Abstract
Transcription by the nuclear factor of activated T cells (NFAT) is regulated by the frequency of Ca2+ oscillation. However, why and how Ca2+ oscillation regulates NFAT activity remain elusive. NFAT is dephosphorylated by Ca2+-dependent phosphatase calcineurin and translocates from the cytoplasm to the nucleus to initiate transcription. We analyzed the kinetics of dephosphorylation and translocation of NFAT. We show that Ca2+-dependent dephosphoryl ation proceeds rapidly, while the rephosphorylation and nuclear transport of NFAT proceed slowly. Therefore, after brief Ca2+ stimulation, dephosphoryl ated NFAT has a lifetime of several minutes in the cytoplasm. Thus, Ca2+ oscillation induces a build-up of dephosphorylated NFAT in the cytoplasm, allowing effective nuclear translocation, provided that the oscillation interval is shorter than the lifetime of dephos phorylated NFAT. We also show that Ca2+ oscillation is more cost-effective in inducing the translocation of NFAT than continuous Ca2+ signaling. Thus, the lifetime of dephosphorylated NFAT functions as a working memory of Ca2+ signals and enables the control of NFAT nuclear translocation by the frequency of Ca2+ oscillation at a reduced cost of Ca2+ signaling.
Keywords: calcineurin/Ca2+ oscillation/frequency dependence/NFAT/nuclear translocation
Introduction
An increase in intracellular Ca2+ concentration ([Ca2+]i) regulates a vast array of important cellular functions including contraction, secretion, synaptic plasticity, fertilization and immune responses (Clapham, 1995; Berridge et al., 2000). The versatility of Ca2+ signals is assumed to be dependent on the spatiotemporal patterns of intracellular Ca2+ signals. One of the most notable spatiotemporal patterns of Ca2+ signals is the oscillatory change in [Ca2+]i, or Ca2+ oscillation (Fewtrell, 1993; Berridge et al., 2000). Many cellular functions are thought to be regulated by the Ca2+ oscillation frequency (Negulescu et al., 1994; De Koninck and Schulman, 1998; Dolmetsch et al., 1998; Li et al., 1998; Oancea and Meyer, 1998). However, the molecular mechanism underlying the Ca2+ oscillation frequency dependence of the activation of specific cellular functions has remained elusive.
Gene transcription due to the nuclear factor of activated T cells (NFAT) is one of the cellular responses regulated by Ca2+ oscillation frequency (Dolmetsch et al., 1998; Li et al., 1998). NFAT remains in the cytoplasm when phosphorylated within unstimulated cells. Following an increase in [Ca2+]i, NFAT is dephosphorylated by the Ca2+-dependent phosphatase, calcineurin (Klee et al., 1998; Rusnak and Mertz, 2000). Then, NFAT translocates to the nucleus to initiate the transcription of a set of genes (Graef et al., 2001). Although several subtypes of NFAT with different tissue distributions have been discovered, conventional NFATs (NFAT1–4) share a common Ca2+-dependent property (Kiani et al., 2000). However, the molecular basis of the decoding mechanism of Ca2+ oscillation frequency dependence remains to be elucidated. In this study, we carried out continuous imaging of the subcellular distribution of green fluorescent protein (GFP)-tagged NFAT4, while manipulating [Ca2+]i in a time-controlled manner. We also analyzed the time-course of the Ca2+-dependent NFAT dephosphorylation and compared it with that of nuclear translocation. We show that the lifetime of dephosphorylated NFAT in the cytoplasm is the key factor that determines the Ca2+ oscillation frequency dependence of nuclear translocation of NFAT. These results were confirmed by experiments on endogenous NFAT4 in Jurkat T cells. Furthermore, we show that the decoding mechanism makes Ca2+ oscillation more advantageous than a continuous increase in [Ca2+]i in terms of Ca2+ signaling efficiency. The present study clarifies the molecular basis and advantage of the decoding mechanism of Ca2+ oscillation.
Results and discussion
Time-course of Ca2+-dependent NFAT translocation
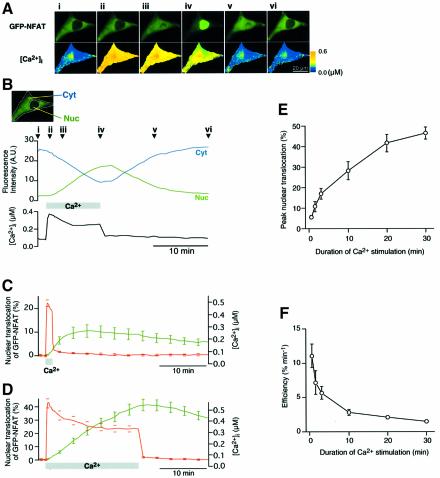
We monitored the NFAT translocation into the nucleus following an increase in intracellular Ca2+ concentration ([Ca2+]i) in baby hamster kidney (BHK) cells expressing GFP-tagged NFAT4 (GFP–NFAT) (Ho et al., 1995; Shibasaki et al., 1996). The Ca2+-clamp method (Dolmetsch et al., 1998) was used to vary [Ca2+]i, which was simultaneously monitored using a fluorescent Ca2+ indicator. Prior to the Ca2+ stimulation, a dominant fraction of GFP–NFAT was present in the cytoplasmic region (Figure 1Ai). Following an increase in [Ca2+]i, GFP–NFAT gradually accumulated in the nuclear region with a concomitant decrease in the amount of cytoplasmic GFP–NFAT (Figure 1Aii–iv and B). Notably, the GFP–NFAT translocation lagged significantly behind the increase in [Ca2+]i. This indicates that there is a slow rate-limiting process leading to the nuclear translocation of GFP–NFAT downstream of Ca2+ signals. Soon after the termination of Ca2+ stimulation, the increase in the amount of nuclear GFP–NFAT stopped, and then GFP–NFAT export from the nucleus began (Figure 1Av and vi, and B). On the other hand, GFP expressed in BHK cells exhibited uniform distribution in and out of the nucleus, and no change in the distribution was observed upon Ca2+ stimulation (data not shown).
Fig. 1. Imaging of Ca2+-dependent nuclear translocation of GFP–NFAT. (A) Time-lapse images of GFP–NFAT and [Ca2+]i at various time points during and after Ca2+ stimulation as indicated in (B). (B) Changes in GFP fluorescence intensity of the cell shown in (A) in the cytoplasmic region (indicated as Cyt) and the nuclear region (Nuc) are plotted with the change in [Ca2+]i. (C and D) Time courses of GFP–NFAT translocation and [Ca2+]i upon Ca2+ stimulation for 1.5 min [(C), n = 10] or 20 min [(D), n = 8] (mean ± SEM). The ranges of errors are shown only at selected time points for clarity. (E) Dependence of the peak extent of GFP–NFAT nuclear translocation on the duration of Ca2+ stimulation (mean ± SEM; n = 8–21). (F) Efficiency of GFP–NFAT nuclear translocation. Peak nuclear translocation of GFP–NFAT per unit duration of Ca2+ stimulation was plotted against the duration of Ca2+ stimulation.
Surprisingly, when brief (1.5 min) Ca2+ stimulation was given, the nuclear transport of GFP–NFAT proceeded even after the termination of Ca2+ stimulation, reaching a peak ∼8 min after Ca2+ stimulation (Figure 1C). This result indicates that GFP–NFAT is capable of storing information carried by the transient increase in [Ca2+]i as a working memory and that the slow GFP–NFAT transport across the nuclear membrane proceeds in a Ca2+-independent manner. We then examined the effects of the duration of Ca2+ stimulation on the extent of GFP–NFAT translocation. With 1.5 min of stimulation, ∼10% of GFP–NFAT translocated into the nucleus (Figure 1C). When the duration of Ca2+ stimulation was increased to 20 min, ∼40% of GFP–NFAT translocated into the nucleus (Figure 1D). We varied the duration of Ca2+ stimulation between 0.5 and 30 min. The results obtained showed that the peak nuclear translocation of GFP–NFAT increased, but not linearly, with the duration of Ca2+ stimulation (Figure 1E). We also examined the nuclear translocation of GFP–NFAT at different [Ca2+]i and found that the effect of [Ca2+]i levels off above 0.3 µM (data not shown). Therefore, although there is a gradual decline in [Ca2+]i during the long Ca2+ stimulation (Figure 1D), this should not have any major effects on our results, because [Ca2+]i was maintained above 0.3 µM.
NFAT dephosphorylation and nuclear translocation
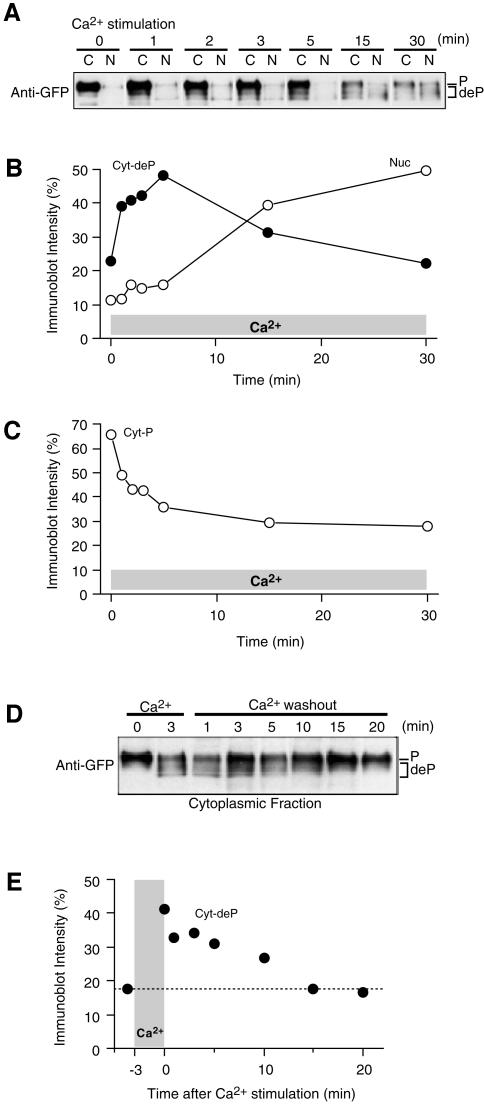
Since NFAT is dephosphorylated by calcineurin before its nuclear translocation, we analyzed the kinetics of dephosphorylation to elucidate the mechanism that dissociates the time courses of [Ca2+]i and the nuclear translocation of NFAT. At different times after the initiation of Ca2+ stimulation, cells were collected to separate cytoplasmic and nuclear fractions. The relative amounts of the phosphorylated and dephosphorylated forms of GFP–NFAT were determined in each fraction on the basis of the change in the relative molecular weight by SDS–PAGE (Ruff and Leach, 1995; Shibasaki et al., 1996). Before Ca2+ stimulation, a major GFP–NFAT band with an apparent molecular weight of ∼150 kDa was observed in the cytoplasmic fraction (Figure 2A), indicating that the predominant fraction of GFP–NFAT is present in the cytoplasm in a phosphorylated form at rest. Upon Ca2+ stimulation, the amount of nuclear GFP–NFAT increased gradually (Figure 2A and B, open circles), with a time-course consistent with that obtained by fluorescence imaging (Figure 1D). In contrast, the amount of dephosphorylated GFP–NFAT rapidly increased in the cytoplasmic fraction within a few minutes after the initiation of Ca2+ stimulation, as shown by the increase in the mobility of the GFP–NFAT band, and then gradually decreased (Figure 2A and B, filled circles). The widening of the dephosphorylated band may reflect the presence of multiple phosphorylation sites on each NFAT molecule (Chow et al., 1997; Zhu et al., 1998; Okamura et al., 2000; Marin et al., 2002). On the other hand, the amount of phosphorylated GFP–NFAT in the cytoplasm decreased rapidly within 5 min of Ca2+ stimulation, but slowly afterwards (Figure 2A and C). These results indicate that GFP–NFAT is rapidly dephosphorylated in the cytoplasm after Ca2+ stimulation and becomes nuclear transport ready, but that its nuclear transport proceeds very slowly, causing the temporal dissociation between Ca2+ signals and translocation.
Fig. 2. Analysis of dephosphorylation of GFP–NFAT. (A) Dephosphorylation and nuclear translocation of GFP–NFAT upon Ca2+ stimulation. The phosphorylation level of GFP–NFAT was analyzed by western blotting with the anti-GFP antibody after the rapid fractionation of BHK cells during Ca2+ stimulation. C, cytoplasmic fraction; N, nuclear fraction. Representative results of three experiments. (B and C) Time-course of the densitometric signals of cytoplasmic-dephosphorylated and nuclear GFP–NFAT (B) and cytoplasmic-phosphorylated GFP–NFAT (C) normalized by total density. (D) Decay of dephosphorylated GFP–NFAT in the cytoplasm after the termination of Ca2+ signaling. Cells were first stimulated with Ca2+ for 3 min, then fractionated at the indicated time-points after Ca2+ removal. Representative results of three experiments are shown. (E) Time-course of the densitometric signal of cytoplasmic dephosphorylated GFP–NFAT normalized by total density.
We also examined the time-course of the amount of dephosphorylated GFP–NFAT in the cytoplasmic fraction after the termination of brief (3 min) Ca2+ stimulation. Dephosphorylated GFP–NFAT remained detectable in the cytoplasm for more than 10 min (Figure 2D and E). This is consistent with the observation that the nuclear translocation of GFP–NFAT continues for several minutes after the termination of brief Ca2+ stimulation (Figure 1C). Thus, the dephosphorylated, nuclear-transport-ready GFP–NFAT in the cytoplasm functions as a working memory of Ca2+ signals.
Model calculation
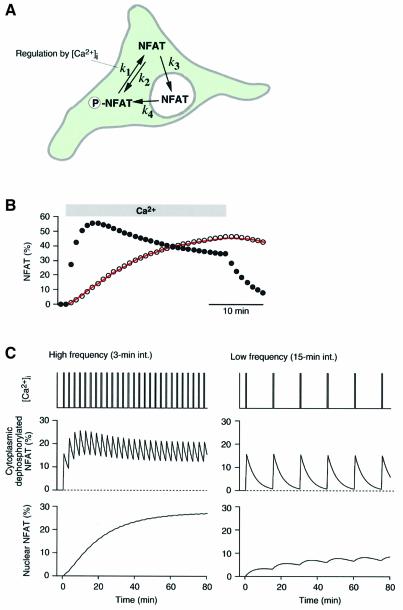
For more quantitative analysis, we examined whether we could model the nuclear translocation processes of NFAT. We assumed that NFAT is present in three states (cytoplasmic phosphorylated, cytoplasmic dephosphoryl ated and nuclear transported) and that there are transitions between the states, as indicated by the arrows in Figure 3A. The transition rate constants were assumed to be constant except for the dephosphorylation rate constant (k1), which was made to be under on–off regulation by [Ca2+]i. The solutions of two exponential terms can be obtained to describe the time-course of each state by solving differential equations (see Materials and methods). By finding an appropriate set of rate constants, we were able to fit the imaging data with reasonable accuracy using the model (Figure 3B, open circles; see legend for rate constants). Furthermore, the model provided the time-course of the concentration of dephosphorylated, nuclear-transport-ready NFAT (Figure 3B, filled circles), which showed reasonable correlation with the actual measurement of the dephosphorylation time-course during Ca2+ stimulation (Figure 2B, filled circles). Therefore, the model is successful in describing the basic features of the Ca2+-dependent nuclear translocation of NFAT.
Fig. 3. Model of Ca2+-dependent nuclear translocation of NFAT. (A) Schematic illustration of a model of NFAT translocation. NFAT assumes one of the three states, cytoplasmic phosphorylated, cytoplasmic dephosphorylated or nuclear transported. Rate constants are defined as indicated. The dephosphorylation rate constant (k1) was assumed to be regulated by [Ca2+]i. (B) Model fitting (open circles) to the nuclear translocation time-course of NFAT (continuous trace). Model prediction of the time-course of cytoplasmic-dephosphorylated NFAT is also shown (filled circles). Model para meters used: k1, 0.359/min (during Ca2+ stimulation); k2, 0.147/min; k3, 0.060/min; and k4, 0.035/min. (C) Model prediction of NFAT translocation during Ca2+ oscillation. High-frequency Ca2+ oscillation maintains dephosphorylated NFAT in the cytoplasm and induces significant nuclear translocation. Low-frequency oscillation results in intermittent dephosphorylation and induces only slight nuclear translocation of NFAT. Model parameters were the same as those used in (B).
We further examined the behavior of the model when an oscillatory change in [Ca2+]i was applied. As shown in Figure 3C (left panel), a high-frequency oscillation of 0.5 min Ca2+ spikes maintains NFAT dephosphorylation, since NFAT is repetitively dephosphorylated before the dephosphorylated NFAT (working memory) disappears from the cytoplasm. Therefore, a high-level nuclear translocation occurs. On the other hand, a low-frequency Ca2+ oscillation induces the intermittent dephosphoryl ation of NFAT and a low-level nuclear translocation (Figure 3C, right panel). Thus, the model succeeds in decoding Ca2+ oscillation frequency. We then tested these model predictions experimentally.
Ca2+ oscillation frequency and nuclear translocation of NFAT
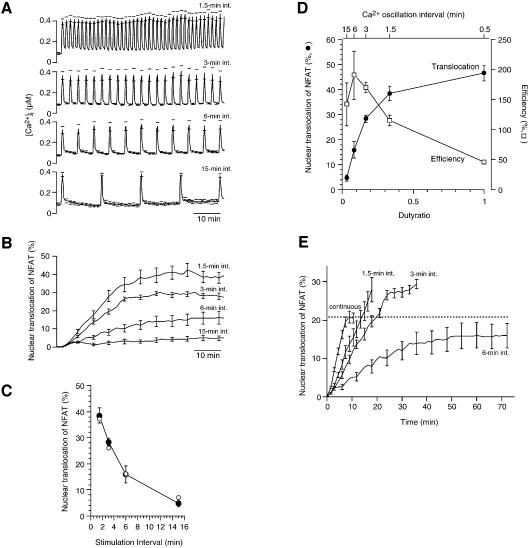
We challenged the cells with Ca2+ stimulation of 0.5 min duration at intervals varying between 1.5 and 15 min (Figure 4A), which include the physiological range of Ca2+ oscillation intervals observed in activated T cells (Lewis and Cahalan, 1989; Donnadieu et al., 1992). The Ca2+ oscillations induced the nuclear translocation of GFP–NFAT in a frequency-dependent manner (Figure 4B). The extent of steady-state nuclear translocation of GFP–NFAT decreased with increasing stimulation interval (Figure 4C, filled circles), in general agreement with the model prediction (Figure 4C, open circles). These results provide direct evidence that NFAT translates different Ca2+ oscillation frequencies into its nuclear localization level.
Fig. 4. Ca2+ oscillation frequency dependence of GFP–NFAT nuclear translocation. (A) [Ca2+]i in GFP–NFAT expressing BHK cells challenged with Ca2+ pulses of 0.5 min duration at different intervals (1.5–15 min). (B) GFP–NFAT translocation during oscillatory Ca2+ stimulation. Increase in GFP fluorescence intensity of the nuclear region normalized by that of the entire cell region. (C) Steady-state nuclear translocation of GFP–NFAT is plotted against Ca2+ oscillation interval. Filled circles, experimental results; open circles, model predictions. (D) Dependence of nuclear translocation effectiveness and efficiency on the duty ratio of Ca2+ oscillation. Filled circles, steady-state nuclear translocation level; open squares, efficiency of Ca2+ signals expressed as nuclear translocation divided by duty ratio. (E) Nuclear translocation of GFP–NFAT during Ca2+ stimulation of the same total duration but different patterns. Ca2+ stimulation was applied as either a single continuous 6 min pulse or 12 pulses of 0.5 min duration at intervals of 1.5, 3 or 6 min. The mean ± SEM (n = 5–10) is shown for all results. The ranges of errors are shown only at selected time points for clarity in (A), (B) and (E).
Efficiency of signal transmission and Ca2+ oscillation frequency
The generation of Ca2+ signals imposes various costs to cells, such as the metabolism of signaling molecules, activation of Ca2+ pump ATPase and Ca2+-mediated cell death (Shibasaki and McKeon, 1995; Berridge et al., 1998). It would be beneficial for cells to increase the efficiency of Ca2+ signaling to maximize the effect at a minimized cost associated with Ca2+ signaling. The efficiency of Ca2+ signals was evaluated as the extent of nuclear translocation of GFP–NFAT per unit duration of Ca2+ signals, and it decreased with Ca2+ signal duration (Figure 1E). Since short Ca2+ pulses are more efficient than long Ca2+ pulses, it is conceivable that oscillatory changes in [Ca2+]i can be more efficient than a continuous increase in translocating NFAT. The efficiency of Ca2+ oscillation can be evaluated by the extent of steady-state nuclear translocation divided by the duty ratio, which is the ratio of the duration of individual Ca2+ stimulation (i.e. 0.5 min in the present experiment) to the oscillation interval and is proportional to the frequency of Ca2+ oscillation. Our results show that the extent of nuclear translocation of GFP–NFAT is enhanced with the increase in duty ratio (Figure 4D, filled circles), while the efficiency of Ca2+ signaling decreases (Figure 4D, open squares). Thus, Ca2+ oscillations with the duty ratios of 0.17 and 0.33 (3 and 1.5 min intervals) resulted in ∼60 and 80%, respectively, of the maximum nuclear translocation of GFP–NFAT at more than 3- or 2-fold higher efficiency than continuous Ca2+ signaling.
The efficiency of Ca2+ oscillation has been analyzed from a different viewpoint. Li et al. (1998) studied NFAT-mediated gene expression in response to Ca2+ release from intracellular stores due to the photolysis of caged inositol 1,4,5-trisphosphate (IP3). The same total amount of caged IP3 elicited a more enhanced gene expression when intermittently photolyzed at 1 min intervals than when photolyzed at shorter or longer intervals, or continuously. This study suggests that the effect of Ca2+ oscillation can be maximized within a certain range of frequencies at the same cost of IP3 generation. However, the mechanism underlying this phenomenon has not been elucidated. We carried out experiments along this line, and Ca2+ stimulation of the same total duration was applied to the cells as either a single continuous 6 min stimulation or 12 pulses of 0.5 min duration at intervals of 1.5, 3 or 6 min. Ca2+ oscillation at 1.5 or 3 min intervals produced a higher-peak nuclear translocation of GFP–NFAT than the continuous increase or oscillation at 6 min intervals (Figure 4E). These results demonstrate that the cost-effectiveness of Ca2+ signaling increases during Ca2+ oscillation at optimal frequencies. For this property of the Ca2+-oscillation-mediated regulation of cell functions, the presence of a working memory is essential. If there were no working memory, i.e. if the nuclear translocation of NFAT proceeded only during [Ca2+]i increase, the maximum nuclear translocation would be limited by the total duration of [Ca2+]i increase. Under such conditions, oscillatory increases in [Ca2+]i would always cause a lower extent of final nuclear transport than a continuous increase in [Ca2+]i, because NFAT would escape from the nucleus during interoscillation periods.
Ca2+-dependent translocation of endogenous NFAT in Jurkat T cells
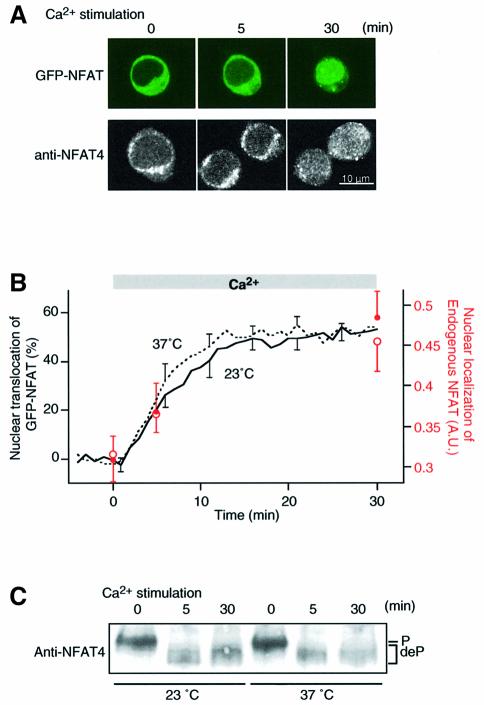
We examined whether the properties of exogenously expressed GFP-tagged NFAT are applicable to the physiological behavior of endogenous NFAT. Since endogenous NFAT4 is expressed in the human T cell line, Jurkat (Lyakh et al., 1997), we compared Ca2+-dependent nuclear translocation of endogenous NFAT with that of GFP–NFAT expressed in these cells. GFP–NFAT expressed in Jurkat cells also showed cytoplasmic localization at rest (Figure 5A, upper panel). Upon Ca2+ stimulation, GFP–NFAT gradually translocated into the nucleus (Figure 5A and B, continuous line), and the time-course was similar to that of GFP–NFAT expressed in BHK cells (Figure 1D). We then observed the translocation of endogenous NFAT by performing anti-NFAT4 immunostaining at various time points after Ca2+ stimulation. Endogenous NFAT also showed cytoplasmic localization at rest (Figure 5A, lower panel). Upon Ca2+ stimulation, nuclear staining intensity increased with a time-course similar to that of GFP–NFAT expressed in Jurkat cells (Figure 5A, lower panel, and B, red open symbols). We also carried out the same experiments at 37°C and found approximately the same time-course between the nuclear translocation of GFP–NFAT (Figure 5B, dashed line) and that of endogenous NFAT (Figure 5B, red filled symbols). We further examined the kinetics of dephosphorylation of endogenous NFAT upon Ca2+ stimulation. Endogenous NFAT4 was rapidly dephosphorylated within 5 min of Ca2+ stimulation at both room temperature and 37°C (Figure 5B), as was the case with GFP–NFAT in BHK cells at room temperature (Figure 2). These results demonstrate that endogenous NFAT in Jurkat cells undergoes rapid dephosphorylation and slow nuclear translocation, as seen in GFP–NFAT expressed in either BHK cells or Jurkat cells. We observed in Jurkat cells parallel increases in the steady-state endogenous NFAT translocation and NFAT reporter gene activity with increasing amplitude of Ca2+ stimulation (data not shown). Indeed, the oscillation interval dependence of nuclear translocation (Figure 4C) is generally consistent with that of the expression of an NFAT reporter gene in Jurkat cells (Dolmetsch et al., 1998).
Fig. 5. Activation kinetics of endogenous NFAT in Jurkat T cells. (A) Time-lapse images of GFP–NFAT translocation (upper panel) and samples of anti-NFAT4 immunofluorescence images (lower panel) obtained at the indicated time-points after Ca2+ stimulation. (B) Time courses of nuclear translocation of endogenous NFAT4 (circles) and GFP–NFAT (lines) upon Ca2+ stimulation. Experiments were carried out at room temperature (open circles, n = 9–12; continuous line, n = 7) and 37°C (filled circles, n = 11–17; dashed line, n = 6) (mean ± SEM). The ranges of errors are shown only at selected time points for clarity in the GFP–NFAT experiments. (C) Dephosphorylation of endogenous NFAT4 following Ca2+ stimulation. Whole-cell lysate prepared from Jurkat cells stimulated with Ca2+ at either room temperature or 37°C were used for western blotting with rabbit antiserum raised against NFAT4. Dephosphorylation of NFAT4 resulted in the mobility shift of the immunoblot bands.
Ca2+ oscillation frequency-dependent translocation of NFAT
The present results clarified the molecular basis of the decoding mechanism of Ca2+ oscillation by NFAT. NFAT is rapidly dephosphorylated upon an increase in [Ca2+]i and a significant fraction of NFAT becomes nuclear transport ready. Since the rephosphorylation of NFAT in the cytoplasm proceeds slowly even after a brief Ca2+ stimulation, dephosphorylated transport-ready NFAT remains in the cytoplasm for several minutes and functions as a working memory of Ca2+ signals. However, transport across the nuclear membrane proceeds extremely slow and each transport-ready NFAT molecule has a low chance of undergoing nuclear translocation. When Ca2+ stimuli are repeatedly applied at an interval shorter than the lifetime of dephosphorylated NFAT, there is a build-up of transport-ready NFAT in the cytoplasm. This promotes the nuclear translocation of NFAT during Ca2+ oscillation. Furthermore, our results demonstrated that, due to the presence of a working memory, Ca2+ oscillation can be more cost-effective for the cell than a continuous increase in [Ca2+]i.
In conclusion, the present study shows that the working memory of brief Ca2+ signaling (as a modification of NFAT phosphorylation) upstream of a rate-limiting step plays a key role in decoding Ca2+ oscillation frequency and improving the efficiency of Ca2+ signaling. This principle may be applicable to other cellular functions that are regulated by Ca2+ oscillation.
Materials and methods
Cells and transfection
BHK cells were maintained in modified Eagle’s medium (Gibco-BRL, Gaithersberg, MD) containing 5% fetal calf serum (FCS) and supplemented with 100 U/ml penicillin and 100 µg/ml streptomycin. The transfection of pCMV-GFP-NFAT4 (Shibasaki et al., 1996) or pCMV-GFP was carried out by the calcium-phosphate method (Shibasaki and McKeon, 1995). Jurkat cells (E6-1) were maintained in RPMI medium (Gibco-BRL) containing 10% FCS and supplemented with 100 U/ml penicillin and 100 µg/ml streptomycin. The transfection of pCMV-GFP-NFAT4 was carried out using LipofectAMINE 2000 (Invitrogen, Carlsbad, CA). Cells were cultured for 30 h after transfection before use.
Fluorescence imaging
GFP–NFAT expressing BHK cells on poly-d-lysine-coated glass-bottomed dish (MatTek, Ashland, MA) were loaded at room temperature with 5 µM Fura-PE3AM (TefLabs, Austin, TX) in physiological salt solution (PSS) containing 150 mM NaCl, 4 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 5.6 mM glucose, 5 mM HEPES pH 7.4 and 5% FCS. The fluorescence images were captured with an inverted microscope (IX70; Olympus, Tokyo, Japan), equipped with a 100× objective (NA 1.35), a cooled charge-coupled device (CCD) camera (Photometrics, Tucson, AZ) and a polychromatic illumination system (T.I.L.L. Photonics, Planegg, Germany), at a rate of one triplet of frames for every 10 s at excitation wavelengths of 355 and 380 nm for Fura-PE3 and 470 nm for GFP. The image-capture interval was changed to 60 s after the Fura-PE3 signal reached the steady-state level. Nuclear translocation of GFP–NFAT was evaluated based on the ratio of the fluorescence intensity of the nuclear region to the fluorescence intensity of the entire region of the cell. The intracellular Ca2+ concentration of the Fura-PE3-loaded cells was calculated using a previously reported equation (Grynkiewicz et al., 1985). All experiments using BHK cells were carried out at room temperature (23°C).
Jurkat cells expressing GFP–NFAT were adhered onto poly-d-lysine-coated glass-bottomed dish by low-speed centrifugation (200 g for 4 min). The fluorescence images were captured every 60 s using a confocal microscope (Fluoview; Olympus) equipped with a 100× objective (NA 1.35) with excitation at 488 nm. Experiments were carried out at either room temperature or 37°C. The nuclear translocation of GFP–NFAT was evaluated based on the ratio of the fluorescence intensity of the nuclear region to that of the entire region of the cell on a confocal plane.
Ca2+-clamp method
[Ca2+]i was controlled by the Ca2+-clamp method (Dolmetsch et al., 1998). Briefly, cells were pretreated with 1 µM thapsigargin to deplete the intracellular Ca2+ store and to activate store-operated Ca2+ channels. For the regulation of [Ca2+]i during fluorescence imaging, a computer-controlled puffing pipette was used to treat the cells with PSS containing either 10 mM Ca2+ to increase [Ca2+]i or 5 mM EGTA to decrease [Ca2+]i. When clamping of [Ca2+]i at intermediate concentrations was necessary, PSS containing <10 mM Ca2+ was used. For experiments at 37°C, the bath solution was warmed using a stage heater (Kitazato Supply, Tokyo, Japan) and the puffing solution was also prewarmed.
Cell fractionation
BHK cells were grown on 12-well culture plates, and the bath solution was rapidly changed to control [Ca2+]i using the Ca2+-clamp method. At various time-points during Ca2+ stimulation, the cells were washed with ice-cold phosphate-buffered saline (PBS) and resuspended in prechilled lysis buffer (10 mM NaCl, 0.5 mM dithiothreitol, and 20 mM Tris–Cl, pH 7.5) supplemented with protease inhibitors (complete protease inhibitor cocktail; Roche, Mannheim, Germany), 1 µM pepstatin and 0.1% Nonidet P-40. The cells were then transferred to microcentrifuge tubes, vortexed for 10 s, and centrifuged for 15 s at 17 400 g. The supernatant was recovered as a cytoplasmic fraction. The pellet was washed in the above buffer minus the detergent, and resuspended as the nuclear fraction in a buffer containing 0.1% Nonidet P-40. Both fractions were immediately mixed with one-third volume of 4× SDS sample buffer (8% SDS, 20% sucrose, 20% mercaptoethanol, 0.008% bromophenol blue and 250 mM Tris–HCl pH 6.8) and the mixture was boiled at 95°C for 10 min. Equivalent amounts of cytoplasmic and nuclear fractions from ∼3 × 105 cells were electrophoresed on 8.5% SDS–polyacrylamide gels and subjected to western blotting. The purity of the fractions were checked by western blotting using the antibodies against HSP90 (cytoplasmic marker) and Sp-1 (nuclear marker) (Santa Cruz Biotechnology, Santa Cruz, CA), and 86.4 ± 1.4% (mean ± SEM, n = 15) Sp-1 was recovered in the nuclear fraction, and 88.6 ± 1.6% (n = 15) HSP90 in the cytoplasmic fraction.
Western blotting
After electrophoresis, the proteins were transferred onto a PVDF membrane (Bio-Rad, Hercules, CA), and non-specific sites were blocked with 5% non-fat dry milk in Tris-buffered saline (100 mM NaCl and 20 mM Tris–HCl pH 7.8). For the detection of GFP–NFAT, the blot was probed with 1:3000 anti-GFP polyclonal antibody (MBL, Nagoya, Japan) and 1:8000 anti-rabbit IgG horseradish peroxidase (MBL) diluted in the blocking buffer, then developed using chemiluminescence reagents (Western Lightning™; PerkinElmer Life Sciences, Boston, MA). The quantitative determination of the immunoblot signal intenisty was carried out using Mac BAS software (Fuji Film, Tokyo, Japan). NFAT dephosphorylation and nuclear translocation were analyzed based on the relative immunoblot intensity of cytoplasmic phosphorylated form, dephosphorylated form and nuclear form. For the analysis of endogenous NFAT in Jurkat cell lysate, proteins were probed with 1:2000 rabbit antiserum raised against recombinant human NFAT4 protein and 1:8000 anti-rabbit IgG conjugated with horseradish peroxidase (MBL).
Model calculation
A model of NFAT translocation is described in the text (see Figure 3A). Designating the fractions of cytoplasmic-phosphorylated NFAT, cytoplasmic-dephosphorylated NFAT and nuclear-transported NFAT as x, y and 1 – x – y, respectively, we obtained the following differential equations:
dx/dt = –k1 x + k2 y + k4 (1 – x – y)
dy/dt = k1 x – (k2 + k3) y
where k1 is assumed to be 0 in the absence of Ca2+ stimulation. Analytical solutions for x and y were obtained using the initial conditions that x = 1 and y = 0 at t = 0, and were fitted to the time-course of the nuclear translocation of GFP–NFAT (Figure 1D) to estimate k1, k2, k3 and k4. KaleidaGraph (Synergy Software, Reading, PA) and Mathematica (Wolfram Research, Champaign, IL) were used for curve fitting and numerical calculations.
Immunocytochemistry and reporter gene assay
Jurkat cells adhering to the poly-d-lysine-coated glass-bottomed dish were stimulated by the Ca2+-clamp method. After stimulation, the cells were rapidly washed with ice-cold PBS, then fixed with 4% paraformaldehyde, and permeabilized with 0.1% Triton X-100. After blocking with 5% bovine serum albumin in PBS, endogenous NFAT was detected using anti-NFAT4 rabbit antiserum and Alexa 546-conjugated anti-rabbit IgG antibody (Molecular Probes, Eugene, OR). NFAT subcellular localization was visualized with a confocal laser-scanning microscope (Fluoview; Olympus). For evaluating NFAT localization, the fluorescence intensity of the nuclear region was normalized by that of the entire cell region. NFAT transcription activity was studied in Jurkat cells transfected with an NFAT luciferase reporter plasmid. After Ca2+ stimulation in the presence of PMA (50 ng/ml) for 3 h at 37°C, the cells were subjected to luciferase assay.
Analysis of endogenous NFAT dephosphorylation
Jurkat cells were stimulated by the Ca2+-clamp method in the presence of 5% FCS. The reaction was stopped by placing on ice and the cells were rapidly pelletted (17 500 g for 15 s). The cell pellet was washed once with ice-cold PBS and lysed in preheated (70°C) SDS sample buffer and boiled at 95°C for 10 min. The samples of the whole-cell lysate from ∼7.5 × 105 cells were electrophoresed on 8.5% SDS–polyacrylamide gels and subjected to western blotting.
Acknowledgments
Acknowledgements
We thank Drs A.Hashimoto and S.Namiki for reading the manuscript. This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology.
References
- Berridge M.J., Bootman,M.D. and Lipp,P. (1998) Calcium—a life and death signal. Nature, 395, 645–648. [DOI] [PubMed] [Google Scholar]
- Berridge M.J., Lipp,P. and Bootman,M.D. (2000) The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell. Biol., 1, 11–21. [DOI] [PubMed] [Google Scholar]
- Chow C.W., Rincon,M., Cavanagh,J., Dickens,M. and Davis,R.J. (1997) Nuclear accumulation of NFAT4 opposed by the JNK signal transduction pathway. Science, 278, 1638–1641. [DOI] [PubMed] [Google Scholar]
- Clapham D.E. (1995) Calcium signaling. Cell, 80, 259–268. [DOI] [PubMed] [Google Scholar]
- De Koninck P. and Schulman,H. (1998) Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations. Science, 279, 227–230. [DOI] [PubMed] [Google Scholar]
- Dolmetsch R.E., Xu,K. and Lewis,R.S. (1998) Calcium oscillations increase the efficiency and specificity of gene expression. Nature, 392, 933–936. [DOI] [PubMed] [Google Scholar]
- Donnadieu E., Cefai,D., Tan,Y.P., Paresys,G., Bismuth,G. and Trautmann,A. (1992) Imaging early steps of human T cell activation by antigen-presenting cells. J. Immunol., 148, 2643–2653. [PubMed] [Google Scholar]
- Fewtrell C. (1993) Ca2+ oscillations in non-excitable cells. Annu. Rev. Physiol., 55, 427–454. [DOI] [PubMed] [Google Scholar]
- Graef I.A., Chen,F. and Crabtree,G.R. (2001) NFAT signaling in vertebrate development. Curr. Opin. Genet. Dev., 11, 505–512. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie,M. and Tsien,R.Y. (1985) A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem., 260, 3440–3450. [PubMed] [Google Scholar]
- Ho S.N., Thomas,D.J., Timmerman,L.A., Li,X., Francke,U. and Crabtree,G.R. (1995) NFATc3, a lymphoid-specific NFATc family member that is calcium-regulated and exhibits distinct DNA binding specificity. J. Biol. Chem., 270, 19898–19907. [DOI] [PubMed] [Google Scholar]
- Kiani A., Rao,A. and Aramburu,J. (2000) Manipulating immune responses with immunosuppressive agents that target NFAT. Immunity, 12, 359–372. [DOI] [PubMed] [Google Scholar]
- Klee C.B., Ren,H. and Wang,X. (1998) Regulation of the calmodulin-stimulated protein phosphatase, calcineurin. J. Biol. Chem., 273, 13367–13370. [DOI] [PubMed] [Google Scholar]
- Lewis R.S. and Cahalan,M.D. (1989) Mitogen-induced oscillations of cytosolic Ca2+ and transmembrane Ca2+ current in human leukemic T cells. Cell Regul., 1, 99–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Llopis,J., Whitney,M., Zlokarnik,G. and Tsien,R.Y. (1998) Cell-permeant caged InsP3 ester shows that Ca2+ spike frequency can optimize gene expression. Nature, 392, 936–941. [DOI] [PubMed] [Google Scholar]
- Lyakh L., Ghosh,P. and Rice,N.R. (1997) Expression of NFAT-family proteins in normal human T cells. Mol. Cell. Biol., 17, 2475–2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin O., Burzio,V., Boschetti,M., Meggio,F., Allende,C.C., Allende,J.E. and Pinna,L.A. (2002) Structural features underlying the multisite phosphorylation of the A domain of the NF-AT4 transcription factor by protein kinase CK1. Biochemistry, 41, 618–627. [DOI] [PubMed] [Google Scholar]
- Negulescu P.A., Shastri,N. and Cahalan,M.D. (1994) Intracellular calcium dependence of gene expression in single T lymphocytes. Proc. Natl Acad. Sci. USA, 91, 2873–2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oancea E. and Meyer,T. (1998) Protein kinase C as a molecular machine for decoding calcium and diacylglycerol signals. Cell, 95, 307–318. [DOI] [PubMed] [Google Scholar]
- Okamura H. et al. (2000) Concerted dephosphorylation of the transcription factor NFAT1 induces a conformational switch that regulates transcriptional activity. Mol. Cell, 6, 539–550. [DOI] [PubMed] [Google Scholar]
- Ruff V.A. and Leach,K.L. (1995) Direct demonstration of NFATp dephosphorylation and nuclear localization in activated HT-2 cells using a specific NFATp polyclonal antibody. J. Biol. Chem., 270, 22602–22607. [DOI] [PubMed] [Google Scholar]
- Rusnak F. and Mertz,P. (2000) Calcineurin: form and function. Physiol. Rev., 80, 1483–1521. [DOI] [PubMed] [Google Scholar]
- Shibasaki F. and McKeon,F. (1995) Calcineurin functions in Ca2+-activated cell death in mammalian cells. J. Cell Biol., 131, 735–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki F., Price,E.R., Milan,D. and McKeon,F. (1996) Role of kinases and the phosphatase calcineurin in the nuclear shuttling of transcription factor NF-AT4. Nature, 382, 370–373. [DOI] [PubMed] [Google Scholar]
- Zhu J., Shibasaki,F., Price,R., Guillemot,J.C., Yano,T., Dotsch,V., Wagner,G., Ferrara,P. and McKeon,F. (1998) Intramolecular masking of nuclear import signal on NF-AT4 by casein kinase I and MEKK1. Cell, 93, 851–861. [DOI] [PubMed] [Google Scholar]