Abstract
NF-κB downregulates tumor necrosis factor (TNF)-induced c-Jun N-terminal kinase (JNK) activation that promotes cell death, but the mechanism is not yet fully understood. By using murine embryonic fibroblasts (MEFs) that are deficient in TNF receptor-associated factor (TRAF) 2 and TRAF5 (DKO) or p65 NF-κB subunit (p65KO), we demonstrate here that TNF stimulation leads to accumulation of reactive oxygen species (ROS), which is essential for prolonged mitogen-activated protein kinase (MAPK) activation and cell death. Interestingly, dying cells show necrotic as well as apoptotic morphological changes as assessed by electron microscopy and flow cytometry, and necrotic, but not apoptotic, cell death is substantially inhibited by antioxidant. Importantly, TNF does not induce ROS accumulation or prolonged MAPK activation in wild-type MEFs, indicating that TRAF-mediated NF-κB activation normally suppresses the TNF-induced ROS accumulation that subsequently induces prolonged MAPK activation and necrotic cell death
Keywords: MAPK/NF-κB/ROS/TNF/TRAF
Introduction
Tumor necrosis factor (TNF) exerts a variety of biological effects, including production of inflammatory cytokines, upregulation of adhesion molecules, proliferation, differentiation and cell death (Tracey and Cerami, 1994). While such pleiotropic effects are mediated by two cognate TNF receptors, TNF-R1 and TNF-R2, TNF-induced cell death is mainly mediated by TNF-R1. In response to TNF, TNF-R1 is trimerized and recruits an adapter molecule, TRADD. In the apoptotic signaling pathway, the recruited TRADD interacts with FADD, which then recruits and activates caspase-8 (Mak and Yeh, 2002; Wallach et al., 2002). The activated caspase-8 in turn activates effector caspases, such as caspase-3 and -7, resulting in apoptosis. On the other hand, recruited TRADD directly or indirectly interacts with RIP, TNF receptor-associated factor (TRAF) 2 and TRAF5, which are implicated in nuclear factor (NF)-κB and c-Jun N-terminal kinase (JNK) activation (Mak and Yeh, 2002; Wallach et al., 2002). TRAFs have been identified as being signaling intermediates in both TNF receptor and interleukin (IL)-1 receptor/Toll-like receptor (TLR) superfamilies (Inoue et al., 2000; Chung et al., 2002). TRAFs interact directly or indirectly with several mitogen-activated protein kinase (MAPK) kinase kinases (MAPKKKs), including apoptosis signal-regulating kinase (ASK) 1, TAK1 and MEKK1, and thereby activate MAPK cascades (Nishitoh et al., 1998; Baud et al., 1999; Ninomiya-Tsuji et al., 1999). TRAF2, TRAF5 and TRAF6 have been shown to be involved in TNFR- and IL-1R/TLR-mediated NF-κB and MAPK activation (Yeh et al., 1997; Lomaga et al., 1999; Naito et al., 1999; Tada et al., 2001).
The MAPK cascades are activated by various cellular stresses and growth factors, and are involved in various biological responses such as cytokine production, differentiation, proliferation and cell death (Ichijo, 1999; Davis, 2000). In mammals, MAPK cascades are composed of three distinct signaling modules, JNK, p38 MAPK and extracellular signal-regulated kinase (ERK). Upon cytokine or growth factor stimulation, or in response to various stresses, MAPKKKs are rapidly activated by oligomerization or undefined mechanisms, and phosphorylate downstream MAP kinases, called MAPK kinases (MAPKKs) (Ichijo, 1999; Davis, 2000). Then, activated MAPKKs finally phosphorylate and activate MAPKs, which in turn phosphorylate specific targets and activate their transcriptional activities. TNF- and IL-1-induced MAPK activation are usually rapid and transient with a peak at ∼10 min and then declining to basal level by 60 min. On the other hand, genotoxic stresses such as UV or γ-irradiation induce long-lasting or prolonged MAPK activation. Several lines of evidences suggested that transient MAPK activation is associated with gene expression, proliferation or differentiation, whereas prolonged MAPK activation promotes cell death in a cell type- and stimulus-dependent manner (Xia et al., 1995; Chen et al., 1996; Guo et al., 1998).
NF-κB is a transcriptional factor that regulates expression of various inflammatory cytokines, chemokines, and adhesion molecules (Ghosh et al., 1998). NF-κB is activated by inflammatory cytokines and cellular stresses, including TNF, IL-1, lipopolysaccharide (LPS), UV, or γ-irradiation. NF-κB also plays a critical role in protection from TNF-induced cell death (Barkett and Gilmore, 1999; Karin and Lin, 2002). Cells lacking NF-κB subunit p65 (p65), IκB kinase β (IKKβ) and IκB kinase γ (IKKγ)/NEMO show increased sensitivity to TNF-induced cell death (Barkett and Gilmore, 1999; Karin and Lin, 2002). One of the mechanisms by which NF-κB inhibits TNF-induced cell death is to upregulate anti-apoptotic genes including the Bcl-2 and XIAP families, and FLIP (Barkett and Gilmore, 1999; Karin and Lin, 2002). However, the survival signals elicited by NF-κB are not fully understood. Recently, several groups reported that TNF, but not IL-1, induces prolonged JNK activation in cells lacking p65, IKKβ, or stably expressing super-repressor of NF-κB, and this prolonged JNK activation participates in TNF-induced cell death (De Smaele et al., 2001; Javelaud and Besancon, 2001; Tang et al., 2001). These studies also revealed that NF-κB upregulates the expression of XIAP and GADD45β, which downregulate TNF-induced JNK activation. However, the molecular mechanism by which XIAP and GADD45β inhibit TNF-induced JNK activation remains unknown.
Reactive oxygen species (ROS), including superoxide anions, hydrogen peroxide and hydroxyl radicals, are normally generated in the mitochondria and act as signaling intermediates (Adler et al., 1999b; Thannickal and Fanburg, 2000). Under physiological conditions, generated ROS are rapidly eliminated by antioxidant enzymes, including superoxide dismutases (SODs), catalase, glutathione peroxidases (GPxs) and peroxiredoxins (PRxs) (Thannickal and Fanburg, 2000). In various pathological conditions such as ischemia, excessively accumulated ROS induce apoptosis or necrosis by activating MAPK, caspase cascades, and/or disruption of mitochondrial membrane potential (Fiers et al., 1999). However, it remains controversial whether ROS play a critical role in cytokine-induced MAPK activation under physiological conditions.
We previously demonstrated that murine embryonic fibroblasts (MEFs) derived from TRAF2 and TRAF5 double knockout (DKO) mice are defective in TNF-induced NF-κB activation and rapid/transient JNK activation, and are highly susceptible to TNF-induced cell death (Tada et al., 2001). In the present study, we found that TNF stimulation induces delayed/prolonged MAPK activation in DKO MEFs, as well as in p65KO MEFs, but not in wild-type MEFs. We have further investigated the molecular mechanism by which NF-κB inhibits TNF-induced prolonged JNK activation and cell death. We report here that TNF induces ROS accumulation in DKO and p65KO, but not wild-type, MEFs, which is essential for TNF-induced prolonged MAPK activation and cell death. Electron microscopy and flow cytometry analyses show that TNF induces necrosis as well as apoptosis in DKO and p65KO MEFs, and necrosis is inhibited by anti-oxidant. Collectively, these results suggested that one of the pro-survival functions of NF-κB is to inhibit TNF-induced ROS accumulation that mediates prolonged MAPK activation and necrotic cell death.
Results
TNF induces delayed and prolonged MAPK activation in TRAF-deficient MEFs
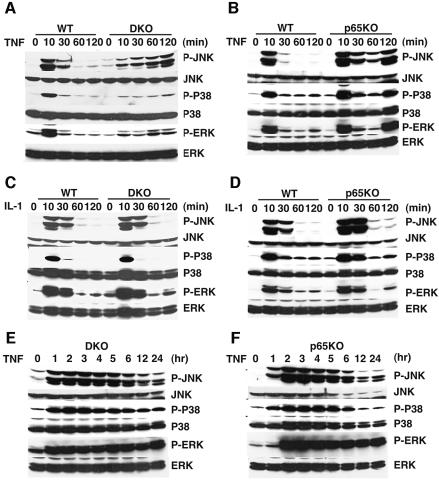
Prolonged JNK activation has been implicated in increased sensitivity to TNF-induced cell death. We previously demonstrated that MEFs derived from TRAF2 and TRAF5 DKO mice have a defect in TNF-induced NF-κB and JNK activation, and show increased sensitivity to TNF-induced cell death (Tada et al., 2001). Given that rapid and transient MAPK activation depends on TRAFs, it is intriguing to test whether prolonged JNK activation is induced in TRAF-deficient cells. Thus, we first examined the time-course of JNK phosphorylation in DKO MEFs after TNF stimulation. As previously reported (Tada et al., 2001), phosphorylation of JNK at 10 min was severely impaired in DKO MEFs compared with wild-type MEFs (Figure 1A). Unexpectedly, however, phosphorylation of JNK gradually increased thereafter up to 120 min after TNF stimulation (Figure 1A). Moreover, in addition to JNK, we detected prolonged phosphorylation of p38 and ERK (Figure 1A). We also confirmed that kinase activities of JNK and ERK were prolonged in DKO MEFs by immune complex kinase assays using glutathione S-transferase (GST)–c-Jun and MBP as substrates, respectively (data not shown).
Fig. 1. Prolonged MAPK activation by TNF, but not IL-1, in DKO and p65KO MEFs. (A, B, E and F) Wild-type, DKO and p65KO MEFs were stimulated with TNF (50 ng/ml) for the indicated time periods, and then the lysates were blotted with antibodies specific for the activated form of JNK (phospho-JNK), p38 (phospho-p38) or ERK (phospho-ERK). The membranes were reblotted with antibodies to total JNK, p38 or ERK. (C and D) Wild-type, DKO and p65KO MEFs were stimulated with IL-1 (50 ng/ml) for the indicated time periods and the lysates were blotted as above.
We next investigated whether prolonged activation of p38 and ERK as well as JNK was also induced in p65KO MEFs, since previous studies showed that activation of JNK, but not ERK or p38, was prolonged in TNF-stimulated p65KO or IKKβ KO MEFs (De Smaele et al., 2001; Tang et al., 2001). In contrast to these previous reports, activation of both p38 and ERK as well as JNK was also prolonged in TNF-stimulated p65KO MEFs (Figure 1B). On the other hand, IL-1 did not induce such a prolonged MAPK activation in DKO and p65KO MEFs (Figure 1C and D).
We also investigated duration of MAPK activation in TNF-stimulated DKO and p65KO MEFs. In both MEFs, phosphorylation of MAPKs peaked at 2 h and persisted up to 5 h, and then gradually declined (Figure 1E and F). The later decline reflected the decreased protein levels of MAPKs due to cell death (Figure 1E and F).
Prolonged MAPK activation is mediated by ROS
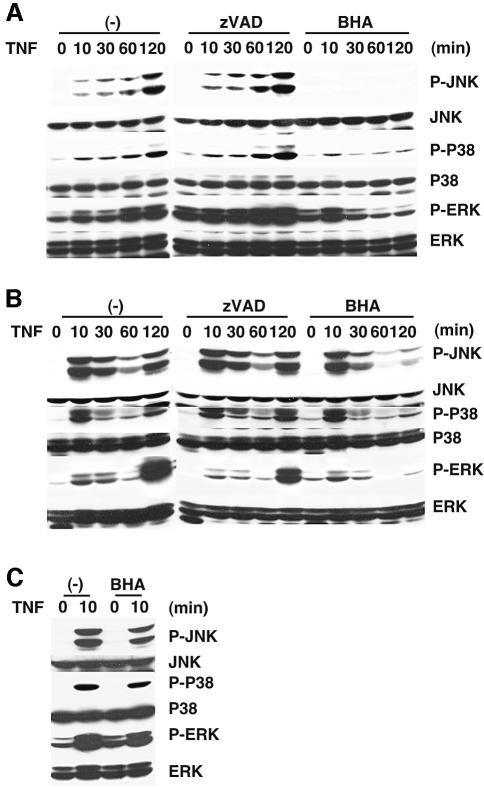
To explore the molecular mechanism for prolonged MAPK activation, we pretreated the cells with a broad caspase inhibitor, z-VAD-fmk, or an antioxidant, butylated hydroxyanisole (BHA), before TNF stimulation. Previous studies showed that BHA inhibits TNF-induced necrosis in murine fibrosarcoma, L929 (Vercammen et al., 1998; Fiers et al., 1999). As shown in Figure 2A and B, BHA, but not z-VAD-fmk, almost completely inhibited prolonged MAPK activation in DKO and p65KO MEFs. Interestingly, in contrast to the strong inhibitory effect of BHA on prolonged MAPK activation at 30 min and later, BHA only slightly inhibited early MAPK activation at 10 min in both DKO and p65KO MEFs (Figure 2A and B). Similarly, BHA only slightly inhibited activation of MAPKs at 10 min after TNF stimulation in wild-type MEFs (Figure 2C). These weak inhibitory effects of BHA on rapid and transient MAPK activation are partly consistent with previous reports demonstrating inhibitory effect of antioxidants on TNF-induced MAPK activation (Gotoh and Cooper, 1998; Saitoh et al., 1998). Collectively, these results demonstrate that transient activation and prolonged activation of MAPKs are induced by different mechanisms.
Fig. 2. BHA, but not z-VAD-fmk, inhibits prolonged MAPK activation by TNF in DKO and p65KO MEFs. (A and B) DKO (A) and p65KO (B) MEFs were untreated or pretreated with z-VAD-fmk (10 µM) or BHA (100 µM) for 20 min, then stimulated with TNF (50 ng/ml) for the indicated time periods. The cell lysates were analyzed as described in Figure 1. (C) Wild-type MEFs were untreated or treated with BHA (100 µM) for 20 min, then stimulated with TNF (50 ng/ml) for 10 min. The cell lysates were analyzed as described in Figure 1.
TNF induces accumulation of ROS in DKO and p65KO MEFs, but not wild-type MEFs
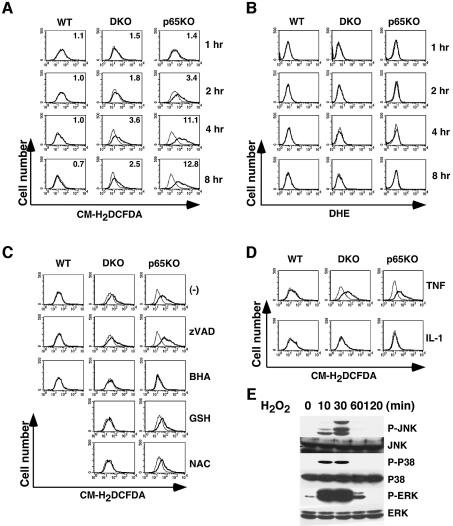
The fact that BHA treatment inhibited prolonged MAPK activation in DKO and p65KO MEFs prompted us to examine whether TNF stimulation induces ROS accumulation in these cells. It is still controversial whether TNF induces ROS accumulation in primary fibroblasts. Wild-type, DKO and p65KO MEFs were stimulated with TNF for 1–8 h, then the cells were labeled with cell-permeable fluorescent dyes, 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescence diacetate (CM-H2DCFDA) or dihydroethidium (DHE). When these dyes are oxidized by ROS in the cells, their fluorescent signals increase. While CM-H2DCFDA is mainly oxidized by hydrogen peroxides (H2O2) and hydroxyl radical, DHE is oxidized by superoxide anion (O2–). Fluorescent signals were analyzed by flow cytometry. Importantly, no substantial increase in fluorescent signals of either CM-H2DCFDA or DHE was observed in wild-type MEFs up to 24 h after TNF stimulation, indicating that TNF does not induce ROS accumulation in wild-type MEFs (Figure 3A and B; data not shown). In contrast, in DKO and p65KO MEFs, fluorescent intensity of CM-H2DCFDA, but not DHE, slightly increased at 2 h, and peaked at 4–8 h after TNF stimulation (Figure 3A). We next examined whether BHA or z-VAD-fmk inhibits ROS accumulation in DKO and p65KO MEFs. As shown in Figure 3C, BHA almost completely inhibited ROS accumulation in DKO and p65KO MEFs, while z-VAD-fmk did not (Figure 3C). We also observed inhibitory effects of other antioxidants, including glutathione (GSH) and N-acetyl cystein (NAC), although their effects were not complete in p65KO MEFs compared with BHA (Figure 3C). Consistent with the partial inhibitory effect of NAC on TNF-induced ROS accumulation, NAC only partially inhibited TNF-induced MAPK activation (see Supplementary figure 1, available at The EMBO Journal Online). On the other hand, IL-1 stimulation did not induce ROS accumulation in wild-type, DKO or p65KO MEFs (Figure 3D). These results demonstrate that accumulation of ROS perfectly coincides with prolonged MAPK activation.
Fig. 3. TNF, but not IL-1, induces accumulation of ROS in DKO and p65KO MEFs. (A and B) Wild-type, DKO and p65KO MEFs were unstimulated (thin line) or stimulated (bold line) with TNF (10 ng/ml) for the indicated time periods, then the cells were labeled with CM-H2DCFDA (1 µM) (A) or DHE (1 µM) (B) for the last 30 min, and analyzed by flow cytometry. The ratios of mean fluorescent intensity of stimulated cells to unstimulated cells are indicated at the right upper corner (A). (C) Wild-type, DKO and p65KO MEFs were untreated or pretreated with z-VAD-fmk (10 µM), BHA (100 µM), GSH (5 mM) or NAC (10 mM) for 20 min, then stimulated with TNF (10 ng/ml) for 4 h, labeled with CM-H2DCFDA and analyzed as described in (A). (D) Wild-type, DKO and p65KO MEFs were stimulated with IL-1 (10 ng/ml) for 4 h, labeled with CM-H2DCFDA and the fluorescent signals analyzed as described in (A). (E) Wild-type MEFs were stimulated with H2O2 (1 mM) for the indicated time periods, and the lysates were analyzed as described in Figure 1.
Previous studies showed that ROS including H2O2 activates JNK, p38 and ERK depending on the cell-type (Adler et al., 1999b; Thannickal and Fanburg, 2000). Thus, we tested whether exogenously added H2O2 could activate JNK, p38 and ERK in MEFs. As shown in Figure 3E, H2O2 potently activated JNK, p38 and ERK in wild-type MEFs. Together, these results substantiate the fact that accumulated ROS mediate prolonged MAPK activation in DKO and p65KO MEFs upon TNF stimulation.
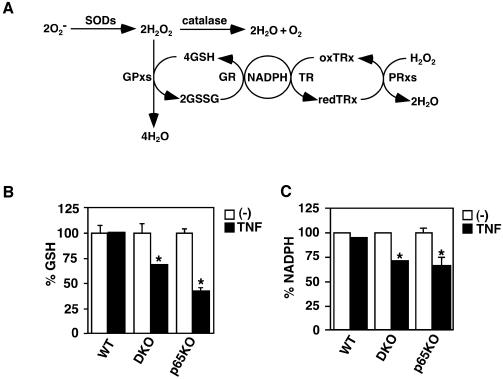
Cellular GSH and NADPH levels are decreased in DKO and p65KO, but not wild-type, MEFs after TNF stimulation
Under normal conditions, ROS generated in mitochondria are rapidly eliminated by cellular antioxidants. In general, O2– is dismutated to H2O2 by SODs. H2O2 is subsequently eliminated by catalase, GPxs and PRxs (Figure 4A). Optimum cellular GSH level is essential for maintaining the activities of GPxs. NADPH is also required for converting oxidized glutathione disulfide form (GSSG) to reduced form GSH. Given that TNF induced accumulation of ROS in DKO and p65KO MEFs, we examined the cellular levels of GSH and NADPH before and after TNF stimulation. While either GSH or NADPH was not significantly altered in wild-type MEFs before and after TNF stimulation, both GSH and NADPH levels were significantly reduced in DKO and p65KO MEFs after stimulation (Figure 4B and C). These results suggest that the machinery to maintain the cellular GSH and NADPH levels is compromised in DKO and p65KO MEFs. Consistent with this notion, exogenously added GSH inhibited TNF-induced ROS accumulation in DKO and p65KO MEFs (Figure 3C).
Fig. 4. TNF induces reduction of GSH and NADPH levels in DKO and p65KO MEFs. (A) Pathways of ROS elimination by cellular antioxidants. O2– is converted into H2O2 by SODs. Then, H2O2 is eliminated by catalase, GPxs and PRxs. During elimination of H2O2, reduced glutathione (GSH) is converted to disulfide form (GSSG) by GPxs, and then GSSG is recycled to GSH by glutathione reductase (GR). On the other hand, PRxs also catalyze conversion of H2O2 into H2O by reduced thioredoxin (TRx). Oxidized TRx is recylcled back to redTRx by thioredoxin reductase (TR). NADPH is essential for both recycling reactions. (B) Wild-type, DKO and p65KO MEFs were unstimulated (open columns) or stimulated (closed columns) with TNF (10 ng/ml) for 4 h, and then the cellular levels of GSH were measured as described in Materials and methods. The levels of GSH were normalized by protein contents. GSH levels are presented as percentage reduction in stimulated cells compared with unstimulated cells. Results are presented as mean ± SEs of triplicate samples and represent two independent experiments with similar results. *P < 0.05 compared with unstimulated cells. (C) Wild-type, DKO and p65KO MEFs were unstimulated (open columns) or stimulated (closed columns) with TNF (10 ng/ml) for 4 h, and then the cellular levels of NADPH were measured as described in Materials and methods. NADPH levels are presented as percentage reduction in stimulated cells compared with unstimulated cells. Results are presented as mean ± SEs of triplicate samples and represent two independent experiments with similar results. *P < 0.05 compared with unstimulated cells.
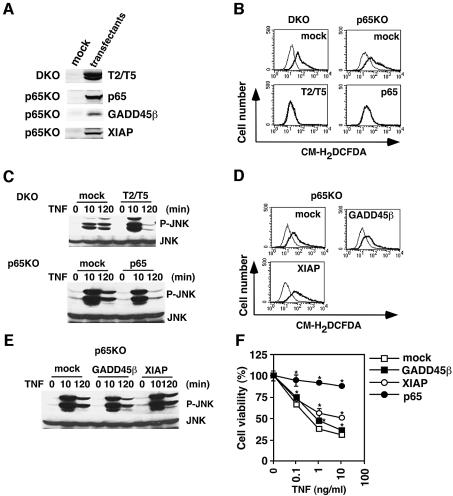
Ectopic expression of TRAFs or p65, but not GADD45β or XIAP, inhibits TNF-induced ROS accumulation, prolonged MAPK activation, and reduction of GSH and NADPH levels in DKO and p65KO MEFs
To rule out the possibility that some other defects in the TNF-induced signaling pathways are present in DKO and p65KO MEFs, we tested whether TNF-induced ROS accumulation, prolonged MAPK activation, and reduction of GSH and NADPH levels could be suppressed by ectopic expression of TRAF2 and TRAF5 or p65. To do this, we stably transfected DKO MEFs with TRAF2 and TRAF5, and p65KO MEFs with p65. Expression of the transfected genes was verified by western blotting with anti-Flag (for TRAF2 and TRAF5) or anti-HA (for p65) antibody (Figure 5A). Reconstitution of TRAF2 and TRAF5 in DKO MEFs or p65 in p65KO MEFs almost completely inhibited TNF-induced ROS accumulation, prolonged JNK activation, and reduction of GSH and NADPH levels (Figure 5B and C; Supplementary figure 2). These results verify that the accumulation of ROS, prolonged MAPK activation, reduction of GSH and NADPH levels in DKO and p65KO MEFs are due to the absence of TRAF2 and TRAF5 or p65.
Fig. 5. Introduction of TRAF2 and TRAF5 or p65, but not GADD45β or XIAP, inhibits TNF-induced ROS accumulation, prolonged JNK activation, and reduction of GSH and NADPH levels in DKO and p65KO MEFs. (A) Total cell lysates were blotted with anti-Flag (for TRAF2, TRAF5, GADD45β and XIAP) or anti-HA antibody (for p65). (B and D) Mock- or TRAF2- and TRAF5 (T2/T5)-transfected DKO MEFs, and mock-, p65-, GADD45β- or XIAP-transfected p65KO MEFs were stimulated with TNF (10 ng/ml) for 4 h and analyzed as described in Figure 3. (C and E) Transfectants were stimulated with TNF (50 ng/ml) for the indicated time periods, and the lysates were analyzed as described in Figure 1. (F) Mock-, GADD45β-, XIAP- or p65-transfected p65KO MEFs were stimulated with the indicated doses of TNF for 16 h. Cell viability was determined by WST assay. Results are presented as mean ± SEs of triplicate samples and represent four independent experiments with similar results. *P < 0.05 compared with mock transfectants.
Previous studies showed that GADD45β and XIAP were induced by TNF in an NF-κB-dependent manner and inhibited JNK activation in p65KO MEFs (De Smaele et al., 2001; Tang et al., 2001). Thus, we next examined whether ectopic expression of GADD45β or XIAP might inhibit ROS accumulation and prolonged JNK activation. Expression of the transfected genes was verified by western blotting with anti-Flag antibody (Figure 5A). In contrast to the previous data, transfection of either GADD45β or XIAP did not inhibit TNF-induced ROS accumulation or prolonged MAPK activation in p65KO MEFs (Figure 5D and E). However, expression of XIAP, but not GADD45β, substantially inhibited TNF-induced cell death (Figure 5F).
Contribution of ROS and MAPKs to TNF-induced cell death in DKO and p65KO MEFs
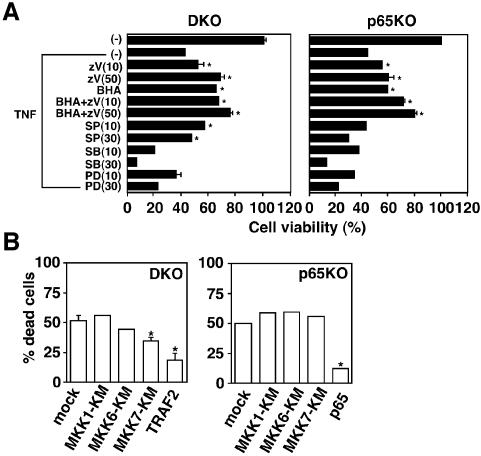
We next examined whether accumulated ROS per se or prolonged MAPK activation participates in TNF-induced cell death. We stimulated DKO and p65KO MEFs with TNF in the presence or absence of inhibitors for caspases, ROS or MAP kinases. As shown in Figure 6A, BHA or zVAD-fmk alone substantially increased cell viability of DKO and p65KO MEFs. Moreover, a combined treatment with BHA and z-VAD-fmk further increased cell viability, suggesting that ROS and caspases induce cell death independently, at least in part. SB203580 (specific inhibitor for p38) and PD98059 (specific inhibitor for ERK) did not increase cell viability, but rather enhanced TNF-induced death of DKO and p65KO MEFs. In contrast, SP600125 (specific inhibitor for JNK) significantly increased viability of DKO, but not p65KO, MEFs. We verified that these inhibitors actually inhibited MAP kinase activities by using antibodies specific for phosphorylated form of JNK, ERK, or specific substrate of p38 (MAPKAP2) (Supplementary figure 3). On the other hand, these MAPK inhibitors did not affect TNF-induced ROS accumulation (data not shown), indicating that prolonged MAPK activation is a downstream event of ROS accumulation.
Fig. 6. Inhibition of TNF-induced cell death in DKO and p65KO MEFs by BHA, z-VAD-fmk and MAPK inhibitors. (A) DKO and p65KO MEFs were stimulated with TNF (10 ng/ml) in the presence of z-VAD-fmk (10 or 50 µM), BHA (100 µM), BHA (100 µM) + z-VAD-fmk (10 or 50 µM), SP600125 (10 or 30 µM), SB203580 (10 or 30 µM) or PD98059 (10 or 30 µM) for 16 h. Cell viability was determined by WST assay as described in Figure 5. (B) DKO and p65KO MEFs were co-transfected with the indicated expression vectors (0.75 µg) along with pEGFP (0.25 µg). After 24 h, the cells were treated with TNF (10 ng/ml) for 16 h, and then counted by fluorescent microscopy. The percentage of dead cells among GFP-positive cells was calculated by counting >100 cells in randomly selected areas. Results are presented as means ± SEs of triplicate samples and represent two individual experiments. *P < 0.05 compared with mock transfectants.
To further evaluate the contribution of MAPK cascades to TNF-induced cell death, we inhibited each MAPK cascade by using dominant-negative mutants of upstream activators, including MKK1-KM (for ERK), MKK7-KM (for JNK) and MKK6-KM (for p38). We transiently transfected DKO and p65KO MEFs with these dominant-negative kinases along with an expression vector for green fluorescence protein (GFP). We also transfected expression vectors for TRAF2 or p65 as positive control. Expression of transfected genes was verified by western blotting (data not shown). Then, cells were treated with TNF, and the morphology of GFP-positive cells was examined. As expected, transient expression of TRAF2 or p65 into DKO or p65KO MEFs, respectively, substantially reduced dead cells (Figure 6B). In contrast, only MKK7-KM partially decreased dead cells in DKO, but not p65KO, MEFs. Collectively, these results indicate that ROS are involved in enhanced TNF-induced cell death of DKO and p65KO MEFs, but contribution of prolonged JNK activation to TNF-induced cell death is relatively cell dependent.
TNF induces both necrosis and apoptosis in DKO and p65KO MEFs, and necrosis is mainly mediated by ROS
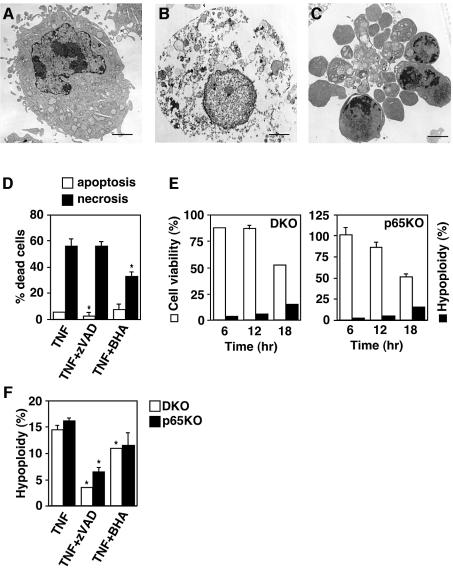
Since ROS have been reported to induce both apoptosis and necrosis depending on the cellular context (Fiers et al., 1999), we examined the morphology of dying cells by transmission electron microscopy. Notably, compared with the unstimulated DKO MEFs (Figure 7A), some dying cells showed a necrotic morphological change, which was characterized by loss of membrane integrity without apparent damage of nuclei (Figure 7B), whereas other cells showed typical apoptotic changes such as chromatin condensation, cell shrinking and apoptotic bodies (Figure 7C). Furthermore, number of necrotic cells was substantially reduced in the presence of BHA, but not zVAD-fmk (Figure 7D). Similar morphological changes and inhibitory effects were also observed in p65KO MEFs (data not shown).
Fig. 7. TNF induces both necrosis and apoptosis in DKO and p65KO MEFs. (A–C) DKO MEFs were unstimulated (A) or stimulated (B and C) with TNF (10 ng/ml) for 18 h. The morphological changes were observed by transmission electron microscopy. The scale bar represents 1 µm. Similar morphological changes were observed in p65KO MEFs (data not shown). (D) DKO MEFs were stimulated with TNF (10 ng/ml) alone or in the presence of zVAD-fmk (50 µM) or BHA (100 µM) for 18 h, then the samples were prepared for the electron microscope. The percentages of cell numbers showing apoptotic (open columns) and necrotic (closed columns) morphological changes were calculated by counting four randomly selected areas (40–70 cells per areas). Results are presented as mean ± SE. *P < 0.05 compared with TNF stimulation alone. (E) DKO and p65KO MEFs were stimulated with TNF (10 ng/ml) for the indicated time periods. Cell viability (open columns) and DNA hypoploidy (closed columns) were estimated by WST assay and flow cytometry, respectively. Data are represented as the as mean ± SE of triplicate samples. Similar results were obtained in three independent experiments. (F) DKO MEFs (open columns) and p65KO MEFs (closed columns) were stimulated with TNF (10 ng/ml) alone or in the presence of zVAD-fmk (50 µM) or BHA (100 µM) for 18 h, then DNA hypoploidy were measured by flow cytometry. Results are presented as mean ± SEs of triplicate samples. *P < 0.05 compared with TNF alone.
To assess the contribution of apoptosis and necrosis to TNF-induced cell death more quantitatively, we performed time-course analyses of TNF-induced DNA hypoploidy as an indication of apoptosis using flow cytometry. In parallel with these experiments, we also examined the cell viability by WST assay. Viability of DKO and p65KO MEFs gradually decreased to 50% at 18 h after TNF stimulation (Figure 7E). Although the cells with DNA hypoploidy increased to 15% at 18 h, this population was substantially less than the dead cell population (Figure 7E). These results, in combination with the electron microscopy data, indicate that a substantial part of DKO and p65KO MEFs died of necrosis after TNF stimulation (Figure 7E). We also tested the effects of BHA and z-VAD-fmk on TNF-induced apoptosis. As expected, the TNF-induced apoptosis was markedly inhibited by z-VAD-fmk. In contrast, BHA treatment substantially increased cell viability (Figure 6A), BHA only slightly decreased population of DNA hypoploidy (Figure 7F).
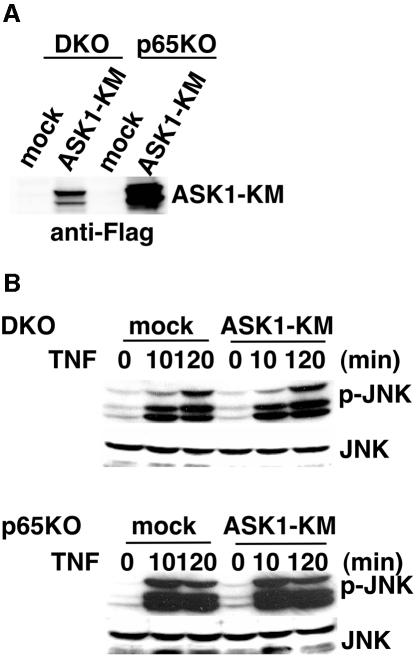
A kinase-inactive mutant of ASK1 does not inhibit prolonged JNK activation in DKO and p65KO MEFs
Previous studies have shown that ASK1 is activated by H2O2 and involved in prolonged JNK activation in some cells (Gotoh and Cooper, 1998; Saitoh et al., 1998). Thus, we next examined the contribution of ASK1 to prolonged JNK activation in DKO and p65KO MEFs after TNF stimulation. We first tried an in vitro kinase assay using immunoprecipitates with anti-ASK1 antibody or western blotting with antibody specific for phosphorylated ASK1 antibody. However, since the expression level of ASK1 in MEFs was low (H.Ichijo, unpublished result), we could not detect substantial levels of ASK1 kinase activities or phosphorylation of ASK1 after TNF stimulation (data not shown). Next, we stably transfected kinase-inactive mutant of ASK1 (ASK1-KM) as a dominant-negative inhibitor in DKO and p65KO MEFs. Expression of ASK1-KM was verified by western blotting with anti-Flag antibody (Figure 8A). As shown in Figure 8B, ASK1-KM did not inhibit TNF-induced prolonged JNK activation in DKO and p65KO MEFs.
Fig. 8. Introduction of ASK1-KM does not inhibit TNF-induced prolonged JNK activation in DKO and p65KO MEFs. (A) Total cell lysates were blotted with anti-Flag antibody. (B) Mock- or ASK1-KM-transfected DKO and p65KO MEFs were stimulated with TNF (50 ng/ml) for the indicated time periods, and the lysates were analyzed as described in Figure 1.
Discussion
In the present study, we investigated the mechanism by which TNF induces cell death in DKO and p65KO MEFs that are deficient in TNF-induced NF-κB activation. TNF, but not IL-1, induced prolonged MAPK activation due to ROS accumulation in DKO and p65KO MEFs, but not wild-type MEFs. Accumulation of ROS was associated with reduced levels of cellular antioxidants, GSH and NADPH. An antioxidant, BHA, inhibited not only TNF-induced ROS accumulation and prolonged MAPK activation, but also TNF-induced cell death. Interestingly, dying cells showed necrotic as well as apoptotic morphological changes. Collectively, these results demonstrate that NF-κB inhibits TNF-induced ROS accumulation, which mediates prolonged MAPK activation and cell death, under normal conditions.
It has been reported that NF-κB-inducible genes such as XIAP and GADD45β play an important role in suppressing prolonged JNK activation (De Smaele et al., 2001; Tang et al., 2001). However, the molecular mechanism by which XIAP and GADD45β inhibit JNK activation remains obscure. We previously identified GADD45β as a molecule interacting with MTK1 and showed that overexpression of GADD45β potently activates JNK and p38 pathways (Takekawa and Saito, 1998). Consistent with these results, stable expression of GADD45β in p65KO MEFs did not substantially inhibit TNF-induced ROS accumulation or prolonged MAPK activation, and cell death (Figure 5D–F). Similarly, XIAP did not inhibit TNF-induced ROS accumulation or prolonged MAPK activation, although XIAP substantially increased cell viability of TNF-stimulated cells (Figure 5D–F). These discrepancies might result from cell-type differences to generate GADD45β transfectants (T cell clone versus MEF) and/or differences in experimental systems (transient versus stable transfection of XIAP).
Previous studies suggested an important role of ROS in TNF-induced MAPK activation (Gotoh and Cooper, 1998; Saitoh et al., 1998). However, at least under our experimental conditions, we could not detect ROS accumulation in TNF-stimulated wild-type MEFs (Figure 3A). While BHA almost completely inhibited both ROS accumulation and prolonged MAPK activation in DKO and p65KO MEFs, BHA only slightly inhibited TNF-induced rapid/transient MAPK activation (Figures 2 and 3). Together, ROS appear not to play an essential role in TNF-induced transient MAPK activation in MEFs. However, we cannot exclude the possibility that ROS might play a critical role in other types of cells. In contrast to previous studies (De Smaele et al., 2001; Javelaud and Besancon, 2001; Tang et al., 2001), activation of not only JNK, but also p38 and ERK, was prolonged after TNF stimulation in DKO and p65KO MEFs (Figure 1A and B), which were all abrogated by BHA (Figure 2). Thus, accumulated ROS appear to activate all three MAPK cascades. Consistent with this notion, exogenously added ROS actually activated three MAPK cascades in MEFs (Figure 3E). Concerning the molecular mechanism by which ROS mediate prolonged JNK activation, ASK1 might participate in this process (Tobiume et al., 2001). However, under our experimental conditions, expression of ASK1-KM did not inhibit TNF-induced prolonged JNK activation in DKO and p65KO MEFs (Figure 8). These results suggested that a kinase other than ASK1, such as MEKK1, might be also involved in the ROS-induced JNK activation. It has been also reported that under non-stressed conditions, JNK binds cellular JNK inhibitor, GST, and this interaction is disrupted by ROS, resulting in activation of JNK (Adler et al., 1999a). Although these scenarios could explain the mechanism for prolonged JNK activation, they cannot explain the mechanism for prolonged ERK activation. In this respect, it is noteworthy that ROS- and UV-induced ERK activation has been reported to be mediated by c-src- and EGF receptor-dependent pathways (Chen et al., 2001; Kitagawa et al., 2002). We are currently pursuing these possibilities.
While TNF or IL-1 stimulation generally induces rapid and transient MAPK activation, genotoxic stresses such as UV and γ-irradiation induce prolonged MAPK activation that promotes cell death (Xia et al., 1995; Chen et al., 1996; Guo et al., 1998). Consistent with these studies, prolonged JNK activation partly contributed to TNF-induced cell death in DKO MEFs, as demonstrated by the inhibitory effect of a JNK inhibitor, SP600125 (Figure 6A). However, SP600125 did not inhibit TNF-induced cell death of p65KO MEFs (Figure 6A). Consistent with the data using MAPK inhibitors, expression of MKK7-KM only partially inhibited TNF-induced cell death of DKO, but not p65KO, MEFs (Figure 6B). The fact that inhibition of JNK cascade did not affect TNF-induced cell death of p65KO MEFs may be explained by the fact that TRAF-mediated transient JNK activation was intact in p65KO MEFs but not DKO MEFs (Figure 1A and B). Given that transient JNK activation transmits survival signals (Davis, 2000), SP600125 or MKK7-KM might cancel both survival and apoptotic signals in p65KO MEFs, resulting in no apparent inhibitory effect on TNF-induced cell death.
Our present study also demonstrates that TNF-induced ROS accumulation can be induced independently of TRAF2 and TRAF5. In addition, IL-1 signaling that is mediated by TRAF6 could not induce ROS accumulation (Figure 3D). Together, TRAF family proteins appear not to be essential for ROS accumulation. Given that z-VAD-fmk did not inhibit TNF-induced ROS accumulation (Figure 3C), TRADD, FADD or RIP, but not caspase-8, might play a critical role in this process. In this respect, RIP has been reported to be responsible for Fas-, TNF- or TRAIL-induced necrosis (Holler et al., 2000), but the contribution of ROS to RIP-mediated cell death has not been determined. Further study is required to address whether RIP is essential for TNF-induced ROS accumulation.
Here we show that reduced cellular levels of GSH and NADPH are correlated with accumulation of ROS in TNF-stimulated DKO and p65KO MEFs. ROS are generated in mitochondria and rapidly eliminated by antioxidants under normal conditions. Thus, the function of some antioxidant enzyme, the expression level or activity of which is regulated by NF-κB, might be impaired in DKO and p65KO MEFs. Previous studies showed that manganese-dependent SOD (MnSOD) is upregulated by TNF in an NF-κB-dependent manner, and overexpression of MnSOD inhibits ROS-induced apoptosis (Tanaka et al., 2002). While basal expression levels of MnSOD mRNA were not different between wild-type and DKO or p65KO MEFs, induction of MnSOD mRNA by TNF was substantially impaired in DKO and p65KO MEFs (data not shown). However, stable transfection of MnSOD into DKO or p65KO MEFs did not significantly inhibit TNF-induced ROS accumulation or cell death (data not shown). Taken that the primary target of MnSOD is O2– and the main accumulated ROS in DKO and p65KO MEFs were H2O2 or hydroxyl radical, the basal expression level of MnSOD might be sufficient for elimination of O2–. Regarding scavengers to detoxify H2O2, there are many antioxidant enzymes, including catalase, GPxs and PRxs (Thannickal and Fanburg, 2000). So far, we have observed no significant inhibitory effect of such antioxidant enzymes, including GPx1, GPx2, PRx1 and PRx2 on TNF-induced ROS accumulation in DKO or p65KO MEFs after stable transfection (data not shown). Further study is now underway to identify an antioxidant enzyme responsible for the ROS accumulation.
Another interesting point from this study is that a substantial part of DKO and p65KO MEFs died by necrosis after TNF stimulation, as assessed by electron microscopy and flow cytometry (Figure 7A–E). Furthermore, taken that BHA only marginally inhibited the emergence of apoptotic cells (Figure 7F), but substantially inhibited necrotic morphological changes (Figure 7D), ROS mainly, but not exclusively, contribute to necrotic cell death. These results are reminiscent of murine fibrosarcoma cell line, L929, in which TNF induces ROS-dependent necrosis (Vercammen et al., 1998). On the other hand, previous studies using p65KO mice implied an important role of NF-κB in protection from TNF-induced apoptosis of hepatocytes (Beg and Baltimore, 1996; Doi et al., 1999). It will be interesting to determine whether necrotic morphological changes are also observed in hepatocytes in p65KO mice.
In summary, our present study demonstrates a critical role of NF-κB to inhibit TNF-induced ROS accumulation under normal conditions. Elucidation of the mechanism by which NF-κB eliminates ROS would be helpful in manipulating various pathological conditions, such as ischemia or aging, in which ROS are deeply implicated in the pathogenesis.
Materials and methods
Reagents and cell culture
Recombinant murine TNF and murine IL-1β were purchased from BD PharMingen. NAC and reduced form of GSH were purchased from Sigma. SP600125, SB203580 and PD98059 were purchased from Biomol and Calbiochem. Antibodies specific for phospho-JNK, phospho-p38, phospho-ERK, phospho-MAPKAP2, JNK, p38, ERK and MAPKAP2 were purchased from Cell Signaling. Anti-Flag and anti-HA antibodies were purchased from Sigma and Roche Diagnostics Corp., respectively. BHA, z-VAD-fmk, CM-H2DCFDA and DHE were purchased from Wako Pure Chemicals, Peptide Institute and Molecular Probes. Wild-type, DKO and p65KO MEFs, and Phoenix-Eco cells were cultured in high-glucose DMEM containing 10% fetal calf serum (FCS).
Western blot analysis
MEFs (1 × 106 cells) were lysed in a buffer containing 50 mM Tris–HCl pH 8.0, 150 mM NaCl, 1% NP-40, 0.5% deoxycholate, 0.1% SDS, 1 mM phenylmethylsulfonyl fluoride and 1 µg/ml aprotinin. After centrifugation, cell lysates were subjected to 10% SDS–PAGE and transferred onto polyvinylidene difluoride membranes (Millipore). The membranes were blotted with antibodies to phospho-JNK, phospho-p38, phospho-ERK or phospho-MAPKAP2, and reblotted with antibodies to total JNK, total p38, total ERK or total MAPKAP2, respectively. To detect transfected gene products, the membranes were blotted with anti-Flag or anti-HA antibody. The membranes were developed with Enhanced Chemiluminescence (ECL) Western Blotting Detection System Plus (Amersham Pharmacia Biotech.) according to the manufacturer’s instruction.
Measurement of ROS accumulation
MEFs (2 × 105 cells) were plated in six-well plates and stimulated with TNF or IL-1 in phenol red-free medium for the indicated time periods. After stimulation, the cells were incubated with CM-H2DCFDA or DHE in the dark for 30 min at 37°C. Then, the cells were harvested and analyzed on a flow cytometer (FACSCalibur; BD Biosciences). Data were processed by using the CellQuest program (BD Biosciences).
Generation of stable transfectants
Generation of stable transfectants by using retroviral vectors was performed as described previously (Sasazuki et al., 2002). Retroviral expression vectors for TRAF2, TRAF5, p65, GADD45β, XIAP or ASK1-KM were constructed by inserting each cDNA into pMX-puro. Phoenix-Eco cells (1.5 × 106 cells) were transiently transfected with 3 µg of the indicated vectors using LipofectAMINE according to the manufacturer’s instruction (Invitrogen) to generate viral supernatants. After infection, DKO and p65KO MEFs were selected with 2.5 µg/ml puromycin (Sigma) to isolate stable transfectants. Puromycin-resistant pools were used for the experiments.
Measurement of GSH
Intracellular GSH levels were measured by using a colorimetric assay kit (OxisResearch™) according to the manufacturer’s instruction. Briefly, MEFs (2 × 106 cells) were unstimulated or stimulated with TNF for 4 h and harvested. The cells were resuspended in 500 µl of 5% meta phosphoric acid solution and sonicated using an ultrasonic sonicator (Astrason™). After centrifugation, the supernatants were sequentially incubated with 4-chloro-1-methyl-7-trifluromethyl-quindinium methylsulfate and NaOH, and then the absorbance at 400 nm was measured on a spectrophotometer. The pellets were lysed in the lysis buffer and the protein concentration was measured by the Bradford method. GSH contents were normalized by the protein concentration of each sample and are presented as GSH level per microgram of cell lysate.
Measurement of NADPH
NADPH levels were measured by the method of Gibon and Larher (1997). Briefly, MEFs (2.5 × 106 cells) were unstimulated or stimulated with TNF for 4 h. The cells were harvested and resuspended in 250 µl of 0.1 N NaOH, followed by brief homogenization. The homogenates were heated at 95°C for 5 min. After centrifugation, the supernatants were neutralized with 250 µl of 0.1 N HCl. Then, the supernatants were incubated with a buffer containing 400 mM NaCl, 100 mM Tris–HCl pH. 8.0, 4 mM EDTA, 0.42 mM 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT), 1.66 mM phenzine ethosulfate (PES), 2.5 mM NADP+ and 14 U of glucose 6-phosphate dehydrogenase (G6PDH) at 37°C for 40 min. The reaction was stopped by addition of 666 µl of 5 M NaCl, then centrifuged at 17 400 g at 4°C for 5 min. The pellets were lysed in 1 ml of 98% ethanol and the absorbance at 570 nm was measured on a spectrophotometer. The cellular NADPH contents were determined by using an NADPH standard curve.
WST assay
MEFs (5 × 103 cells) were plated in 96-well plates and cultured for 12 h in DMEM containing 10% FCS. The cells were then stimulated with TNF in the presence or absence of various inhibitors for 16 h. Cell viability was determined by WST assay using a Cell Counting kit (Dojindo).
Cell death assay
MEFs (1 × 105 cells) were cotransfected with the indicated expression vectors along with an expression vector for GFP (pEGFP) (Clontech) using LipofectAMINE. Expression vectors for MKK1-KM, MKK7-KM and MKK6-KM were constructed by inserting each cDNA into pcDNA3.1 (Invitrogen). After 24 h, cells were treated with TNF for 16 h, then GFP-positive cells were counted by fluorescent microscopy. Cells showing flat or round morphology were considered to be live or dead, respectively. Expression of transfected gene products was verified by western blotting with anti-Myc (for MKK1-KM and MKK7-KM) or anti-HA (for MKK6, TRAF2 and p65) antibody (data not shown).
Electron microscopy
MEFs (4 × 106 cells) were unstimulated or stimulated with TNF in the presence or absence of various inhibitors for 18 h, and then serially fixed with 2% glutaraldehyde in phosphate-buffered saline for 2 h and then with 2% OsO4 for 2 h before embedding in Epon 812. Thin sections were prepared using a MT-5000 ultramicrotome (Dupont Pharmaceuticals), stained with uranyl acetate followed by lead citrate, and then observed on a JEM1230 electron transmission microscope (JEOL). To evaluate the percentages of cell numbers showing apoptotic and necrotic morphological changes, sections were stained with toluidine blue and used for cell counting.
Statistical analysis
Statistical analysis was performed by Student’s t-test. A P value <0.05 was considered to be significant.
Supplementary data
Supplementary data are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We thank H.Sakurai, R.Takahashi, T.Kitamura, E.Nishida, C.Marshall, G.P.Nolan, W.-C.Yeh and T.W.Mak for providing reagents and Traf2–/– mice. We also thank H.Ichijo and H.Nishina for helpful discussion, and A.Matsuzawa and H.Nishitoh for technical advice. This work was supported in part by Grants-in-Aid for Scientific Research on Priority Areas (C) from the Ministry of Education, Culture, Sports, Science and Technology, Grant-in-Aid for Scientific Research (B) from Japan Society for the Promotion of Science, Japan, and by a Grant from Human Frontier Science Program (HFSP).
References
- Adler V. et al. (1999a) Regulation of JNK signaling by GSTp. EMBO J., 18, 1321–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler V., Yin,Z., Tew,K.D. and Ronai,Z. (1999b) Role of redox potential and reactive oxygen species in stress signaling. Oncogene, 18, 6104–6111. [DOI] [PubMed] [Google Scholar]
- Barkett M. and Gilmore,T.D. (1999) Control of apoptosis by Rel/NF-κB transcription factors. Oncogene, 18, 6910–6924. [DOI] [PubMed] [Google Scholar]
- Baud V., Liu,Z.G., Bennett,B., Suzuki,N., Xia,Y. and Karin,M. (1999) Signaling by proinflammatory cytokines: oligomerization of TRAF2 and TRAF6 is sufficient for JNK and IKK activation and target gene induction via an amino-terminal effector domain. Genes Dev., 13, 1297–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beg A.A. and Baltimore,D. (1996) An essential role for NF-κB in preventing TNF-α-induced cell death. Science, 274, 782–784. [DOI] [PubMed] [Google Scholar]
- Chen K., Vita,J.A., Berk,B.C. and Keaney,J.F.,Jr (2001) c-Jun N-terminal kinase activation by hydrogen peroxide in endothelial cells involves SRC-dependent epidermal growth factor receptor transactivation. J. Biol. Chem., 276, 16045–16050. [DOI] [PubMed] [Google Scholar]
- Chen Y.R., Wang,X., Templeton,D., Davis,R.J. and Tan,T.H. (1996) The role of c-Jun N-terminal kinase (JNK) in apoptosis induced by ultraviolet C and gamma radiation. Duration of JNK activation may determine cell death and proliferation. J. Biol. Chem., 271, 31929–31936. [DOI] [PubMed] [Google Scholar]
- Chung J.Y., Park,Y.C., Ye,H. and Wu,H. (2002) All TRAFs are not created equal: common and distinct molecular mechanisms of TRAF-mediated signal transduction. J. Cell Sci., 115, 679–688. [DOI] [PubMed] [Google Scholar]
- Davis R.J. (2000) Signal transduction by the JNK group of MAP kinases. Cell, 103, 239–252. [DOI] [PubMed] [Google Scholar]
- De Smaele E., Zazzeroni,F., Papa,S., Nguyen,D.U., Jin,R., Jones,J., Cong,R. and Franzoso,G. (2001) Induction of gadd45β by NF-κB downregulates pro-apoptotic JNK signalling. Nature, 414, 308–313. [DOI] [PubMed] [Google Scholar]
- Doi T.S., Marino,M.W., Takahashi,T., Yoshida,T., Sakakura,T., Old,L.J. and Obata,Y. (1999) Absence of tumor necrosis factor rescues RelA-deficient mice from embryonic lethality. Proc. Natl Acad. Sci. USA, 96, 2994–2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiers W., Beyaert,R., Declercq,W. and Vandenabeele,P. (1999) More than one way to die: apoptosis, necrosis and reactive oxygen damage. Oncogene, 18, 7719–7730. [DOI] [PubMed] [Google Scholar]
- Ghosh S., May,M.J. and Kopp,E.B. (1998) NF-κB and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol., 16, 225–260. [DOI] [PubMed] [Google Scholar]
- Gibon Y. and Larher,F. (1997) Cycling assay for nicotinamide adenine dinucleotides: NaCl precipitation and ethanol solubilization of the reduced tetrazolium. Anal. Biochem., 251, 153–157. [DOI] [PubMed] [Google Scholar]
- Gotoh Y. and Cooper,J.A. (1998) Reactive oxygen species- and dimerization-induced activation of apoptosis signal-regulating kinase 1 in tumor necrosis factor-alpha signal transduction. J. Biol. Chem., 273, 17477–17482. [DOI] [PubMed] [Google Scholar]
- Guo Y.L., Baysal,K., Kang,B., Yang,L.J. and Williamson,J.R. (1998) Correlation between sustained c-Jun N-terminal protein kinase activation and apoptosis induced by tumor necrosis factor-alpha in rat mesangial cells. J. Biol. Chem., 273, 4027–4034. [DOI] [PubMed] [Google Scholar]
- Holler N. et al. (2000) Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat. Immunol., 1, 489–495. [DOI] [PubMed] [Google Scholar]
- Ichijo H. (1999) From receptors to stress-activated MAP kinases. Oncogene, 18, 6087–6093. [DOI] [PubMed] [Google Scholar]
- Inoue J., Ishida,T., Tsukamoto,N., Kobayashi,N., Naito,A., Azuma,S. and Yamamoto,T. (2000) Tumor necrosis factor receptor-associated factor (TRAF) family: adapter proteins that mediate cytokine signaling. Exp. Cell Res., 254, 14–24. [DOI] [PubMed] [Google Scholar]
- Javelaud D. and Besancon,F. (2001) NF-κB activation results in rapid inactivation of JNK in TNFα-treated Ewing sarcoma cells: a mechanism for the anti-apoptotic effect of NF-kappa B. Oncogene, 20, 4365–4372. [DOI] [PubMed] [Google Scholar]
- Karin M. and Lin,A. (2002) NF-κB at the crossroads of life and death. Nat. Immunol., 3, 221–227. [DOI] [PubMed] [Google Scholar]
- Kitagawa D. et al. (2002) Activation of extracellular signal-regulated kinase by ultraviolet is mediated through Src-dependent epidermal growth factor receptor phosphorylation. Its implication in an anti-apoptotic function. J. Biol. Chem., 277, 366–371. [DOI] [PubMed] [Google Scholar]
- Lomaga M.A. et al. (1999) TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40 and LPS signaling. Genes Dev., 13, 1015–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak T.W. and Yeh,W.C. (2002) Signaling for survival and apoptosis in the immune system. Arthritis Res., 4, S243–S252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito A. et al. (1999) Severe osteopetrosis, defective interleukin-1 signalling and lymph node organogenesis in TRAF6-deficient mice. Genes Cells, 4, 353–362. [DOI] [PubMed] [Google Scholar]
- Ninomiya-Tsuji J., Kishimoto,K., Hiyama,A., Inoue,J., Cao,Z. and Matsumoto,K. (1999) The kinase TAK1 can activate the NIK-IκB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature, 398, 252–256. [DOI] [PubMed] [Google Scholar]
- Nishitoh H., Saitoh,M., Mochida,Y., Takeda,K., Nakano,H., Rothe,M., Miyazono,K. and Ichijo,H. (1998) ASK1 is essential for JNK/SAPK activation by TRAF2. Mol. Cell, 2, 389–395. [DOI] [PubMed] [Google Scholar]
- Saitoh M., Nishitoh,H., Fujii,M., Takeda,K., Tobiume,K., Sawada,Y., Kawabata,M., Miyazono,K. and Ichijo,H. (1998) Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J., 17, 2596–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasazuki T. et al. (2002) Identification of a novel transcriptional activator, BSAC, by a functional cloning to inhibit tumor necrosis factor-induced cell death. J. Biol. Chem., 277, 28853–28860. [DOI] [PubMed] [Google Scholar]
- Tada K. et al. (2001) Critical roles of TRAF2 and TRAF5 in tumor necrosis factor-induced NF-κB activation and protection from cell death. J. Biol. Chem., 276, 36530–36534. [DOI] [PubMed] [Google Scholar]
- Takekawa M. and Saito,H. (1998) A family of stress-inducible GADD45-like proteins mediate activation of the stress-responsive MTK1/MEKK4 MAPKKK. Cell, 95, 521–530. [DOI] [PubMed] [Google Scholar]
- Tanaka H., Matsumura,I., Ezoe,S., Satoh,Y., Sakamaki,T., Albanese,C., Machii,T., Pestell,R.G. and Kanakura,Y. (2002) E2F1 and c-Myc potentiate apoptosis through inhibition of NF-κB activity that facilitates MnSOD-mediated ROS elimination. Mol. Cell, 9, 1017–1029. [DOI] [PubMed] [Google Scholar]
- Tang G., Minemoto,Y., Dibling,B., Purcell,N.H., Li,Z., Karin,M. and Lin,A. (2001) Inhibition of JNK activation through NF-κB target genes. Nature, 414, 313–317. [DOI] [PubMed] [Google Scholar]
- Thannickal V.J. and Fanburg,B.L. (2000) Reactive oxygen species in cell signaling. Am. J. Physiol. Lung Cell. Mol. Physiol., 279, L1005–L1028. [DOI] [PubMed] [Google Scholar]
- Tobiume K. et al. (2001) ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep., 2, 222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey K.J. and Cerami,A. (1994) TUMOR NECROSIS FACTOR: A pleiotropic cytokine and therapeutic target. Annu. Rev. Immunol., 45, 491–503. [DOI] [PubMed] [Google Scholar]
- Vercammen D., Beyaert,R., Denecker,G., Goossens,V., Van Loo,G., Declercq,W., Grooten,J., Fiers,W. and Vandenabeele,P. (1998) Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J. Exp. Med., 187, 1477–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallach D. et al. (2002) How are the regulators regulated? The search for mechanisms that impose specificity on induction of cell death and NF-κB activation by members of the TNF/NGF receptor family. Arthritis Res., 4, S189–S196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z., Dickens,M., Raingeaud,J., Davis,R.J. and Greenberg,M.E. (1995) Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science, 270, 1326–1331. [DOI] [PubMed] [Google Scholar]
- Yeh W.-C. et al. (1997) Early lethality, functional NF-κB activation and increased sensitivity to TNF-induced cell death in TRAF2-deficient mice. Immunity, 7, 715–725. [DOI] [PubMed] [Google Scholar]