Abstract
Objective: To describe a patient with treatment-refractory pyoderma gangrenosum and the outcome of a novel therapeutic approach. Methods: Case report and review of the literature. Results: A patient with inflammatory bowel disease developed severe pyoderma gangrenosum while receiving treatment with the chimeric anti-TNF-α antibody infliximab. Despite subsequent trials of numerous immunosuppressive and immunomodulatory medications, the dermatologic disease progressed. The patient's ulcers finally resolved when treatment with adalimumab, a fully humanized monoclonal antibody specific for TNF-α, was initiated. Conclusions: We report a novel application of the TNF-α inhibitor, adalimumab, in the treatment of pyoderma gangrenosum.
Pyoderma gangrenosum (PG) is a neutrophilic dermatosis associated in 70% of cases with underlying systemic disease such as inflammatory bowel disease (IBD), rheumatoid arthritis (RA), monoclonal gammopathy, or malignancy.1,2 Treatment of the underlying disease process promotes PG resolution in many cases. Corticosteroids are considered first-line therapy for PG.3 Other immunosuppressive and immunomodulatory agents, including cyclosporine, thalidomide, tacrolimus, mycophenolate mofetil, and azathioprine, are used as well. Despite multiple therapeutic options, many cases of PG are refractory to treatment.
Pharmacologic inhibition of the pro-inflammatory cytokine TNF-α has demonstrated efficacy for a wide range of inflammatory conditions, including IBD, RA, and psoriasis. There are three TNF-α inhibitors commercially available: etanercept (Enbrel, Immunex Corporation, Thousand Oaks, CA), a fusion protein dimer of the human TNF-α receptor; infliximab (Remicade, Centocor Incorporated, Horsham, PA), a chimeric mouse-human monoclonal antibody to TNF-α; and adalimumab (Humira, Abbott Laboratories, Abbott Park, IL), a fully human monoclonal antibody to TNF-α. There have been multiple recent reports of PG successfully treated with infliximab4–12 and etanercept,13–15 including one randomized controlled trial of infliximab.4 There is also one case report of adalimumab for a patient with idiopathic PG who had previously suffered an anaphylactoid reaction to infliximab and failed etanercept therapy.16
Here we present the case of a patient with IBD who developed PG despite receiving treatment with infliximab. This patient subsequently failed numerous trials of various other immunosuppressive and immunomodulatory regimens. We describe our experiences with the use of adalimumab in this patient.
REPORT OF A CASE
A 38-year-old white woman with a 2-year history of IBD developed a rapidly enlarging, painful ulcer on her anterior left thigh. For the preceding 6 months, the patient had been on azathioprine 100 mg daily and infliximab 5 mg/kg infusions once every 8 weeks for active lymphocytic ileitis. Physical examination revealed a solitary, deep ulcer on her anterior thigh that was 2.3 cm in diameter, with a characteristic violaceous undermined border and a painful zone of induration extending 1 cm beyond the ulcer rim. Tissue cultures from a biopsy of the ulcer edge were negative for bacteria, mycobacteria, and fungi. The skin biopsy showed necrosis, a mixed inflammatory infiltrate, and a small-vessel leukocytoclastic vasculitis consistent with PG. Special stains for organisms were negative.
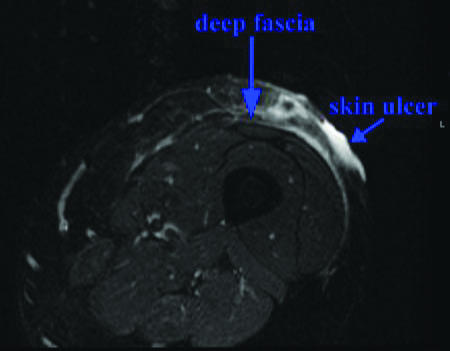
The patient's PG was resistant to multiple treatment regimens. In addition to the immunosuppressive regimen for her IBD (azathioprine and infliximab), local treatments were initiated, including triamcinolone injections (5 mg/mL) to the ulcer site, topical tacrolimus 0.1% ointment twice daily, and conservative wound care including oral antibiotics. Initially, the decision was made not to administer systemic corticosteroids as the patient had previously developed pseudotumor cerebri with ocular manifestations and headache while receiving prednisone for her IBD. The PG continued to progress over the next month, so the frequency of infliximab 5 mg/kg infusions was increased to monthly administration. Monthly high-dose intravenous immunoglobulin (IVIG, 2 g/kg administered in divided doses over 3 days) was also initiated. When the ulcer depth progressed to the level of the deep fascia (Fig 1), requiring significant narcotic analgesia for pain control, cyclosporine 3 mg/kg daily was added as well.
Figure 1.
Limb MRI. STIR axial MRI of the left lower extremity demonstrating superficial T2 bright signal compatible with inflammation involving the subcutaneous tissue down to the level of the deep fascia.
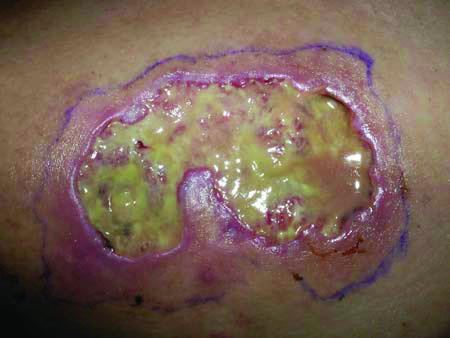
The regimen of cyclosporine, infliximab, azathioprine, and monthly IVIG initially resulted in improvement of the PG and some re-epithelialization; however, the immunosuppression had to be temporarily withdrawn after the patient was hospitalized for aseptic meningitis. After discharge, the IVIG was restarted, but was then stopped after 2 episodes of intractable nausea following IVIG infusions. Despite reinitiating cyclosporine and infliximab, the ulceration and surrounding inflammation progressed, eventually involving an area 8 cm in diameter on her left thigh (Fig 2). A trial of sulfasalazine 2000 mg (or 2 grams) per day was ineffective as well. Given that her PG was not responding to multiple immunosuppressive medications, prednisone 20 mg daily was initiated in consultation with ophthalmology. Higher doses of prednisone were attempted, but the patient again developed symptoms of pseudotumor cerebri. Because of the patient's persistent headaches and visual changes, the prednisone was tapered.
Figure 2.
Pyoderma gangrenosum. Large purulent ulcer with violaceous undermined borders.
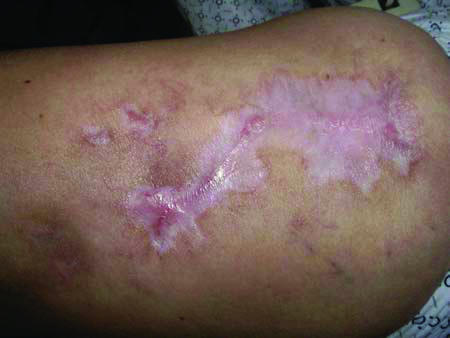
While on infliximab, cyclosporine, and azathioprine, the patient required hospitalization for a flare of her IBD and intractable pain from her PG. Adalimumab was then initiated at 80 mg subcutaneous (SQ) injections every other week in combination with cyclosporine, prednisone, and sulfasalazine. The ulcer and gastrointestinal disease responded rapidly (Fig 3), and the patient was discharged on cyclosporine, prednisone, and adalimumab. Over the next 3 months, cyclosporine and prednisone were tapered and eventually discontinued as the PG continued to improve. The patient has experienced no recurrence of her PG during 4 months on adalimumab monotherapy (80 mg SQ every other week), though there have been interval flares of her bowel disease.
Figure 3.
Healed wound. Residual scarring at area of pyoderma gangrenosum resolution.
DISCUSSION
Pyoderma gangrenosum is a type of painful cutaneous ulcer associated with underlying systemic disease in the majority of cases. Though the pathophysiology is poorly understood, PG is thought to be related to immune dysregulation, including defects in neutrophil chemotaxis, neutrophil hyperreactivity and overexpression of cytokines such as interleukin-8.17,18 Many of these effects may be mediated by the proinflammatory cytokine TNF-α.
TNF-α has been shown to enhance neutrophil activation, upregulate the expression of adhesion molecules, and induce the release of chemokines and cytokines from fibroblasts.13,19 The TNF-α inhibitors infliximab, etanercept, and adalimumab reduce inflammation by binding and inactivating free TNF-α.20 Infliximab and adalimumab have the additional ability to bind surface-bound TNF-α, with subsequent complement fixation and cell lysis, potentially suppressing T cells and other cells expressing surface TNF.21
Here we describe a patient who developed severe PG despite receiving treatment with infliximab and azathioprine, whose skin disease proved refractory to multiple subsequent trials of other immunomodulatory agents as well. Ultimately, this patient's PG responded rapidly and dramatically to adalimumab therapy. This case is intriguing because of the patient's disparate responses to the 2 anti-TNF-α agents.
We at first suspected that infliximab failed to suppress PG in our patient because of its immunogenicity as a mouse-human chimeric protein and the resultant formation of human antichimeric antibodies (HACAs), as infliximab had been administered for 6 months when the lower-extremity ulceration first appeared. In studies, 10%–61% of patients receiving infliximab therapy develop HACAs, which have been associated with lower serum infliximab concentrations, decreased duration of treatment response, and decreased infliximab efficacy.22–25 In contrast, the fully human agent adalimumab has the theoretical advantage of causing less antidrug antibody formation, a notion supported by experimental assays demonstrating that only 6%–12% of patients develop anti-adalimumab antibodies.26,27 However, serum assays performed 7 months after infliximab discontinuation in this patient revealed no detectable HACAs. It is possible that HACAs once present had since disappeared, but is perhaps more likely that another, as yet uncharacterized, difference between infliximab and adalimumab contributed to their divergent effects in our patient.
Studies suggest that of the TNF-α inhibitors, infliximab may be most prone to loss of efficacy over time. Finckh et al28 found that in patients with RA, the problem of gradual drug failure is greater for infliximab than for etanercept or adalimumab, although this study did not assess levels of antidrug antibodies. Cases of RA and Crohn's disease that become refractory to infliximab over time often respond well to adalimumab.26,29–32 In fact, the level of therapeutic efficacy achieved with adalimumab in infliximab-resistant patients is comparable to the improvement achieved when adalimumab is used in TNF-α-inhibitor–naïve patients.29,31 Thus, adalimumab may be a valuable treatment for PG patients who fail to respond to infliximab and other conventional immunosuppressive regimens.
REFERENCES
- 1.Su WP, Davis MD, Weenig RH, et al. Pyoderma gangrenosum: clinicopathologic correlation and proposed diagnostic criteria. Int J Dermatol. 2004;43:790–800. doi: 10.1111/j.1365-4632.2004.02128.x. [DOI] [PubMed] [Google Scholar]
- 2.Powell FC, O'Kane M. Management of pyoderma gangrenosum. Dermatol Clin. 2002;20:347–355. doi: 10.1016/s0733-8635(01)00029-8. [DOI] [PubMed] [Google Scholar]
- 3.Reichrath J, Bens G, Bonwitz A, et al. Treatment recommendations for pyoderma gangrenosum: an evidence-based review of the literature based on more than 350 patients. J Am Acad Dermatol. 2005;53:273–283. doi: 10.1016/j.jaad.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Brooklyn TN, Dunnill MG, Shetty A, et al. Infliximab for the treatment of pyoderma gangrenosum: a randomised, double blind, placebo controlled trial. Gut. 2006;55:505–509. doi: 10.1136/gut.2005.074815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaur MR, Lewis HM. Severe recalcitrant pyoderma gangrenosum treated with infliximab. Br J Dermatol. 2005;153:689–691. doi: 10.1111/j.1365-2133.2005.06812.x. [DOI] [PubMed] [Google Scholar]
- 6.Swale VJ, Saha M, Kapur N, et al. Pyoderma gangrenosum outside the context of inflammatory bowel disease treated successfully with infliximab. Clin Exp Dermatol. 2005;30:134–136. doi: 10.1111/j.1365-2230.2004.01681.x. [DOI] [PubMed] [Google Scholar]
- 7.Singh M, Andrew SM, Lear JT. Infliximab as a treatment for recalcitrant pyoderma gangrenosum. Clin Exp Dermatol. 2004;29:196–197. doi: 10.1111/j.1365-2230.2004.01470.x. [DOI] [PubMed] [Google Scholar]
- 8.Regueiro M, Valentine J, Plevy S, et al. Infliximab for treatment of pyoderma gangrenosum associated with inflammatory bowel disease. Am J Gastroenterol. 2003;98:1821–1826. doi: 10.1111/j.1572-0241.2003.07581.x. [DOI] [PubMed] [Google Scholar]
- 9.Zaccagna A, Bertone A, Puiatti P, et al. Anti-tumor necrosis factor alpha monoclonal antibody (infliximab) for the treatment of pyoderma gangrenosum associated with Crohn's disease. Eur J Dermatol. 2003;13:258–260. [PubMed] [Google Scholar]
- 10.Ljung T, Staun M, Grove O, et al. Pyoderma gangrenosum associated with Crohn disease: effect of TNF-α blockade with infliximab. Scand J Gastroenterol. 2002;37:1108–1110. doi: 10.1080/003655202320378338. [DOI] [PubMed] [Google Scholar]
- 11.Romero-Gómez M, Sánchez-Muñoz D. Infliximab induces remission of pyoderma gangrenosum. Eur J Gastroenterol Hepatol. 2002;14:907. doi: 10.1097/00042737-200208000-00021. [DOI] [PubMed] [Google Scholar]
- 12.Triantafillidis JK, Cheracakis P, Sklavaina M, et al. Favorable response to infliximab treatment in a patient with active Crohn disease and pyoderma gangrenosum. Scand J Gastroenterol. 2002;37:863–865. [PubMed] [Google Scholar]
- 13.Roy DB, Conte ET, Cohen DJ. The treatment of pyoderma gangrenosum using etanercept. J Am Acad Dermatol. 2006;54:S128–S134. doi: 10.1016/j.jaad.2005.10.058. [DOI] [PubMed] [Google Scholar]
- 14.Pastor N, Betlloch I, Pascual JC, et al. Pyoderma gangrenosum treated with anti-TNF alpha therapy (etanercept) Clin Exp Dermatol. 2005;31:152–153. doi: 10.1111/j.1365-2230.2005.01972.x. [DOI] [PubMed] [Google Scholar]
- 15.McGowan JW, Johnson CA, Lynn A. Treatment of pyoderma gangrenosum with etanercept. J Drugs Dermatol. 2004;3:441–444. [PubMed] [Google Scholar]
- 16.Hubbard VG, Friedmann AC, Goldsmith P. Systemic pyoderma gangrenosum responding to infliximab and adalimumab. Br J Dermatol. 2005;152:1059–1061. doi: 10.1111/j.1365-2133.2005.06467.x. [DOI] [PubMed] [Google Scholar]
- 17.Adachi Y, Kindzelskii AL, Cookingham G, et al. Aberrant neutrophil trafficking and metabolic oscillations in severe pyoderma gangrenosum. J Invest Dermatol. 1998;111:259–268. doi: 10.1046/j.1523-1747.1998.00311.x. [DOI] [PubMed] [Google Scholar]
- 18.Oka M, Berking C, Nesbit M, et al. Interleukin-8 overexpression is present in pyoderma gangrenosum ulcers and leads to ulcer formation in human skin xenografts. Lab Invest. 2000;80:595–604. doi: 10.1038/labinvest.3780064. [DOI] [PubMed] [Google Scholar]
- 19.Norris P, Poston RN, Thomas DS, et al. The expression of endothelial leukocyte adhesion molecule-1 (ELAM-1), intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1) in experimental cutaneous inflammation: a comparison of ultraviolet B erythema and delayed hypersensitivity. J Invest Dermatol. 1991;96:763–770. doi: 10.1111/1523-1747.ep12471720. [DOI] [PubMed] [Google Scholar]
- 20.Mpofu S, Fatima F, Moots RJ. Anti-TNF-α therapies: they are all the same (aren't they?) Rheumatology (Oxford) 2005;44:271–273. doi: 10.1093/rheumatology/keh483. [DOI] [PubMed] [Google Scholar]
- 21.Scallon BJ, Moore MA, Trinh H, et al. Chimeric anti-TNF-α monoclonal antibody cA2 binds recombinant transmembrane TNF-α and activates immune effector functions. Cytokine. 1995;7:251–259. doi: 10.1006/cyto.1995.0029. [DOI] [PubMed] [Google Scholar]
- 22.Wagner CL, Schantz A, Barnathan E, et al. Consequences of immunogenicity to the therapeutic monoclonal antibodies ReoPro and Remicade. Dev Bio. (Basel) 2003;112:37–53. [PubMed] [Google Scholar]
- 23.Baert F, Norman M, Vermeire S, et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn's disease. N Engl J Med. 2003;348:601–608. doi: 10.1056/NEJMoa020888. [DOI] [PubMed] [Google Scholar]
- 24.Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–1549. doi: 10.1016/S0140-6736(02)08512-4. [DOI] [PubMed] [Google Scholar]
- 25.Haraoui B, Cameron L, Ouellet M, et al. Anti-infliximab antibodies in patients with rheumatoid arthritis who require higher doses of infliximab to achieve or maintain a clinical response. J Rheumatol. 2006;33:31–36. [PubMed] [Google Scholar]
- 26.van de Putte LB, Atkins C, Malaise M, et al. Efficacy and safety of adalimumab as monotherapy in patients with rheumatoid arthritis for whom previous disease modifying antirheumatic drug treatment has failed. Ann Rhuem Dis. 2004;63:508–516. doi: 10.1136/ard.2003.013052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson PJ. Tumor necrosis factor inhibitors: clinical implications of their different immunogenicity profiles. Semin Arthritis Rheum. 2005;34:S19–S22. doi: 10.1016/j.semarthrit.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Finckh A, Simard JF, Gabay C, et al. Evidence for differential acquired drug resistance to anti-tumor necrosis factor agents in rheumatoid arthritis. Ann Rheum Dis. 2006;65:746–752. doi: 10.1136/ard.2005.045062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nikas SN, Voulgari PV, Alamanos Y, et al. Efficacy and safety of switching from infliximab to adalimumab: a comparative controlled study. Ann Rheum Dis. 2006;65:257–260. doi: 10.1136/ard.2005.039099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Papadakis KA, Shaye OA, Vasiliauskas EA, et al. Safety and efficacy of adalimumab (D2E7) in Crohn's disease patients with an attenuated response to infliximab. Am J Gastroenterol. 2005;100:75–79. doi: 10.1111/j.1572-0241.2005.40647.x. [DOI] [PubMed] [Google Scholar]
- 31.Wick MC, Ernestam S, Lindblad S, et al. Adalimumab (Humira®) restores clinical response in patients with secondary loss of efficacy from infliximab (Remicade®) or etanercept (Enbrel®): results from the STURE registry at Karolinska University Hospital. Scand J Rheumatol. 2005;34:353–358. doi: 10.1080/03009740510026887. [DOI] [PubMed] [Google Scholar]
- 32.Sandborn WJ, Hanauer S, Loftus EV, et al. An open-label study of the human anti-TNF monoclonal antibody adalimumab in subjects with prior loss of response or intolerance to infliximab for Crohn's disease. Am J Gastroenterol. 2004;99:1984–1989. doi: 10.1111/j.1572-0241.2004.40462.x. [DOI] [PubMed] [Google Scholar]