Abstract
Dengue viruses and malaria protozoa are of increasing global concern in public health. The diseases caused by these pathogens often show regular seasonal patterns in incidence because of the sensitivity of their mosquito vectors to climate. Between years in endemic areas, however, there can be further significant variation in case numbers for which public health systems are generally unprepared. There is an acute need for reliable predictions of within-year and between-year epidemic events. The prerequisite for developing any system of early warning is a detailed understanding of the factors involved in epidemic genesis. In this report we discuss the potential causes of the interepidemic periods in dengue hemorrhagic fever in Bangkok and of Plasmodium falciparum malaria in a highland area of western Kenya. The alternative causes are distinguished by a retrospective analysis of two unique and contemporaneous 33-year time series of epidemiological and associated meteorological data recorded at these two sites. We conclude that intrinsic population dynamics offer the most parsimonious explanation for the observed interepidemic periods of disease in these locations.
Dengue fever is caused by infection with dengue viruses of the family Flaviviridae, transmitted principally by Aedes aegypti mosquitoes in the tropical and subtropical regions of the world (1, 2). There are four antigenically related but distinct dengue virus serotypes, each causing strong homologous immunity but short-lived cross-immunity to reinfection (3). Fifty to one hundred million cases of dengue fever are estimated to occur annually, along with several hundred thousand cases of the life-threatening form of the disease, dengue hemorrhagic fever (DHF) (4). The geographic range of dengue has expanded over the last two decades, primarily because of the spread of its principal vector, Aedes aegypti (5). Furthermore, dengue has shown a dramatic increase in incidence within its range, with many large urban centers becoming newly endemic for the disease (6). Continued trends of rapid population growth, increasing aggregation in urban centers, and ever larger volumes of international travel, combined with a lack of effective vector control, have encouraged rapid viral evolution (7) and collectively augur an increasingly serious public health problem.
Plasmodium falciparum malaria parasites are transmitted mainly by the Anopheles gambiae complex in rural Africa. In this region alone P. falciparum infections are thought to result in approximately 200 million clinical events and 1 million deaths per annum, approximately 75% of which are children (8). In addition, there is growing concern that patterns of malaria on the African continent are changing, with increased risk for severe complicated disease and mortality (9). Changes in environmental management, population migration patterns, focal breakdown in health service provision, rising drug resistance, and global warming have all been suggested as contributing to these changes (10–13). These themes again point to an increasing problem for the global health agenda.
The mosquito vectors of dengue and malaria parasites are extremely sensitive to climate. Meteorological conditions directly influence vector reproduction and mortality rates and thereby control population distribution and abundance. Moreover, they indirectly affect epidemiologically significant factors such as the blood feeding frequency of the vector and the extrinsic incubation period of the pathogen (12, 14). These effects often result in predictable annual cycling, or seasonality, in mosquito-borne diseases (15). In addition to this seasonal variation, however, there are also occasional large increases in clinical burden, “epidemics,” that often overwhelm health care services (2, 13). In endemic disease settings, periodic superannual fluctuations in incidence have been observed in a variety of vector-borne diseases (16–21).
Epidemics of dengue fever (22, 23) and historical and contemporary malaria epidemics (24–29) have also more recently been associated with the El Niño phase of the Southern Oscillation (ENSO). The Southern Oscillation is a periodic interannual biphasic variation in sea-level pressure across the Pacific Ocean that drives a complex global system of meteorological perturbations (30, 31). The El Niño period is characterized by high surface pressure over the western Pacific and low surface pressure over the southeastern Pacific; the complementary phase is termed La Niña. During El Niño, conditions are usually said to be drier and hotter in Thailand and wetter in Kenya (32, 33), although recent evidence suggests that coupling of the ENSO and climate around the Indian Ocean region is not as strong as previously suggested (34). In addition to the claimed links with dengue and malaria, the Southern Oscillation has also been related to outbreaks of mosquito-borne Murray Valley encephalitis in southeastern Australia (35, 36), bluetongue virus in northern and eastern Australia (37), and Rift Valley fever in Kenya (38).
In sharp contrast to the studies that have emphasized climatic determinants of vector-borne disease epidemics, epidemiological theory has modeled these periodic phenomena as resulting from the dynamic interaction between host and parasite/pathogen populations (39). In unforced Susceptible, Exposed, Infectious, Recovered (SEIR) models of directly transmitted diseases, incidence is predicted to exhibit damped oscillations with an approximate interepidemic period (T):
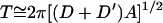 |
1 |
where D is the latent interval and D′ is the infectious interval of the disease and A is the average age of first infection. In models with demographic or environmental stochasticity, resonance ensures that these oscillations are sustained, provided the population is large enough for the pathogen to avoid extinction. Such interactions occur regardless of changes in the abiotic environment, although many forcing mechanisms, including seasonality, have been suggested to be able to maintain these oscillations (40).
Recent extensions of this work allow for diseases, such as P. falciparum malaria, that do not confer life-long immunity on first infection and whose macro-epidemiology reflects a disease spectrum of independently transmitted “strains” (41). Simple dynamic models of negative strain interactions on the transmission dynamics of antigenically diverse pathogens, such as dengue viruses and P. falciparum, demonstrate that sustained cyclical or chaotic dynamics can result from moderate levels of strain cross-immunity (42). These dynamics are determined by the duration of infectiousness within the human host; short-period cycles of a few years result from infectious intervals of a few days, whereas long-period cycles of many years occur for diseases with infectious durations approaching a month or longer. Models of positive “strain” interactions such as antibody-dependent enhancement in dengue can also result in persistent cyclical and chaotic behavior (43). In modeling a two-strain system, researchers have found stable interepidemic periods of 0–5 years across a wide range of antibody-dependent enhancement. This work has been further corroborated by analyses of age-stratified seroprevalence data of dengue in Thailand, in which the force of infection was found to vary over a 3- to 4-year period (44).
There are, therefore, two possible sets of causes of regular between-year epidemic events, one extrinsic and associated with ENSO-type climate phenomena, the other intrinsic and associated with host pathogen population dynamics. It is essential to determine the etiology of interepidemic periods and the relative importance of intrinsic and extrinsic influences, to develop accurate early warning systems for these diseases. These factors are distinguished here, using two unique epidemiological time series: one a record of DHF in the Bangkok–Thonburi metropolitan area (hereafter Bangkok) and the other a record of P. falciparum malaria in the rural highlands of Kericho in western Kenya.
Total monthly admissions of patients with DHF were recorded at various hospitals in the Bangkok area (13° 50" N, 100° 40" E) from 1966 to 1998 inclusive. DHF cases throughout the observation period were based on clinical diagnoses (high fever, plasma leakage, and hemoconcentration) defined formally later by the World Health Organization (45). Subsequent serological studies in Bangkok have shown such clinical diagnoses for DHF to be highly specific (46, 47). It was not possible to determine whether the DHF cases reported represent the total burden of DHF in Bangkok or a proportion of the DHF cases were acquired elsewhere. There is no reason to assume, however, that these factors introduced significant biases during the observation period; the DHF data presented therefore represent a reliable sample of the disease in Bangkok. Furthermore, other potentially complicating factors, such as intermittent control activities (48) and increased affluence leading to increased screening of households, have not stopped the increase in the incidence of DHF in Bangkok during the observation period (49). Mean monthly temperature (oC) and total monthly rainfall (mm) data were measured for the same period at the Sukhumvit Road meteorological station in central Bangkok. The DHF case data were converted to incidence figures, using census information provided for 1960, 1970, 1980, and 1990, a period during which the population of Bangkok increased from approximately 2 million to 5 million.
The malaria data originate from the health care system established by Brooke Bond Kenya Ltd. at its tea estates in Kericho (0° 22" S, 35° 17" E), located in the Rift Valley highlands of western Kenya (9). Monthly hospital admissions of P. falciparum malaria cases, confirmed through microscopy, were systematically recorded in ward registers for 1966–1998 inclusive. Case numbers in Kericho were treated as incidence figures, because the population eligible for health care remained approximately 100,000 throughout the recording period. Climate data were also available from 1966 to 1998 as mean monthly temperature (oC) and monthly rainfall totals (mm) recorded at the center of the estates by the Tea Research Foundation. No preventative chemoprophylaxis, house spraying with residual insecticides, or bed net distribution has been implemented since the late 1950s (50). Patients whose ethnic group did not originate in the highlands were excluded from the analyses, as they were frequently found to acquire malaria while on leave. The Multivariate El Niño Southern Oscillation Index (MENSOI) (51, 52) (available at http://www.cdc.noaa.gov/∼kew/MEI/) was also obtained for the 1966–1998 period. This index uses six variables over the tropical Pacific to parameterize the Southern Oscillation: sea-level pressure, zonal and meridional components of the surface wind, sea surface temperature, surface air temperature, and total cloudiness fraction of the sky. The MENSOI index is the unrotated first principal component of the combined fields standardized by season and a 1950–1993 reference period (51). Positive values represent El Niño and negative values represent La Niña.
The time-series technique of spectral density analysis (SDA) (53) was then used to investigate periodicity in both the epidemiological and meteorological data, after insights gained from its application to directly transmitted diseases (54, 55). The SDA was implemented with the trends 6.1 module of the Statistics Package for the Social Sciences (SPSS, Chicago). The monthly epidemiological and meteorological time series for Bangkok and Kericho, as well as the MENSOI data, were first made stationary (detrended) by using a 61-point moving average. This approximately 5-year moving average was used because cycles of any longer duration would not be detected in a 32-year time series. Moving averages of 3–141 months were applied and the SDA was repeated, to check that the detrending did not influence the results. SDA partitions the total variance of a series into orthogonal sinusoidal components at different frequencies, and the squared amplitudes of these frequencies represent their contribution to the total variance of the data set. Spectral density plots of these squared amplitudes are smoothed and plotted either against frequency (in which case the areas under different segments of the curve represent the total contribution of those frequencies to the total variance) or against period, which generally makes interpretation easier. The latter are shown for the present data sets in Figs. 1 b–d, 2 b–d, and 3b.
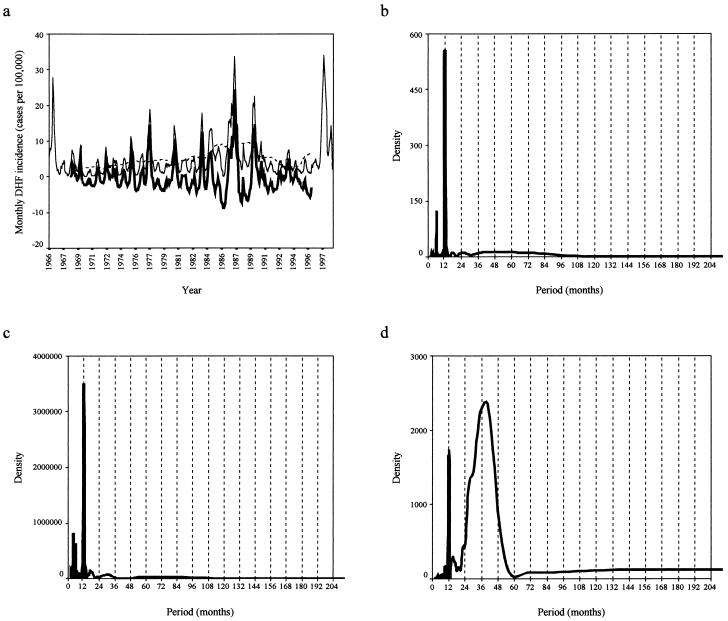
Figure 1.
(a) A line graph showing the monthly incidence (cases per 100,000) of DHF in Bangkok from January 1966 to December 1998. The dashed line shows a moving average of 61 months and the bold line, the stationary DHF incidence series (original value − moving average) on which SDA was performed. (b–d) The spectral density plots of mean monthly temperature (b), total monthly rainfall (c), and total DHF incidence (d) for Bangkok are shown. A Tukey–Hamming window of three points was applied to smooth the spectral density plots. Details of the variance structure from the periodograms (unsmoothed spectral density plots of frequency) show that annual and short frequencies account for 31.5% of the total variance in the DHF time series and superannual frequencies account for 68.5%. For the temperature and rainfall time series, the annual/superannual variance split is 92.5:7.5 and 94.0:6.0, respectively.
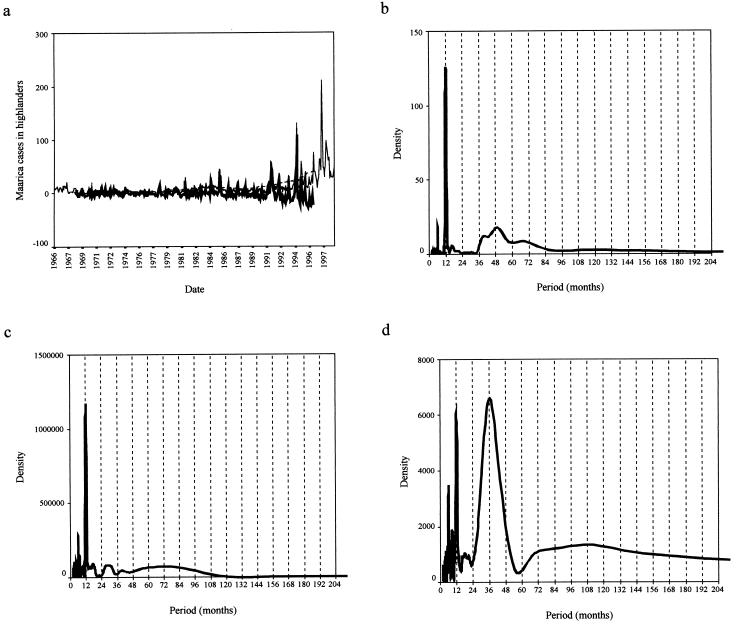
Figure 2.
(a) A line graph showing the monthly incidence (cases per 100,000) of P. falciparum malaria incidence (cases per 100,000) in Kericho from January 1966 to December 1998. (b–d) The SDA of mean monthly temperature (b), total monthly rainfall (c), and total malaria incidence (d) for Kericho are shown. As for Fig. 1 b–d, except that annual and shorter frequencies account for 69.8% of the total variance in the malaria time series and superannual frequencies account for 30.2%. For the temperature and rainfall time series the annual/superannual variance split is 82.1:17.9 and 89.1:10.9, respectively.
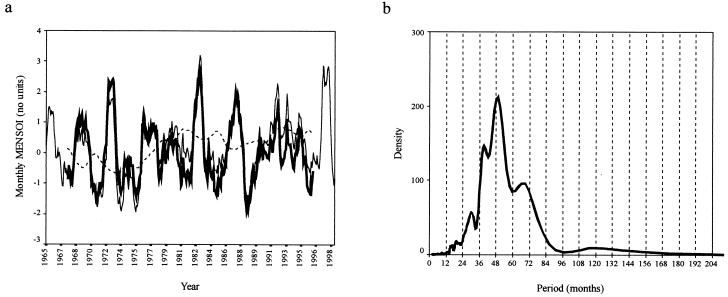
Figure 3.
(a) A line graph showing the monthly MENSOI from January 1966 to December 1998. (b) SDA plot of the MENSOI. As for Fig. 1 b–d, except that details of the variance structure from the periodogram show that annual and shorter frequencies account for 4.5% of the total variance in the MENSOI time series and superannual frequencies account for 95.5%.
The SDA for both temperature (Fig. 1b) and rainfall (Fig. 1c) in Bangkok revealed well-defined peaks at 12 months, which accounted for over 90% of the series variance and illustrated the intense annual seasonality of climate in the region. The DHF incidence data also showed annual variation (frequencies of up to 1 year accounted for ≈30% of series variance), but the majority of change in case numbers (≈70% of series variance) was explained by superannual periods centered around a 3-year periodicity (Fig. 1d). In Kericho, the SDA also showed strong annual variation in the temperature (Fig. 2b) and rainfall (Fig. 2c) data, with 80% and 90% of series variance, respectively, explained by periods of up to 12 months. The malaria incidence data show both annual (70%) and superannual (30%) variation, again with a period of approximately 3 years. In addition to an annual variation, therefore, both diseases have an approximately 3-year interepidemic period that was not found in the contemporaneous climate data. It is interesting to note that despite invalidating the central assumption of life-long immunity on first infection, Eq. 1 predicts an interepidemic period of 35 months for DHF [D = 5 days, D′ = 0.2 month, A = 84 months (56)] and 34 months for P. falciparum malaria [D = 9–10 days, D′ = 9.5 months, A = 3.5 months (57)], which are very close to those resolved by the SDA.
The SDA of the MENSOI (Fig. 3b) for the 1966–1998 period shows peaks of approximately 4-year periodicity. The 3-year period cycle in these data are significantly less pronounced than the longer period cycles. Although cyclical variations are evident in the MENSOI, these clearly do not substantially affect rainfall and temperature in Bangkok or Kericho and thus can exert no “teleconnection” with disease incidence. The lack of a strong superannual periodicity in the climate data and the poor relationship between climate at Bangkok and Kericho and ENSO are not consistent with the hypothesis that interepidemic periods are determined by climate, at least for these mosquito-borne diseases at these locations. We conclude that intrinsic population dynamic processes offer the most parsimonious explanation for the interepidemic periods of DHF incidence in Bangkok and clinical P. falciparum malaria cases in Kericho. We further assert that epidemiological theory offers a more plausible platform than epidemiological teleconnections on which to build models for epidemic prediction. Combining within-year extrinsic and between-year intrinsic determinants of mosquito-borne disease incidence for epidemic prediction should be the focus of future research.
Acknowledgments
We thank Sarah Randolph, Matthew Baylis, Steve Lindsay, Assaf Anyamba, Sunetra Gupta, and two anonymous referees for comments on the manuscript. This work was funded principally by NASA's Interagency Research Partnership for Infectious Diseases (IntRePID) program. S.I.H. is currently supported as an Advanced Training Fellow by The Wellcome Trust (056642). D.W.V., T.E., N.A., and G.D.S. are supported by the U.S. Army Medical Research and Materiel Command, Ft. Detrick, MD. R.W.S. is a Senior Wellcome Trust Fellow (33340) and acknowledges the support of the Kenya Medical Research Institute.
Abbreviations
- DHF
dengue hemorrhagic fever
- ENSO
El Niño phase of the Southern Oscillation
- MENSOI
Multivariate El Niño Southern Oscillation Index
- SDA
spectral density analysis
References
- 1.Holmes E, Bartley L, Garnett G. In: Emerging Infections. Krause R, editor. London: Academic; 1998. pp. 301–325. [Google Scholar]
- 2.Gubler D. Clin Microbiol Rev. 1998;11:480–498. doi: 10.1128/cmr.11.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuno G, Gubler D J, Oliver A. Trans R Soc Trop Med Hyg. 1993;87:103–105. doi: 10.1016/0035-9203(93)90444-u. [DOI] [PubMed] [Google Scholar]
- 4.Halstead S B. In: Dengue and Dengue Hemorrhagic Fever. Gubler D J, Kuno G, editors. Wallingford, U.K.: CAB International; 1997. pp. 23–44. [Google Scholar]
- 5.Gubler D J. In: Dengue and Dengue Hemorrhagic Fever. Gubler D J, Kuno G, editors. Wallingford, U.K.: CAB International; 1997. pp. 1–22. [Google Scholar]
- 6.Rigau-Perez J G, Clark G G, Gubler D J, Reiter P, Sanders R J, Vorndam A V. Lancet. 1998;352:971–977. doi: 10.1016/s0140-6736(97)12483-7. [DOI] [PubMed] [Google Scholar]
- 7.Page R D M, Holmes E C. Molecular Evolution: A Phylogenetic Approach. Oxford: Blackwell Science; 1998. [Google Scholar]
- 8.Snow R W, Craig M, Deichmann U, Marsh K. Bull W H O. 1999;77:624–640. [PMC free article] [PubMed] [Google Scholar]
- 9.Shanks G D, Biomondo K, Hay S I, Snow R W. Trans R Soc Trop Med Hyg. 2000;94:253–255. doi: 10.1016/s0035-9203(00)90310-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenberg A E, Ntumbanzondo M, Ntula N, Mawa L, Howell J, Davachi F. Bull W H O. 1989;67:189–196. [PMC free article] [PubMed] [Google Scholar]
- 11.Loevinsohn M E. Lancet. 1994;343:714–718. doi: 10.1016/s0140-6736(94)91586-5. [DOI] [PubMed] [Google Scholar]
- 12.Lindsay S W, Birley M H. Ann Trop Med Parasitol. 1996;90:573–588. doi: 10.1080/00034983.1996.11813087. [DOI] [PubMed] [Google Scholar]
- 13.Mouchet J, Manguin S, Sircoulon J, Laventure S, Faye O, Onapa A W, Carnevale P, Julvez J, Fontenille D. J Am Mosq Control Assoc. 1998;14:121–130. [PubMed] [Google Scholar]
- 14.Kuno G. In: Dengue and Dengue Hemorrhagic Fever. Gubler D J, Kuno G, editors. Wallingford, U.K.: CAB International; 1997. pp. 61–88. [Google Scholar]
- 15.Hay S I, Snow R W, Rogers D J. Trans R Soc Trop Med Hyg. 1998;92:12–20. doi: 10.1016/s0035-9203(98)90936-1. [DOI] [PubMed] [Google Scholar]
- 16.Gill C A. The Genesis of Epidemics and the Natural History of Disease. New York: Wood; 1928. [Google Scholar]
- 17.Yacob K B M, Swaroop S. J Malaria Inst India. 1945;6:39–51. [PubMed] [Google Scholar]
- 18.Swaroop S. Am J Trop Med Hyg. 1946;29:1–17. doi: 10.4269/ajtmh.1949.s1-29.1. [DOI] [PubMed] [Google Scholar]
- 19.de Zulueta J, Mujtaba S M, Shah I H, de Zulueta J. Trans R Soc Trop Med Hyg. 1980;74:624–632. doi: 10.1016/0035-9203(80)90153-4. [DOI] [PubMed] [Google Scholar]
- 20.Dye C, Wolpert D M. Trans R Soc Trop Med Hyg. 1988;82:843–850. doi: 10.1016/0035-9203(88)90013-2. [DOI] [PubMed] [Google Scholar]
- 21.Rogers D J. Philos Trans R Soc London B. 1988;321:513–539. doi: 10.1098/rstb.1988.0106. [DOI] [PubMed] [Google Scholar]
- 22.Hales S, Weinstein P, Woodward A. Lancet. 1996;348:1664–1665. doi: 10.1016/S0140-6736(05)65737-6. [DOI] [PubMed] [Google Scholar]
- 23.Hales S, Weinstein P, Souares Y, Woodward A. Environ Health Perspect. 1999;107:99–102. doi: 10.1289/ehp.9910799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bouma M J, van der Kaay H J. Lancet. 1994;344:1638–1639. doi: 10.1016/s0140-6736(94)90432-4. [DOI] [PubMed] [Google Scholar]
- 25.Bouma M J, van der Kaay H J. Trop Med Int Health. 1996;1:86–96. doi: 10.1046/j.1365-3156.1996.d01-7.x. [DOI] [PubMed] [Google Scholar]
- 26.Bouma M J, Dye C. J Am Med Assoc. 1997;278:1772–1774. [PubMed] [Google Scholar]
- 27.Bouma M J, Poveda G, Rojas W, Chavasse D, Quiñones M, Cox J, Patz J. Trop Med Int Health. 1997;2:1122–1127. doi: 10.1046/j.1365-3156.1997.d01-210.x. [DOI] [PubMed] [Google Scholar]
- 28.Kilian A H D, Langi P, Talisuna A, Kabagambe G. Trans R Soc Trop Med Hyg. 1999;93:22–23. doi: 10.1016/s0035-9203(99)90165-7. [DOI] [PubMed] [Google Scholar]
- 29.Lindblade K A, Walker E D, Onapa A W, Katungu J, Wilson M L. Trans R Soc Trop Med Hyg. 1999;93:480–487. doi: 10.1016/s0035-9203(99)90344-9. [DOI] [PubMed] [Google Scholar]
- 30.Philander S G. El Niño, La Niña, and the Southern Oscillation. London: Academic; 1990. pp. 9–57. [Google Scholar]
- 31.McPhaden M J. Science. 1999;283:950–954. doi: 10.1126/science.283.5404.950. [DOI] [PubMed] [Google Scholar]
- 32.Ropelewski C F. Nature (London) 1992;356:476–477. [Google Scholar]
- 33.Epstein P R. Science. 1999;285:347–348. doi: 10.1126/science.285.5426.347. [DOI] [PubMed] [Google Scholar]
- 34.Saji N H, Goswami B N, Vinayachndran P N, Yamagata T. Nature (London) 1999;401:360–363. doi: 10.1038/43854. [DOI] [PubMed] [Google Scholar]
- 35.Nicholls N. Aust J Exp Biol Med Sci. 1986;64:587–594. doi: 10.1038/icb.1986.62. [DOI] [PubMed] [Google Scholar]
- 36.Nicholls N. Lancet. 1993;324:1284–1285. doi: 10.1016/0140-6736(93)92368-4. [DOI] [PubMed] [Google Scholar]
- 37.Ward M P, Johnson S J. Prevent Vet Med. 1996;28:57–68. [Google Scholar]
- 38.Linthicum K J, Anyamba A, Tucker C J, Kelley P W, Myers M F, Peters C J. Science. 1999;285:397–400. doi: 10.1126/science.285.5426.397. [DOI] [PubMed] [Google Scholar]
- 39.Anderson R M, May R M. Infectious Diseases of Humans: Dynamics and Control. Oxford: Oxford Univ. Press; 1991. [Google Scholar]
- 40.Aron J L, Schwartz I B. J Theor Biol. 1984;110:665–679. doi: 10.1016/s0022-5193(84)80150-2. [DOI] [PubMed] [Google Scholar]
- 41.Gupta S, Trenholme K, Anderson R M, Day K P. Science. 1994;263:961–963. doi: 10.1126/science.8310293. [DOI] [PubMed] [Google Scholar]
- 42.Gupta S, Ferguson N, Anderson R. Science. 1998;280:912–915. doi: 10.1126/science.280.5365.912. [DOI] [PubMed] [Google Scholar]
- 43.Ferguson N, Anderson R, Gupta S. Proc Natl Acad Sci USA. 1999;96:790–794. doi: 10.1073/pnas.96.2.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ferguson N M, Donnelly C A, Anderson R M. Philos Trans R Soc London B. 1999;354:757–768. doi: 10.1098/rstb.1999.0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.W.H.O. Dengue Haemorrhagic Fever: Diagnosis, Treatment and Control. Geneva: W.H.O.; 1986. [Google Scholar]
- 46.Vaughn D W, Green S, Kalayanarooj S, Innis B L, Nimmannitya S, Suntayakorn S, Rothman A L, Ennis F A, Nisalak A. J Infect Dis. 1997;176:322–330. doi: 10.1086/514048. [DOI] [PubMed] [Google Scholar]
- 47.Vaughn D W, Green S, Kalayanarooj S, Innis B L, Nimmannitya S, Suntayakorn S, Raengsakulrach B, Rothman A L, Ennis F A, Nisalak A. J Infect Dis. 2000;181:2–9. doi: 10.1086/315215. [DOI] [PubMed] [Google Scholar]
- 48.Gratz N G. Med Vet Entomol. 1993;7:1–10. doi: 10.1111/j.1365-2915.1993.tb00644.x. [DOI] [PubMed] [Google Scholar]
- 49.Chareonsook O, Foy H M, Teeraratkul A, Silarug N. Epidemiol Infect. 1999;122:161–166. doi: 10.1017/s0950268898001617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Malakooti M A, Biomndo K, Shanks G D. J Emerging Infect Dis. 1998;4:671–676. doi: 10.3201/eid0404.980422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wolter K. Climate Appl Meteorol. 1987;26:540–558. [Google Scholar]
- 52.Wolter K, Timlin M S. Weather. 1998;53:315–324. [Google Scholar]
- 53.Chatfield C. The Analysis of Time-Series: Theory and Practice. London: Chapman & Hall; 1996. [Google Scholar]
- 54.Anderson R M, Grenfell B T, May R M. J Hyg. 1984;93:587–608. doi: 10.1017/s0022172400065177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sumi A, Ohtomo N, Tanaka Y, Koyama A, Saito K. Jpn J Appl Phys. 1997;36:1303–1318. [Google Scholar]
- 56.Molineaux L, Gramiccia G. The Garki Project. Geneva: W.H.O.; 1980. [Google Scholar]
- 57.Siler J F, Hall M W, Hitchens A P. Philip J Sci. 1926;29:1–304. [Google Scholar]