google Scholar]
- 36.Fosket D E, Morejohn L C. Annu Rev Plant Physiol Plant Mol Biol. 1992;43:201–240. [Google Scholar]
- 37.Brierley H L, Webster P, Long S R. Plant Mol Biol. 1995;27:715–727. doi: 10.1007/BF00020225. [DOI] [PubMed] [Google Scholar]
- 38.Carpenter J L, Ploense S E, Snustad D P, Silflow C D. Plant Cell. 1992;4:557–571. doi: 10.1105/tpc.4.5.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alborn H T, Turlings T C J, Jones T H, Stenhagen G, Loughrin J H, Tumlinson J H. Science. 1997;276:945–949. [Google Scholar]
- 40.Schlotzhauer W S, Pair S D, Horvat R J. J Agric Food Chem. 1996;44:206–209. [Google Scholar]