Abstract
Expression of the large GTPase guanylate binding protein-1 (GBP-1) is induced by inflammatory cytokines (ICs) in endothelial cells (ECs), and the helical domain of the molecule mediates the repression of EC proliferation by ICs. Here we show that the expression of GBP-1 and of the matrix metalloproteinase-1 (MMP-1) are inversely related in vitro and in vivo, and that GBP-1 selectively inhibits the expression of MMP-1 in ECs, but not the expression of other proteases. The GTPase activity of GBP-1 was necessary for this effect, which inhibited invasiveness and tube-forming capability of ECs in three-dimensional collagen-I matrices. A GTPase-deficient mutant (D184N-GBP-1) operated as a transdominant inhibitor of wild-type GBP-1 and rescued MMP-1 expression in the presence of ICs. Expression of D184N-GBP-1, as well as paracrine supplementation of MMP-1, restored the tube-forming capability of ECs in the presence of wild-type GBP-1. The latter finding indicated that the inhibition of capillary formation is specifically due to the repression of MMP-1 expression by GBP-1, and is not affected by the anti-proliferative activity of the helical domain of GBP-1. These findings substantiate the role of GBP-1 as a major regulator of the anti-angiogenic response of ECs to ICs.
Keywords: angiogenesis/collagenase/inflammation/invasion/MMP
Introduction
The guanylate binding proteins (GBPs), including GBP-1 and GBP-2, constitute the most abundant class of proteins induced by IFN-γ (Cheng et al., 1983, 1991). GBPs are GTP-binding proteins with a relative molecular mass of 67 000 (Schwemmle and Staeheli, 1994; Praefcke et al., 1999; van der Bliek, 1999; Prakash et al., 2000a). Based on the crystal structure of GBP-1 and on biochemical considerations (Prakash et al., 2000a, b), it was proposed that GBP-1 belongs to the group of large GTP-binding proteins such as Mx and dynamin, all of which have a similar domain composition and the ability to undergo oligomerization with a high-turnover GTPase activity (van der Bliek, 1999). Structural hallmarks of GBP-1 are a large globular α/β-domain harboring the GTPase activity, and an elongated C-terminal part organized in an α-helical structure with unique features (Prakash et al., 2000a,b).
Angiogenesis in inflammation is an invasive and proliferative process, where the coordinated activation of endothelial cells by inflammatory cytokines (ICs) such as IL-1β, TNF-α and IFN-γ, and angiogenic growth factors (AGF) such as bFGF and VEGF leads to the formation of new vessels and triggers the extravasation of leukocytes into the tissues. Interestingly, the major activation pathways of endothelial cells during inflammatory angiogenesis, namely the IC pathway and the AGF pathway, converge on the regulation of GBP-1 expression. GBP-1 expression in endothelial cells is selectively induced by ICs (IFN-γ, IL-1α, IL-1β and TNF-α), which have been shown to inhibit endothelial cell proliferation and induce adhesion of leukocytes to these cells. In contrast, GBP-1 expression is inhibited by VEGF and bFGF, potent activators of endothelial cell proliferation (Guenzi et al., 2001; Lubeseder-Martellato et al., 2002).
Recently, we have shown that the human GBP-1 characterizes IC-activated endothelial cells in human tissues and is a key mediator of the anti-proliferative effect of IC on endothelial cells (Guenzi et al., 2001; Lubeseder-Martellato et al., 2002). The GBP-1 anti-proliferative activity occurs in the absence of apoptosis, is independent of the GBP-1 GTPase activity and is specifically mediated by the C-terminal helical domain (Guenzi et al., 2001).
In addition to proliferation, invasion of endothelial cells into the extracellular matrix is a key feature of angiogenesis. Invasion is a multistep process in which cell motility is connected to proteolysis (Pepper, 2001; Silletti et al., 2001; Vihinen and Kahari, 2002). Angiogenic proteolysis is dependent on matrix metalloproteinases (MMPs), a family of structurally related, zinc-dependent endopeptidases (Egeblad and Werb, 2002).
At present, 21 different human MMPs have been identified (Egeblad and Werb, 2002). These enzymes are classified according to their structure and substrate specificity into four major subgroups: the interstitial collagenases, gelatinases, stromelysins and membrane-type MMPs (MT-MMPs) (Egeblad and Werb, 2002; Vihinen and Kahari, 2002). Collagenase-1 (MMP-1), collagenase-2 (MMP-8) and collagenase-3 (MMP-13) are the principal secreted neutral proteinases for the degradation of collagens (Kahari and Saarialho-Kere, 1997; Westermarck and Kahari, 1999; Vihinen and Kahari, 2002). The release of MMP-1 by endothelial cells is crucial for angiogenesis (Fisher et al., 1994), and cleavage of collagen type I, a substrate of MMP-1, is required for endothelial cell invasion into the ECM and for vessel formation in vivo (Seandel et al., 2001). Elevated MMP-1 expression correlates with invasiveness of colorectal cancer (Ghilardi et al., 2001) and cutaneous melanoma (Ghilardi et al., 2001; Nikkola et al., 2002), and is associated with lymph node metastasis in stage IB cervical cancer (Moser et al., 1999) and peritoneal metastasis in gastric cancer (Inoue et al., 1999).
The aim of this study was to identify putative GBP-1-regulated genes in endothelial cells in order to further elucidate the cellular function of GBP-1. Here we show that ICs inhibit MMP-1 expression in endothelial cells and that this is mediated by GBP-1. This activity requires the GTPase activity of GBP-1 and efficiently inhibits endothelial cell invasion and their capability to form tubular networks in three-dimensional (3D) collagen I matrices. A GTPase-negative mutant of GBP-1 (D184N-GBP-1) abrogated the inhibitory effect of GBP-1 on MMP-1 expression in endothelial cells in a transdominant-negative manner. Expression of D184N-GBP-1, as well as paracrine supplementation of MMP-1, restored the tube-forming capability of endothelial cells in the presence of wild-type GBP-1. The capability of GBP-1 to inhibit both invasion (via its GTPase activity) and proliferation (via its helical domain) establishes GBP-1 as a key mediator of the anti-angiogenic effects of ICs in endothelial cells.
Results
GBP-1 inhibits MMP-1 expression in endothelial cells in vitro and is inversely related with MMP-1 expression in vivo
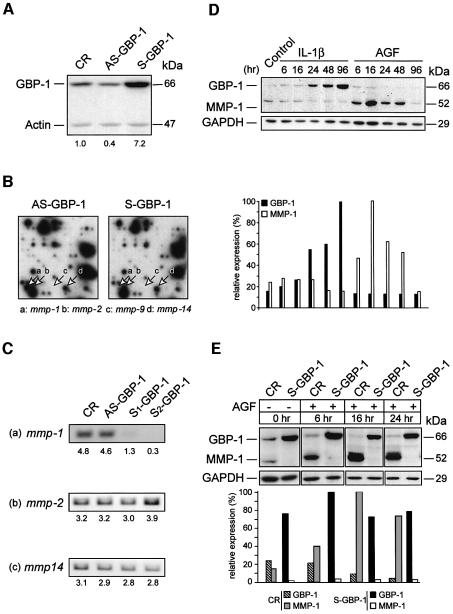
In order to identify genes regulated by GBP-1 in endothelial cells, HUVECs were transduced with retroviral vectors that expressed the GBP-1 full-length cDNA in the sense (S-GBP-1) and antisense (AS-GBP-1) orientations, and with a control vector (CR). AS-GBP-1 was used to repress the expression of endogenous GBP-1 (Guenzi et al., 2001). Expression of GBP-1 protein in S-GBP-1-transduced cells was 7- and 18-fold higher compared with CR- and AS-GBP-1-transduced cells, respectively (Figure 1A). We compared the gene expression profiles of AS-GBP-1- and S-GBP-1-transduced HUVEC using cDNA array technology. A total of 3528 genes were screened. Approximately 30% of the DNA on the arrays hybridized to the cDNA probes. The gene encoding matrix metalloproteinase-1 (mmp-1) was highly expressed in AS-GBP-1-transduced cells, but not in S-GBP-1-transduced cells (Figure 1B, arrow a). RT–PCR analysis confirmed that mmp-1 expression was absent or very low in two independently S-GBP-1-transduced HUVECs cultures, and was high in AS-GBP-1- and CR-transduced cells (Figure 1C, panel a). The expression of mmp-2 and mmp-14 was not affected by GBP-1 (Figure 1B, arrows b and d, and Figure 1C, panels b and c), and expression of mmp-9 was not detected in endothelial cells (Figure 1B, arrow c). In addition, array hybridization did not show signals for other mmps, such as mmp-3, -7, -8, -12, -13, or mt-mmp-2, -3 and -4 (data not shown).
Fig. 1. GBP-1 expression is inversely related with matrix metalloproteinase-1 (MMP-1) expression in endothelial cells. (A) Western blot analysis of GBP-1 expression in CR-, AS-GBP-1- and S-GBP-1-transduced HUVECs using a polyclonal rabbit anti-GBP-1 antibody. Corresponding signal intensities were densitometrically determined and normalized to actin in each lane. The relative increase of GBP-1 expression compared with CR-transduced HUVECs is given below. (B) Gene expression profile of AS-GBP-1- and S-GBP-1-transduced HUVECs using Atlas™ Human 1.2 array I. Signals corresponding to the main matrix metalloproteinases expressed in endothelial cells are indicated by arrows: a, mmp-1; b, mmp-2; c, mmp-9; d, mmp-14. (C) RT–PCR analysis of mmp-1, mmp-2 and mmp-14 expression in CR-, AS-GBP-1- and two independent S-GBP-1-transduced (S1, S2) HUVEC cultures. Corresponding signal intensities were densitometrically determined (arbitrary values) and are given below. (D) Western blot analysis of GBP-1 and MMP-1 expression in HUVECs either unstimulated (control) or stimulated with AGF (VEGF and bFGF, 10 ng/ml each) or IL-1β (200 U/ml) for the indicated periods of time. Corresponding signal intensities were densitometrically determined and normalized to GAPDH for each lane (lower panel). (E) Western blot analyses of GBP-1 and MMP-1 expression in CR- and S-GBP-1-transduced HUVECs incubated for the indicated periods of time with (+) or without (–) AGF (10 ng/ml). Corresponding signal intensities were densitometrically determined and normalized to GAPDH for each lane (lower panel). The monoclonal rat anti-GBP-1 antibody (mAb 1B1) was used for the detection of GBP-1 in (D) and (E).
Expression of GBP-1 and MMP-1 is regulated by IC and AGF (Hanemaaijer et al., 1993; Kumar et al., 1998; Sato, 1998; Cardozo et al., 2001; Guenzi et al., 2001; Lubeseder-Martellato et al., 2002). We analyzed the effect of IL-1β and AGF [bFGF (10 ng/ml) and VEGF (10 ng/ml) combined] on the expression of both proteins in HUVECs. IL-1β, in agreement with previous studies (Guenzi et al., 2001), increased GBP-1 expression after 24 h for up to 4 days (Figure 1D). MMP-1 expression was constantly low in the presence of IL-1β (Figure 1D). In contrast, AGF increased the expression of MMP-1 but not of GBP-1 (Figure 1D). AGF-induced expression of MMP-1 was maximal after 16 h and decreased rapidly afterwards (Figure 1D). Quantitative analysis of band intensities demonstrated that GBP-1 (Figure 1D, black columns) and MMP-1 (Figure 1D, white columns) were differentially expressed in the presence of IC and AGF.
MMP-1 expression was induced by AGF in CR-transduced cells (Figure 1E, light gray columns) with the same kinetics and to the same extent as in non-transduced HUVECs (compare Figure 1D and E), demonstrating that the transduction procedure did not impair endogenous MMP-1 expression. In contrast, MMP-1 expression could not be induced in S-GBP-1-transduced cells (Figure 1E, white columns) that constitutively expressed GBP-1 (Figure 1E, black columns).
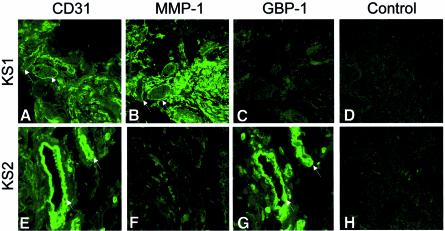
Kaposi’s sarcoma (KS) lesions are highly vascularized (Figure 2A). In the lesions, both AGF and IC are highly expressed, but in a temporally and spatially restricted manner (Guenzi et al., 2001; Stürzl et al., 2001; Lubeseder-Martellato et al., 2002). Expression of the endothelial cell-associated antigen CD31 (Figures 2A and E), of MMP-1 (Figure 2B and F) and of GBP-1 (Figure 2C and G) was analyzed by immunofluorescence in consecutive sections of two different KS biopsies (Figure 2, KS1, KS2). Vessel endothelial cells in the KS1 tumor (Figure 2A) highly expressed MMP-1 (Figure 2B, arrows), but not GBP-1 (Figure 2C). In contrast, in the other tumor (KS2), single blood vessels (Figure 2E) highly expressed GBP-1 (Figure 2G, arrows), but not MMP-1 (Figure 2F). We have shown that GBP-1-positive vessel endothelial cells in KS are exposed to high concentrations of IC, and that GBP-1-negative vessel endothelial cells are proliferating due to AGF activation (Guenzi et al., 2001; Lubeseder-Martellato et al., 2002). Control sections of specimen with MMP-1- (Figure 2D) and GBP-1- (Figure 2H) expressing endothelial cells did not show any staining when the primary anti-GBP-1 or anti-MMP-1 antibody was omitted, respectively.
Fig. 2. GBP-1 expression is inversely related to MMP-1 expression in endothelial cells in Kaposi’s sarcoma (KS). (A–C and E–G) Immunofluorescence staining of consecutive sections of two different KS biopsies (KS1, KS2) for the detection of the endothelial cell-associated antigen CD31, of MMP-1 and of GBP-1. Corresponding CD31- and MMP-1-positive, and CD31- and GBP-1-positive vessel endothelial cells are labeled with an arrow. MMP-1 (D) and GBP-1 (H) expressing KS sections subjected to the staining procedure without the anti-MMP-1 (D) and the anti-GBP-1 (H) antibody, respectively.
Together, these data demonstrate that MMP-1 expression is inversely related to GBP-1 expression in endothelial cells in vitro and in vivo, and that ectopically expressed GBP-1 inhibits the induction of MMP-1 expression by AGF.
GBP-1 mediates the inhibition of MMP-1 expression by IC
In order to determine whether cellular GBP-1 inhibits MMP-1 expression, HUVECs were pre-treated for different times (6.5 h and 24 h) with IL-1β and then incubated with AGF for 16 h to induce MMP-1 expression. MMP-1 expression was induced by AGF in control cells and in cells that were pre-treated for 6.5 h with IL-1β (Figure 3A). In these cells GBP-1 expression was still at background levels (Figure 3A). In contrast, MMP-1 expression could not be induced by AGF in cells that were pre-treated with IL-1β for 24 h, and expressed high levels of GBP-1 (Figure 3B). In addition, pre-treatment for 24 h with other ICs (TNF-α, IFN-γ), which similar to IL-1β induce GBP-1 expression in HUVECs (Guenzi et al., 2001; Lubeseder-Martellato et al., 2002), efficiently blocked the induction of MMP-1 expression by AGF (Figure 3B). After pre-treatment with IL-1β for 24 h, GBP-1 expression was 7-fold higher in CR-transduced HUVECs compared with the AS-GBP-1-transduced HUVECs, in which GBP-1 expression is repressed. Of note, in the latter cells, MMP-1 expression could still be induced under these conditions, but not in CR-transduced cells (Figure 3C). These data indicate that cellular GBP-1 is necessary for the inhibitory effect of IC on AGF-induced MMP-1 expression.
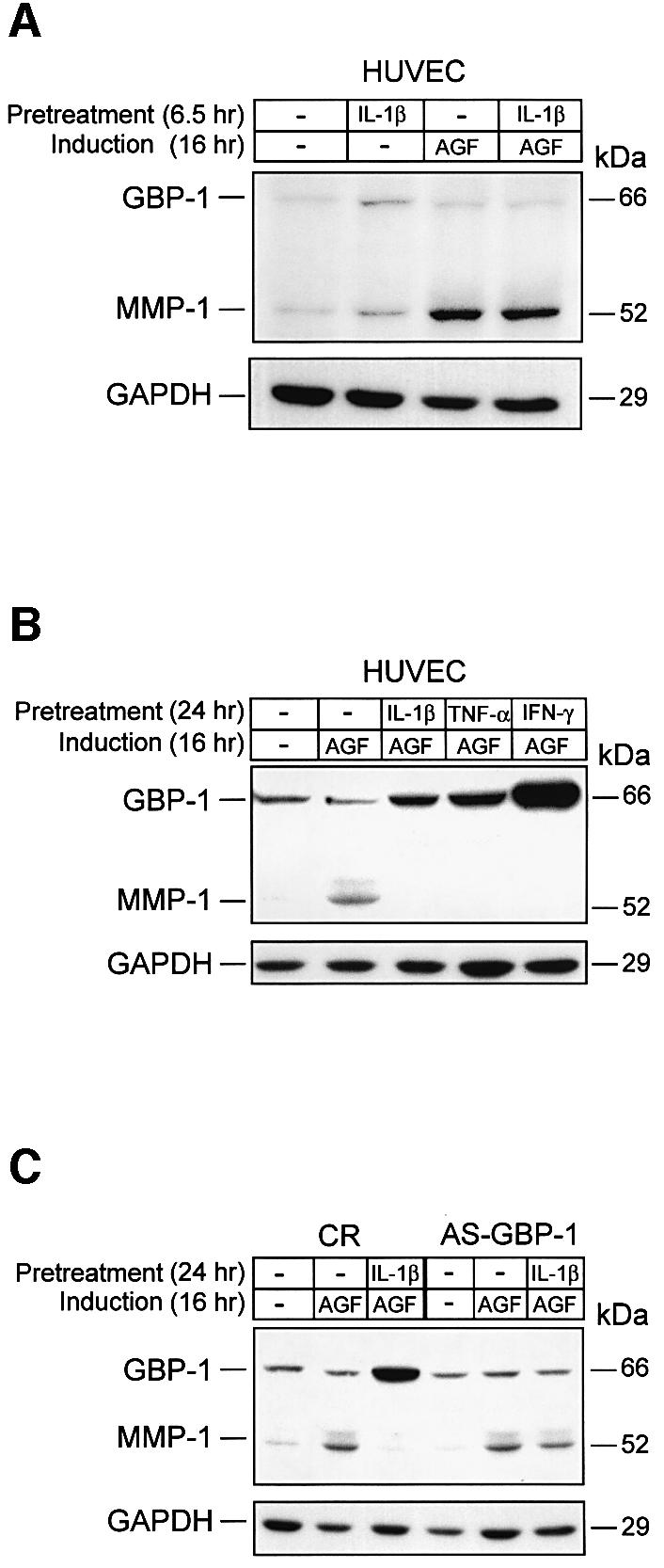
Fig. 3. GBP-1 mediates the IC-induced inhibition of MMP-1 expression in vitro. Western blot analyses of GBP-1 and MMP-1 expression in HUVECs. (A) Cells were pre-treated without (–) or with IL-1β (6.5 h, 200 U/ml) and subsequently incubated without (–) or with AGF (VEGF and bFGF, 10 ng/ml each) for 16 h. (B) Cells were pre-treated without (–) or with IL-1β (200 U/ml), TNF-α (300 U/ml) or IFN-γ (100 U/ml) for 24 h, and subsequently stimulated with AGF (10 ng/ml) for 16 h. (C) CR- and AS-GBP-1-transduced HUVECs were pre-treated without (–) or with IL-1β (24 h, 200 U/ml), and subsequently stimulated with AGF (10 ng/ml) for 16 h. Immunochemical detection of GAPDH indicates, for each lane, the amount of protein blotted onto the membranes.
The GTPase activity of GBP-1 is necessary for the inhibition of MMP-1 expression
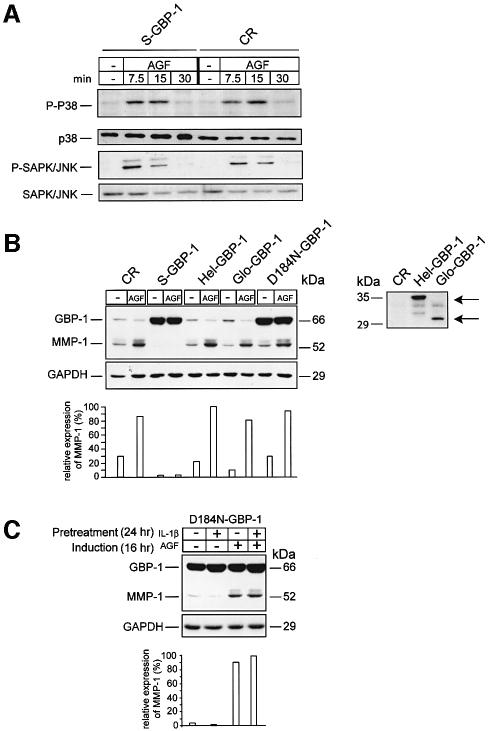
Members of the mitogen-activated protein kinases (MAPK) family, including MAPK/ERK, MAPK p38 and SAPK/JNK, have been shown to be involved in the transcriptional regulation of the mmp-1 gene (Benbow et al., 2002; Vincenti and Brinckerhoff, 2002). However, GBP-1 does not affect the MAPK/ERK signaling cascade (Guenzi et al., 2001). In addition, AGF- (Figure 4A) and IC-induced (data not shown) phosphorylation/activation of MAPK p38 and SAPK/JNK was not impaired in S-GBP-1-transduced HUVECs. Moreover, activation of the transcription factor ATF-2, which acts downstream of p38 MAPK, was not affected by GBP-1 (data not shown). This indicated that GBP-1 may inhibit MMP-1 expression via alternative mechanisms.
Fig. 4. The GTPase activity of GBP-1 is necessary to inhibit MMP-1 expression. (A) Western blot analysis of phospho-P38 (P-P38) and phospho-SAPK/JNK (P-SAPK/JNK) MAPK expression in S- and CR-GBP-1-transduced HUVECs stimulated with AGF (VEGF and bFGF, 10 ng/ml each), except control (–), for the indicated periods of time. (B) Left panel, western blot analyses of GBP-1 and MMP-1 expression in CR-, S-GBP-1-, Hel-GBP-1-, Glo-GBP-1- and D184N-GBP-1-transduced HUVECs stimulated with AGF (10 ng/ml) for 16 h (upper panel). Corresponding signal intensities were densitometrically determined and normalized to GAPDH in each lane (lower panel). Right panel, western blot analyses of the expression of the C-terminal helical domain (Hel-GBP-1, upper arrow) and the N-terminal globular domain (Glo-GBP-1, lower arrow) in HUVECs transduced with the respective retroviral vectors. CR-transduced HUVECs were used as a negative control (CR). A polyclonal rabbit anti-GBP-1 antibody was used. (C) Western blot analyses of GBP-1 and MMP-1 expression in D184N-GBP-1-transduced HUVECs (upper panel). Cells were pre-treated without (–) or with IL-1β (24 h, 200 U/ml), and subsequently incubated without (–) or with AGF (10 ng/ml) for 16 h. The rat monoclonal anti-GBP-1 (mAb, 1B1) antibody was used for detection of GBP-1. Immunochemical detection of GAPDH demonstrates that equal concentrations of protein were blotted onto the membranes (middle panel). Corresponding signal intensities were densitometrically determined and normalized to GAPDH in each lane (lower panel).
In order to identify the structural/functional components of GBP-1 that are required for the inhibition of MMP-1 expression, the effects of three mutants (D184N-GBP-1, Glo-GBP-1 and Hel-GBP-1) of the GBP-1 protein were analyzed. D184N-GBP-1 carries a single amino acid exchange (aspartate to asparagine) at position +184 in the N-terminal globular domain of GBP-1 that abolishes GTPase activity (Praefcke et al., 1999; Guenzi et al., 2001). Glo-GBP-1 represents the isolated N-terminal globular domain of GBP-1 (amino acids 1–290), which fully retains GTPase activity (Guenzi et al., 2001). Hel-GBP-1 represents the C-terminal helical domain (amino acids 288–592) that lacks GTPase activity, but has been shown to inhibit endothelial cell proliferation (Guenzi et al., 2001). The cDNAs encoding these different mutants were expressed in HUVECs by the use of retroviral vectors. The constitutive expression of Hel-GBP-1, Glo-GBP-1 (Figure 4B, right panel) and D184N-GBP-1 (Figure 4B, left panel) was confirmed by western blot analyses. In contrast to S-GBP-1-transduced cells, MMP-1 expression could be induced by AGF in cells expressing the different mutants of GBP-1 (Figure 4B, left panel). These results demonstrate that the GTPase activity is necessary (see D184N-GBP-1), but not sufficient (see Glo-GBP-1), for the inhibition of MMP-1 expression by GBP-1.
The biological activities of two other members of the large GTPases, MxA and dynamin, are inhibited in a transdominant-negative manner by the respective GTPase mutants (Ponten et al., 1997; Lee et al., 1999). By analogy, D184N-GBP-1 may also inhibit the effect of cellular GBP-1 on MMP-1 expression. To investigate this, HUVECs were transduced with D184N-GBP-1. High constitutive expression of D184N-GBP-1 was confirmed by western blot analysis (Figure 4C, lane –,–). In these cells, MMP-1 expression could be induced by AGF, irrespective of whether the cells expressed high amounts of endogenous GBP-1 induced by pre-treatment with IL-1β or not (Figure 4C, right two lanes). These data demonstrate that D184N-GBP-1 can inhibit wild-type GBP-1 activity in a transdominant-negative manner.
GBP-1 inhibits selectively the expression of MMP-1
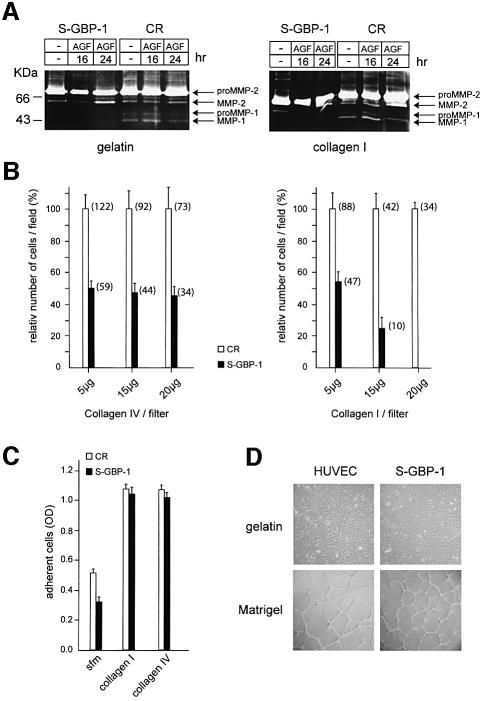
The total proteolytic activities released by CR- and S-GBP-1-transduced HUVECs were analyzed by gel zymography of culture supernatants using either gelatin (Figure 5A, left panel) or collagen I (Figure 5A, right panel) as a substrate. In agreement with previous studies (Zucker et al., 1998), HUVECs secreted large amounts of the zymogen proMMP-2 (72 kDa) (Figure 5A). Addition of AGF enhanced the processing of the zymogen to the active MMP-2 (62 kDa) after 24 h, both in CR- and S-GBP-1-transduced cells (Figure 5A). In addition, CR-transduced HUVECs released a proteolytic activity with a molecular weight of 47 kDa that corresponded to the active form of MMP-1 (Figure 5A). This activity was maximal 16 h after AGF-treatment of the cells (Figure 5A), which is in agreement with the expression kinetics of MMP-1 observed in AGF-treated endothelial cells (compare Figure 1D). In contrast, MMP-1 was never detected in supernatants of S-GBP-1-transduced cells, irrespective of cell stimulation and zymography substrate used (Figure 5A). These findings confirmed at the enzymatic level that GBP-1 selectively inhibits expression of MMP-1.
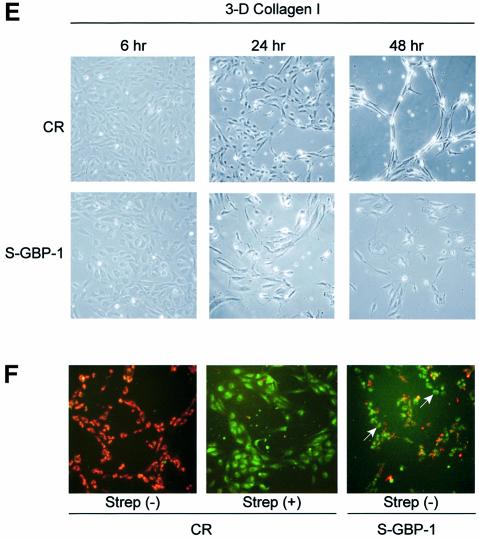
Fig. 5. GBP-1 inhibits migration and tube formation of HUVECs on collagen I matrices. (A) Zymography analysis using gelatin (left panel) or collagen I (right panel) as substrates. Cell culture supernatants of S-GBP-1- and CR-transduced HUVECs stimulated with AGF (VEGF and bFGF, 10 ng/ml each), except control (–), were used for the times indicated. The bands corresponding to the pro- and active forms of MMP-1 and MMP-2 are indicated (arrows). (B) Invasion assay of CR- and S-GBP-1-transduced HUVECs in collagen IV (left panel) and collagen I (right panel). The mean values obtained in three different experiments are shown [± standard deviations (SDs)]. The numbers of transmigrated CR-transduced cells in the respective experiments were set to 100% (white columns), and the relative numbers of S-GBP-1-transduced cells (black columns) were calculated accordingly. The absolute numbers of transmigrated cells are given in parentheses. (C) Adhesion assay of CR- and S-GBP-1-transduced HUVECs to collagen I and collagen IV matrices. The mean values obtained in six independent experiments are shown (± SD). Serum-free medium (sfm) without collagen was used as a control. (D) Phase contrast pictures of HUVECs or S-GBP-1-transduced HUVECs cultured for 24 h on gelatin (upper panel) or Matrigel (lower panel) in the presence of AGF (10 ng/ml). (E) Tube formation of CR- or S-GBP1-transduced HUVECs in 3D collagen I matrices in the presence of AGF (10 ng/ml) and PMA (0.1 µM). (F) In situ detection of apoptosis of CR- and S-GBP1-transduced HUVECs in 3D collagen I matrices after 24 h treated as in (E). Apoptosis was induced by treatment with streptonigrin (Strep, 1 µM) for 3 h. Non-apoptotic, CR-transduced HUVECs are stained red [CR, Strep(–)]. Apoptotic cells are stained green [Strep(+)]. White arrows indicate apoptotic cells (green) in the tube formation assay with S-GBP-1-transduced HUVECs.
GBP-1 inhibits invasion and tube formation of HUVECs in collagen I matrices
We next investigated the effect of GBP-1 on the capability of HUVECs to transmigrate in matrices of increasing concentrations of collagen I and collagen IV. The use of different concentrations of collagen allowed investigation into both the migratory (5 µg collagen) and invasive (15, 20 µg collagen) capability of the cells. At 5 µg collagen I and collagen IV the numbers of transmigrated S-GBP-1-transduced cells (Figure 5B, black columns) were 50% lower compared with CR-transduced cells (Figure 5B, white columns). This indicated that GBP-1 reduces the migratory capability of HUVECs in a substrate independent manner. Increasing the concentrations of collagen I and of collagen IV further reduced the absolute numbers of both transmigrated CR- and S-GBP-1-transduced cells (Figure 5B). Of note, the relative numbers of transmigrated S-GBP-1-transduced cells compared with CR-transduced cells were not further reduced by increasing concentrations of collagen IV (Figure 5B, left panel, black bars). In contrast, increasing concentrations of collagen I completely inhibited the transmigration of S-GBP-1-transduced cells (Figure 5B, right panel, black bars). This effect was due to the inhibition of cell invasiveness by GBP-1 and not due to different adhesion of the transduced cells to the respective matrices (Figure 5C).
When plated on gelatin-coated surfaces, S-GBP-1-transduced cells exhibited the same characteristic cobblestone morphology as non-transduced HUVECs (Figure 5D, upper panels). To investigate whether GBP-1 may affect the angiogenic capability of HUVECs, a capillary formation assay was used. In matrigel matrices, both cell cultures formed the characteristic well elaborated network of capillaries, with similar tube lengths and branching knot frequencies (Figure 5D, lower panels). However, when seeded in 3D collagen I matrices, where MMP-1 proteolytic activity of the cells is required for tube formation (Fisher et al., 1994), only CR-transduced HUVECs formed a clearly elaborated network after 48 h (Figure 5E, CR). In contrast, S-GBP-1-transduced cells only reorganized from a monolayer (Figure 5E, S-GBP-1, 6 h) to structures resembling initiating tubes (Figure 5E, S-GBP-1, 24 h), but these were resolved almost completely after 48 h, concomitant with cell loss (Figure 5E, S-GBP-1, 48 h), probably due to apoptosis (Figure 5F, S-GBP-1). Apoptosis was absent in invasive networks of CR-transduced HUVECs [Figure 5F, CR, Strep(–), red staining], could be induced in these cells by streptonigrin (Strep) treatment [Figure 5F, CR, Strep(+), green staining], and was detected in many cells in initiating networks of S-GBP-1-transduced cells in the absence of streptonigrin [Figure 5F, S-GBP-1, Strep(–), green staining].
GBP-1 mediates the inhibition of capillary formation by IC via inhibition of MMP-1 expression
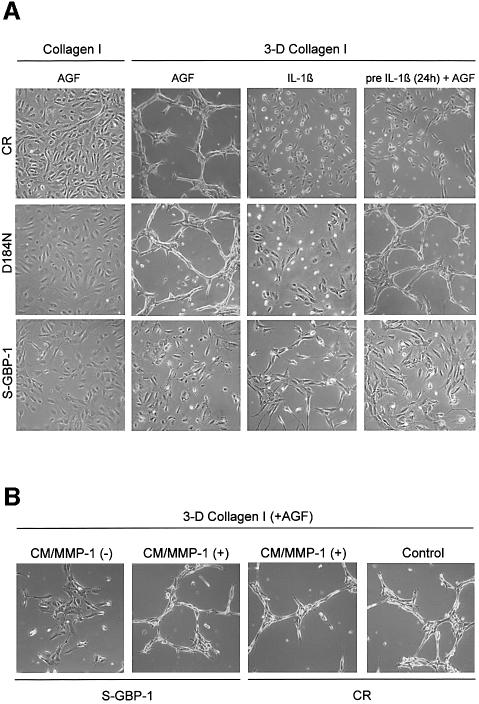
In order to investigate whether GBP-1 may regulate the angiogenic capability of HUVECs in the presence of IC, we analyzed the tube-forming capability of CR-, D184N- and S-GBP-1-transduced HUVECs in 3D collagen I matrices (Figure 6A). When seeded onto collagen I matrices, all of the cells formed monolayers after 48 h in the presence of AGF (Figure 6A, Collagen I, AGF). When seeded into the 3D collagen I matrices, AGF-stimulated CR- and D184N-transduced HUVECs formed elaborated capillary networks, whereas S-GBP-1-transduced cells did not (Figure 6A, 3-D Collagen I, AGF). Of note, IL-1β-stimulated CR-, D184N- and S-GBP-1-transduced HUVECs did not form capillaries in 3D collagen I (Figure 6A, IL-1β).
Fig. 6. Tube formation in 3D collagen I matrices requires MMP-1 expression and is independent of cell proliferation. (A) Left panel, morphology of CR-, D184N- and S-GBP-1-transduced HUVECs cultivated on collagen I for 48 h in the presence of AGF (10 ng/ml). Middle two panels, tube formation of transduced HUVECs in 3D collagen I matrices in the presence of AGF (10 ng/ml) or IL-1β (200 U/ml) for 48 h. Right panels, tube formation of the respective transduced cells pre-treated for 24 h with IL-1β (200 U/ml) and subsequently grown in 3D collagen I matrices for 48 h. (B) Tube formation of S-GBP1- and CR-transduced HUVECs in 3D collagen I matrices. Culture medium (CM) of HUVECs stimulated with AGF for 16 h [CM/MMP-1(+)] or of unstimulated HUVECs [CM/MMP-1(–)] was added to AGF- (10 ng/ml) treated CR- and S-GBP1-transduced HUVECs in collagen I matrices for 48 h. Right, CR-transduced HUVECs in 3D collagen I matrices in the presence of AGF (control).
Interestingly, pre-treatment of CR-transduced HUVECs with IL-1β for 24 h abrogated the capability of these cells to form tubes after subsequent stimulation with AGF (Figure 6A, pre IL-1β + AGF). In contrast, HUVECs expressing the transdominant-negative D184N-GBP-1 formed a well elaborated capillary network under these conditions (Figure 6A, preIL-1β + AGF). These results demonstrated that the tube-forming capability of HUVECs in 3D collagen I matrices is inhibited by GBP-1 and correlates with the expression of MMP-1 in these cells (compare Figure 6A with Figures 3B and 4C).
In agreement with this, the tube-forming capability of AGF-stimulated S-GBP-1-transduced HUVECs could be restored by paracrine supplementation of MMP-1. The addition of conditioned medium (CM) of AGF-stimulated, non-transduced HUVECs, which contain high concentrations of MMP-1, rescued the tube-forming capability of these cells [Figure 6B, S-GBP-1, CM/MMP1(+)]. In contrast, CM obtained from non-stimulated HUVECs that secrete only low concentrations of MMP-1 did not restore capillary formation [Figure 6B, S-GBP-1, CM/MMP-1 (–)]. In a control experiment, CM/MMP1 (+) did not impair the tube-forming capability of AGF-stimulated CR-transduced HUVECs [Figure 6B, CR, CM/MMP-1 (+)]. Altogether these results confirmed the antiangiogenic effect of the GBP-1-mediated inhibition of MMP-1 expression by IC.
Discussion
We have recently shown that GBP-1 characterizes the IC-induced, non-proliferating phenotype of endothelial cells both in vitro and in vivo (Lubeseder-Martellato et al., 2002), and that the helical domain of GBP-1 mediates the anti-proliferative effect of IC on endothelial cells (Guenzi et al., 2001). Here we report that GBP-1, in addition, mediates the inhibition of MMP-1 expression by IC in endothelial cells. This activity requires the GTPase activity of the molecule and efficiently inhibits the invasiveness and tube-forming capability of endothelial cells in 3D collagen I matrices. The capability to inhibit both invasion and proliferation of endothelial cells establishes GBP-1 as a key mediator of the anti-angiogenic effects of IC in endothelial cells.
The inhibitory effect of GBP-1 on MMP-1 expression was supported by several lines of experimental evidence. First, pre-treatment of endothelial cells with IC blocked the subsequent induction of MMP-1 expression by AGF. This effect was only observed at later time points (24 h) after IC treatment, when GBP-1 was strongly expressed, but not at earlier time points (6.5 h) when GBP-1 expression was still at background levels in the cells. Secondly, MMP-1 expression was inhibited by three different ICs (IL-1β, TNF-α, IFN-γ), which exert many different activities on endothelial cells (Mantovani et al., 1992, 1998; Introna and Mantovani, 1997; Pober, 2002), but in common all induce GBP-1 expression. Thirdly, S-GBP-1-transduced HUVECs that constitutively expressed GBP-1 did not express MMP-1, and MMP-1 expression could not be induced by AGF in these cells. Fourthly, the inhibition of GBP-1 expression and activity with GBP-1 antisense RNA and a transdominant-negative GBP-1 molecule (D184N-GBP-1), respectively, abrogated the inhibitory effect of IC on MMP-1 expression. Finally, GBP-1 and MMP-1 expression were inversely related in vessel endothelial cells of KS lesions in vivo. An inverse relation of GBP-1 and MMP-1 expression in vivo was also recently observed by others. For example, in the malignant progression of prostate cancer, a decrease of GBP-1 expression (Virolle et al., 2003) concomitant with an increase of MMP-1 expression (Daja et al., 2003) has been reported. These results congruently indicate that GBP-1 is necessary and sufficient to mediate the inhibition of MMP-1 expression by IC in vitro, and probably also in vivo.
While investigating which structural/functional motifs of GBP-1 may harbor the inhibitory activity on MMP-1 expression, we demonstrated that the exchange of a single amino acid (D184N) fully abrogated the inhibitory effect of GBP-1 on MMP-1 expression. This mutation represses the GTPase activity of the molecule (Praefcke et al., 1999; Guenzi et al., 2001); thus MMP-1 expression is the first cellular target that has been identified to be regulated by the GTPase activity of GBP-1. However, the globular domain alone that retains full GTPase activity did not inhibit MMP-1 expression, suggesting that the GTPase activity of GBP-1 is necessary but not sufficient for this effect.
For the inhibition of MMP-1 expression, the helical domain or parts thereof were also required. Binding sites for other regulatory molecules or for GBP-1 oligomerization may reside in this domain that may be necessary for GBP-1 function. This hypothesis is supported by the fact that the biological activity of two other members of the family of large GTP-binding proteins, MxA and dynamin, is regulated by oligomerization (van der Bliek, 1999; Prakash et al., 2000a; Marks et al., 2001; Kochs et al., 2002). GTPase-defective mutants of both proteins inhibited the biological activity of the respective wild-type protein in a transdominant-negative manner (Ponten et al., 1997; Lee et al., 1999). Our finding that the D184N-GBP-1 had a transdominant-negative effect on cellular GBP-1 supports the idea that oligomerization may regulate GTPase-mediated activities of GBP-1.
During angiogenesis, degradation of the vessel basement membrane and the underlying interstitium by matrix metalloproteinases is required for migration of endothelial cells into the perivascular space (Risau, 1995, 1997; Stetler-Stevenson, 1999; Carmeliet and Jain, 2000). Depending on the stimulus, endothelial cells can synthesize a variety of different MMPs, including MMP-1, -2, -3, -9 and -14 (Herron et al., 1986; Unemori et al., 1992; Fisher et al., 1994; Iwasaka et al., 1996; Duhamel-Clerin et al., 1997; Moses, 1997; Vacca et al., 1997; Haas et al., 1998; Hiraoka et al., 1998; Zucker et al., 1998). GBP-1 selectively inhibited the expression of MMP-1, as demonstrated again by several different experiments. First, expression and activity analyses of MMPs in HUVECs showed that GBP-1 selectively inhibited the expression of MMP-1, whereas expression of other MMPs was not affected (mmp-2 and mmp-14) or could not be detected in these cells (mmp-3, -7, -8, -12, -13, or mt-mmp-2, -3 and -4) with the array technology. Secondly, analyses of the invasive capability of the cells in different substrates showed that the transmigration of S-GBP-1-transduced cells was completely blocked by increasing concentrations of collagen I [a substrate of MMP-1 (Kahari and Saarialho-Kere, 1999; Johansson et al., 2000; Egeblad and Werb, 2002)] but not by collagen IV [a substrate for MMP-2 and MMP-9 (Johansson et al., 2000; Egeblad and Werb, 2002)]. Thirdly, the ability of S-GBP-1-transduced cells to form capillary-like structures in collagen I was blocked, while it was not impaired in Matrigel. Fourthly, the tube-forming capability of highly GBP-1-expressing endothelial cells could be rescued by restoring the AGF-inducibility of MMP-1 expression with a transdominant-negative D184N-GBP-1, and by paracrine supplementation of MMP-1. These results indicate that MMP-1 is required for the formation of angiogenic capillaries in collagen I and that GBP-1 inhibits invasiveness and tube-forming capability of endothelial cells via selective inhibition of MMP-1 expression.
In addition, the rescue of the tube-forming capability of cells that highly express GBP-1 demonstrated that MMP-1 expression and the tube-forming capability of endothelial cells are independent of the inhibition of cell proliferation, which is mediated by the helical domain of GBP-1.
IC induction of GBP-1 expression suggests that IC may harbor anti-angiogenic activities. This apparently contradicts the fact that angiogenesis can occur in inflammatory conditions in vivo (Frater-Schroder et al., 1987; Mahadevan et al., 1989; Montrucchio et al., 1994; Gerol et al., 1998; Torisu et al., 2000). However, it has to be considered that the induction of GBP-1 expression by IC can be inhibited by a surplus of AGF (Guenzi et al., 2001) and in addition requires up to 24 h, after exposure to IC, to reach a high level of expression (Figure 1D). Therefore, in the presence of IC and AGF, endothelial cells can still exert a full range of angiogenic activities during the induction phase of GBP-1 expression. This is confirmed by our finding that the induction of MMP-1 by AGF is significantly faster than the induction of GBP-1 by IC (Figure 1D). However, at later time points, when GBP-1 expression is maximal, this renders the cells refractory to additional stimulation by AGF and terminates angiogenesis. This is in agreement with our findings that GBP-1 expression in inflammatory tissues in vivo is restricted to single non-proliferating and MMP-1-negative vessels (Guenzi et al., 2001; Lubeseder-Martellato et al., 2002). Altogether, this leads to the provocative hypothesis that GBP-1 in inflammatory tissues may regulate the termination of angiogenesis in relation to the balance of IC and AGF at onset. Consequently, the inhibition of GBP-1 activity with transdominant-negative molecules, as described here, may increase angiogenic activity under inflammatory conditions, to support collateral vessel formation in ischemia for example.
Materials and methods
Cell cultures
Primary human macrovascular umbilical vein endothelial cells (HUVECs) were purchased from Clonetics and maintained in endothelial cell basal medium (EBM) supplemented with 5% fetal bovine serum (FBS) and propagated in Roux flasks (Greiner) coated with 1.5% bovine skin gelatin type B (Sigma) in phosphate-buffered saline (PBS). Three independent primary cultures of HUVECs were used for this study. Prior to stimulation, cells were cultured overnight in EBM–0.5% FBS. Recombinant human VEGF121 was purchased from R&D Systems, and recombinant human bFGF, IL-1β, IFN-γ and TNF-α were purchased from Roche.
cDNA array hybridization
Atlas™ Human 1.2 I, II and III cDNA expression array membranes used in this study were purchased from Clontech. The membranes contained 1176 unique human cDNAs (listed at www.clontech.com/atlas/genelists/index.shtml). Primary HUVECs transduced with the amphotropic retroviral vector pBabePuro (Morgenstern and Land, 1990), encoding human GBP-1 in sense (S-GBP-1) or antisense (AS-GBP-1) orientation (Guenzi et al., 2001) were grown to 80% confluency in EBM supplemented with 5% FBS and 0.3 µg/ml puromycin (Sigma). Total RNA was isolated with the RNeasy kit (Qiagen) and treated with DNAse I (1 U/µg RNA; BD Clontech) for 30 min at 37°C. [32P]cDNA probe labeling, hybridization and high-stringency washing were performed following the instructions of the manufacturer. Briefly, S-GBP-1 and AS-GBP-1 cDNA probes of equal activity (5 × 107 c.p.m.) were hybridized to the Atlas™ membranes (BD Clontech) for 20 h at 68°C and subjected to stringent washing. The intensity of hybridization signals was quantified using a PhosphorImager™ (Amersham) and normalized against internal controls (GAPDH and actin). Genes were considered to be up-regulated or down-regulated when the signal intensity of S-GBP-1 and AS-GBP-1 differed by >2-fold. The hybridization was repeated with new probes generated from the original total RNA on a second set of membranes, and only those genes that were reproducibly up- or down-regulated were analyzed further.
RT–PCR
RT–PCR was performed using the Titan one-tube RT–PCR system (Roche) according to the manufacturer’s instructions. The sequences of gene-specific primers for RT–PCR were the same as those of human 1.2 I cDNA arrays (data not shown because of the copyright agreement by Clontech). The cycle number was optimized to ensure amplification stayed in the linear range. PCR products were analyzed by electrophoresis on a 2% agarose gel stained with ethidium bromide. The corresponding signal intensities were densitometrically determined using the Imagequant software (Molecular Dynamics).
Transduction of HUVECs
cDNAs encoding full-length human GBP-1 (S-GBP-1), truncated forms (hel-GBP-1, glo-GBP-1) and GTPase-deficient mutants (D184N-GBP-1) were generated and cloned into the Moloney murine leukemia virus-derived retroviral vector pBabePuro (Morgenstern and Land, 1990) as described previously (Guenzi et al., 2001). PG13/J7 packaging cells (Miller et al., 1991) were used to generate the respective viruses. Retroviral infection of HUVECs was carried out as described previously (Guenzi et al., 2001). Transduction efficiency was between 50 and 70%, as determined by immunochemical detection of GBP-1 at the single-cell level in S-GBP-1-transduced HUVECs. Transduced cells were expanded by cultivation for an additional 10 days in the presence of 0.3 µg/ml puromycin (Sigma) before use. All experiments were repeated with three different cultures of HUVECs.
Western blot analysis
Western blot analysis was performed as described previously (Guenzi et al., 2001). Primary antibodies were diluted in 0.5× Western Blocking Reagent Solution (Roche) containing 0.1% Tween 20 (Sigma) in PBS, as follows: polyclonal rabbit anti-GBP1 (1:5000), polyclonal rabbit anti-actin (1:2000; Sigma), monoclonal rat anti-GBP1 [1:500; mAb 1B1 (Lubeseder-Martellato et al., 2002, p. 187)], monoclonal mouse anti-MMP-1 (1:500; MAB901; R&D Systems), polyclonal rabbit anti-phospho-p38, SAPK/JNK and anti-ATF-2 (1:1000; New England Biolabs).
Peroxidase-conjugated goat anti-mouse, anti-rat or anti-rabbit secondary antibodies (Amersham-Pharmacia Biotech) were diluted 1:5000 in 0.5× Western Blocking Reagent Solution containing 0.1% Tween 20 in PBS. Peroxidase activity was visualized using the enhanced chemiluminescence ECL Kit (Amersham-Pharmacia Biotech) and signals were quantified densitometrically using the Imagequant software.
Zymography
MMP activity in cell culture supernatants of S-GBP-1- and CR-transduced HUVECs was analyzed by substrate-gel electrophoresis (zymography), on a 10% SDS polyacrylamide gel containing either 1 mg/ml gelatin (Sigma) or 1 mg/ml rat tail collagen I (BD Biosciences). Cells were seeded onto 6-well plates in EBM–5% FBS at 2 × 104 cells/cm2, and grown to 80% confluency. Cells were then rinsed twice with serum-free medium (sfm) and incubated for 16 or 24 h in the absence or presence of AGF (bFGF and VEGF, 10 ng/ml). Culture supernatants were collected and centrifuged at 5200 g for 10 min, and Tris–HCl pH 8.0 was added to a final concentration of 50 mM. Equal volumes of the samples were subjected to zymographic analysis as described previously (Lafleur et al., 2001). After electrophoresis the gels were stained with Coomassie Brillant Blue and proteolytic activity was indicated by clear, non-stained bands.
Chemotaxis and chemoinvasion
Chemoinvasion of HUVECs was performed in Boyden chambers (Costar) as described previously (Johansson et al., 2000). Briefly, serum-free NIH 3T3-conditioned medium was used in the lower chamber as chemoattractant. Polycarbonate porous filters (12 µm pore size; Millipore) were pre-coated with 50 µl of 1% acetic acid containing 15 or 20 µg of collagen type I or IV (BD Biosciences), air-dried and re-hydrated in sfm before use. HUVECs were harvested by trypsinization, washed and resuspended in SFM, and 200 µl of cell suspension (5 × 104 cells) were added on the top of the filters. After 6 h of incubation, filters were harvested, cells on the upper surface were removed, and the cells that migrated to the lower surface were immediately fixed with ethanol at 4°C for 20 min and stained with toluidine blue. The number of migrated cells was assessed by counting of five to eight unit fields per filter at a magnification of 160× under a Zeiss microscope. Each test was performed in triplicate. Mean values and standard deviations were calculated.
Chemotaxis was assessed identically, except that filters were pre-coated with 5 µg of collagen I or collagen IV, which do not establish a physical barrier for migrating cells.
Adhesion assay
Adhesion assay of CR- or S-GBP-1-transduced HUVECs to collagen matrices was performed in 96-well plates (Nunc), pre-coated with or without collagen type I or type IV solution (50 µg/ml in 1% acetic acid; Sigma). The matrix solution was removed after 1 h incubation at 37°C, and 100 µl of HUVEC suspension (5 × 103 cells in sfm) were added to each well. Cells were allowed to adhere at 37°C in 5% CO2 for 2 h, supernatants were removed, and cells were fixed and stained in a solution containing formalin 4%, ethanol 32% and crystal violet 0.75%. Adherent cells were quantified spectrophotometrically at 595 nm. Mean values and standard deviations of six independent experiments were calculated and are shown.
Tube formation assay
Tube formation was analyzed in Matrigel (BD Biosciences) and 3D collagen-I substrates. For the Matrigel assay, cells were grown in EBM–0.5% FBS to 80% confluency, trypsinized, and resuspended into EBM–0.5% containing AGF (bFGF and VEGF, 10 ng/ml each). Cells were seeded at a density of 6 × 104 cells/well in 6-well plates, which were pre-coated with 250 µl of Matrigel at 37°C for 1 h. After 24 h of incubation, developing capillaries were photographed under an inverted phase contrast photomicroscope (Zeiss). All assays were performed in triplicate.
For the 3D collagen I assay, highly purified human collagen I (BD Biosciences) was dissolved in serum-free EBM at a concentration of 120 µg/ml, and 200 µl of the solution were added to 12-well dishes (∼0.6 µg/cm2) and incubated overnight at 37°C. Cells grown in low medium supplemented with AGF (bFGF and VEGF, 10 ng/ml each) or with IL-1β (200 U/ml) for 24 h were harvested by trypsinization, washed twice with serum-free EBM and resuspended in 200 µl of EBM–0.5% FBS containing 100 µg/ml collagen-I, and seeded onto the first collagen layer at a density of 3 × 104 cells/cm2. After 30 min, 100 µl of EBM–0.5% FBS with PMA (0.1 µM) and AGF (bFGF and VEGF, 10 ng/ml each) or IL-1β (200 U/ml), respectively, were added (except control). Developing capillaries were photographed after 6, 24 and 48 h under an inverted phase contrast photomicroscope (Zeiss). All assays were performed in triplicate.
Conditioned medium (CM) used for the 3D collagen I assay was prepared as follows: HUVECs were maintained in EBM supplemented with 5% FBS and propagated in Roux flasks (Greiner) coated with 1.5% gelatin. Cells were grown to 80% confluency, rinsed twice with SFM and incubated for 16 h in the absence or presence of AGF (bFGF and VEGF, 10 ng/ml). Culture supernatants were collected, centrifuged at 5200 g for 10 min and used immediately in the assay.
In situ detection of apoptosis
Apoptotic cells in 3D collagen-I substrates were detected using the mitochondrial membrane potential detection kit (Biocarta) as described by the manufacturer. This assay kit uses a unique cationic dye: green fluorescence indicates an increase in the mitochondrial membrane permeability in apoptotic cells, while non-apoptotic cells are stained red. Cells were grown in 3D collagen-I substrates for 24 h, and incubated for 40 min with the dye. Both apoptotic and healthy cells were visualized simultaneously using a fluorescence microscope (Zeiss).
Fluorescence staining of paraffin sections
Stainings of KS tissue sections were performed as described previously (Guenzi et al., 2001; Lubeseder-Martellato et al., 2002). Briefly, eight skin biopsies of acquired immune deficiency syndrome (AIDS)-associated KS were routinely processed, formalin-fixed and paraffin-embedded as described in Stürzl et al. (1999). For staining of CD31, GBP-1 and the MMP-1 antigen, consecutive sections of paraffin-embedded KS lesions were microwave-treated (3×, 10 min, 580 W) in Target Retrieval Solution pH 9.0 (Dako). Slides were then incubated with either mouse anti-CD31 (1:20), rat anti-GBP-1 (1:20) or mouse anti-MMP-1 (1:200; IM352; Oncogene) monoclonal antibodies. Bound primary antibodies were detected with a mixture of highly cross-absorbed goat anti-rat and goat anti-mouse antibodies (1:500) coupled to the fluorochromes AlexaFluor488 and AlexaFluor546 (Molecular Probes Europe), respectively.
Acknowledgments
Acknowledgements
We thank Thomas Grimm and Ilja Quadt (GSF, Neuherberg) for critical reading of the manuscript. This work was supported by grants from the BioFuture program of the German Ministry for Education and Research (BMBF), the Deutsche Forschungsgemeinschaft (DFG-SPP 1130), the Bavarian State Ministry of Sciences, Research and the Arts (Bavaria-Quebec Research Cooperation) and the German Cancer Aid (Deutsche Krebshilfe, Apoptose-Schwerpunktprogramm) to M.S.
References
- Benbow U., Tower,G.B., Wyatt,C.A., Buttice,G. and Brinckerhoff,C.E. (2002) High levels of MMP-1 expression in the absence of the 2G single nucleotide polymorphism is mediated by p38 and ERK1/2 mitogen-activated protein kinases in VMM5 melanoma cells. J. Cell. Biochem., 86, 307–319. [DOI] [PubMed] [Google Scholar]
- Cardozo A.K., Heimberg,H., Heremans,Y., Leeman,R., Kutlu,B., Kruhoffer,M., Orntoft,T. and Eizirik,D.L. (2001) A comprehensive analysis of cytokine-induced and nuclear factor-κB-dependent genes in primary rat pancreatic beta-cells. J. Biol. Chem., 276, 48879–48886. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. and Jain,R.K. (2000) Angiogenesis in cancer and other diseases. Nature, 407, 249–257. [DOI] [PubMed] [Google Scholar]
- Cheng Y.S., Colonno,R.J. and Yin,F.H. (1983) Interferon induction of fibroblast proteins with guanylate binding activity. J. Biol. Chem., 258, 7746–7750. [PubMed] [Google Scholar]
- Cheng Y.S., Patterson,C.E. and Staeheli,P. (1991) Interferon-induced guanylate-binding proteins lack an N(T)KXD consensus motif and bind GMP in addition to GDP and GTP. Mol. Cell. Biol., 11, 4717–4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daja M.M., Niu,X., Zhao,Z., Brown,J.M. and Russell,P.J. (2003) Characterization of expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in prostate cancer cell lines. Prostate Cancer Prostatic Dis., 6, 15–26. [DOI] [PubMed] [Google Scholar]
- Duhamel-Clerin E., Orvain,C., Lanza,F., Cazenave,J.P. and Klein-Soyer,C. (1997) Thrombin receptor-mediated increase of two matrix metalloproteinases, MMP-1 and MMP-3, in human endothelial cells. Arterioscler. Thromb. Vasc. Biol., 17, 1931–1938. [DOI] [PubMed] [Google Scholar]
- Egeblad M. and Werb,Z. (2002) New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer, 2, 161–174. [DOI] [PubMed] [Google Scholar]
- Fisher C., Gilbertson-Beadling,S., Powers,E.A., Petzold,G., Poorman,R. and Mitchell,M.A. (1994) Interstitial collagenase is required for angiogenesis in vitro. Dev. Biol., 162, 499–510. [DOI] [PubMed] [Google Scholar]
- Frater-Schroder M., Risau,W., Hallmann,R., Gautschi,P. and Bohlen,P. (1987) Tumor necrosis factor type α, a potent inhibitor of endothelial cell growth in vitro, is angiogenic in vivo. Proc. Natl Acad. Sci. USA, 84, 5277–5281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerol M., Curry,L., McCarroll,L., Doctrow,S. and Raychaudhury,A. (1998) Growth regulation of cultured endothelial cells by inflammatory cytokines: mitogenic, anti-proliferative and cytotoxic effects. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol., 120, 397–404. [DOI] [PubMed] [Google Scholar]
- Ghilardi G., Biondi,M.L., Mangoni,J., Leviti,S., DeMonti,M., Guagnellini,E. and Scorza,R. (2001) Matrix metalloproteinase-1 promoter polymorphism 1G/2G is correlated with colorectal cancer invasiveness. Clin. Cancer Res., 7, 2344–2346. [PubMed] [Google Scholar]
- Guenzi E. et al. (2001) The helical domain of GBP-1 mediates the inhibition of endothelial cell proliferation by inflammatory cytokines. EMBO J., 20, 5568–5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas T.L., Davis,S.J. and Madri,J.A. (1998) Three-dimensional type I collagen lattices induce coordinate expression of matrix metalloproteinases MT1-MMP and MMP-2 in microvascular endothelial cells. J. Biol. Chem., 273, 3604–3610. [DOI] [PubMed] [Google Scholar]
- Hanemaaijer R., Koolwijk,P., le Clercq,L., de Vree,W.J. and van Hinsbergh,V.W. (1993) Regulation of matrix metalloproteinase expression in human vein and microvascular endothelial cells. Effects of tumour necrosis factor α, interleukin 1 and phorbol ester. Biochem. J., 296, 803–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herron G.S., Werb,Z., Dwyer,K. and Banda,M.J. (1986) Secretion of metalloproteinases by stimulated capillary endothelial cells. I. Production of procollagenase and prostromelysin exceeds expression of proteolytic activity. J. Biol. Chem., 261, 2810–2813. [PubMed] [Google Scholar]
- Hiraoka N., Allen,E., Apel,I.J., Gyetko,M.R. and Weiss,S.J. (1998) Matrix metalloproteinases regulate neovascularization by acting as pericellular fibrinolysins. Cell, 95, 365–377. [DOI] [PubMed] [Google Scholar]
- Inoue T., Yashiro,M., Nishimura,S., Maeda,K., Sawada,T., Ogawa,Y., Sowa,M. and Chung,K.H. (1999) Matrix metalloproteinase-1 expression is a prognostic factor for patients with advanced gastric cancer. Int. J. Mol. Med., 4, 73–77. [DOI] [PubMed] [Google Scholar]
- Introna M. and Mantovani,A. (1997) Early activation signals in endothelial cells. Stimulation by cytokines. Arterioscler. Thromb. Vasc. Biol., 17, 423–428. [DOI] [PubMed] [Google Scholar]
- Iwasaka C., Tanaka,K., Abe,M. and Sato,Y. (1996) Ets-1 regulates angiogenesis by inducing the expression of urokinase- type plasminogen activator and matrix metalloproteinase-1 and the migration of vascular endothelial cells. J. Cell. Physiol., 169, 522–531. [DOI] [PubMed] [Google Scholar]
- Johansson N., Ahonen,M. and Kahari,V.M. (2000) Matrix metalloproteinases in tumor invasion. Cell. Mol. Life Sci., 57, 5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahari V.M. and Saarialho-Kere,U. (1997) Matrix metalloproteinases in skin. Exp. Dermatol., 6, 199–213. [DOI] [PubMed] [Google Scholar]
- Kahari V.M. and Saarialho-Kere,U. (1999) Matrix metalloproteinases and their inhibitors in tumour growth and invasion. Ann. Med., 31, 34–45. [DOI] [PubMed] [Google Scholar]
- Kochs G., Haener,M., Aebi,U. and Haller,O. (2002) Self-assembly of human MxA GTPase into highly ordered dynamin-like oligomers. J. Biol. Chem., 277, 14172–14176. [DOI] [PubMed] [Google Scholar]
- Kumar R., Yoneda,J., Bucana,C.D. and Fidler,I.J. (1998) Regulation of distinct steps of angiogenesis by different angiogenic molecules. Int. J. Oncol., 12, 749–757. [DOI] [PubMed] [Google Scholar]
- Lafleur M.A., Hollenberg,M.D., Atkinson,S.J., Knauper,V., Murphy,G. and Edwards,D.R. (2001) Activation of pro-(matrix metalloproteinase-2) (pro-MMP-2) by thrombin is membrane-type-MMP-dependent in human umbilical vein endothelial cells and generates a distinct 63 kDa active species. Biochem. J., 357, 107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A., Frank,D.W., Marks,M.S. and Lemmon,M.A. (1999) Dominant-negative inhibition of receptor-mediated endocytosis by a dynamin-1 mutant with a defective pleckstrin homology domain. Curr. Biol., 9, 261–264. [DOI] [PubMed] [Google Scholar]
- Lubeseder-Martellato C. et al. (2002) Guanylate-binding protein-1 expression is selectively induced by inflammatory cytokines and is an activation marker of endothelial cells during inflammatory diseases. Am. J. Pathol., 161, 1749–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadevan V., Hart,I.R. and Lewis,G.P. (1989) Factors influencing blood supply in wound granuloma quantitated by a new in vivo technique. Cancer Res., 49, 415–419. [PubMed] [Google Scholar]
- Mantovani A., Bussolino,F. and Dejana,E. (1992) Cytokine regulation of endothelial cell function. FASEB J., 6, 2591–2599. [DOI] [PubMed] [Google Scholar]
- Mantovani A., Garlanda,C., Introna,M. and Vecchi,A. (1998) Regulation of endothelial cell function by pro- and anti-inflammatory cytokines. Transplant. Proc., 30, 4239–4243. [DOI] [PubMed] [Google Scholar]
- Marks B., Stowell,M.H., Vallis,Y., Mills,I.G., Gibson,A., Hopkins,C.R. and McMahon,H.T. (2001) GTPase activity of dynamin and resulting conformation change are essential for endocytosis. Nature, 410, 231–235. [DOI] [PubMed] [Google Scholar]
- Miller A.D., Garcia,J.V., von Suhr,N., Lynch,C.M., Wilson,C. and Eiden,M.V. (1991) Construction and properties of retrovirus packaging cells based on gibbon ape leukemia virus. J. Virol., 65, 2220–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montrucchio G., Lupia,E., Battaglia,E., Passerini,G., Bussolino,F., Emanuelli,G. and Camussi,G. (1994) Tumor necrosis factor α-induced angiogenesis depends on in situ platelet-activating factor biosynthesis. J. Exp. Med., 180, 377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenstern J.P. and Land,H. (1990) Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res., 18, 3587–3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser P.L., Kieback,D.G., Hefler,L., Tempfer,C., Neunteufel,W. and Gitsch,G. (1999) Immunohistochemical detection of matrix metalloproteinases (MMP) 1 and 2 and tissue inhibitor of metalloproteinase 2 (TIMP 2) in stage IB cervical cancer. Anticancer Res., 19, 4391–4393. [PubMed] [Google Scholar]
- Moses M.A. (1997) The regulation of neovascularization of matrix metalloproteinases and their inhibitors. Stem Cells, 15, 180–189. [DOI] [PubMed] [Google Scholar]
- Nikkola J., Vihinen,P., Vlaykova,T., Hahka-Kemppinen,M., Kahari,V.M. and Pyrhonen,S. (2002) High expression levels of collagenase-1 and stromelysin-1 correlate with shorter disease-free survival in human metastatic melanoma. Int. J. Cancer, 97, 432–438. [DOI] [PubMed] [Google Scholar]
- Pepper M.S. (2001) Role of the matrix metalloproteinase and plasminogen activator-plasmin systems in angiogenesis. Arterioscler. Thromb. Vasc. Biol., 21, 1104–1117. [DOI] [PubMed] [Google Scholar]
- Pober J.S. (2002) Endothelial activation: intracellular signaling pathways. Arthritis Res., 4, S109–S116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponten A., Sick,C., Weeber,M., Haller,O. and Kochs,G. (1997) Dominant-negative mutants of human MxA protein: domains in the carboxy-terminal moiety are important for oligomerization and antiviral activity. J. Virol., 71, 2591–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praefcke G.J., Geyer,M., Schwemmle,M., Robert Kalbitzer,H. and Herrmann,C. (1999) Nucleotide-binding characteristics of human guanylate-binding protein 1 (hGBP1) and identification of the third GTP-binding motif. J. Mol. Biol., 292, 321–332. [DOI] [PubMed] [Google Scholar]
- Prakash B., Praefcke,G.J., Renault,L., Wittinghofer,A. and Herrmann,C. (2000a) Structure of human guanylate-binding protein 1 representing a unique class of GTP-binding proteins. Nature, 403, 567–571. [DOI] [PubMed] [Google Scholar]
- Prakash B., Renault,L., Praefcke,G.J., Herrmann,C. and Wittinghofer,A. (2000b) Triphosphate structure of guanylate-binding protein 1 and implications for nucleotide binding and GTPase mechanism. EMBO J., 19, 4555–4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risau W. (1995) Differentiation of endothelium. FASEB J., 9, 926–933. [PubMed] [Google Scholar]
- Risau W. (1997) Mechanisms of angiogenesis. Nature, 386, 671–674. [DOI] [PubMed] [Google Scholar]
- Sato Y. (1998) Transcription factor ETS-1 as a molecular target for angiogenesis inhibition. Hum. Cell, 11, 207–214. [PubMed] [Google Scholar]
- Schwemmle M. and Staeheli,P. (1994) The interferon-induced 67-kDa guanylate-binding protein (hGBP1) is a GTPase that converts GTP to GMP. J. Biol. Chem., 269, 11299–11305. [PubMed] [Google Scholar]
- Seandel M., Noack-Kunnmann,K., Zhu,D., Aimes,R.T. and Quigley,J.P. (2001) Growth factor-induced angiogenesis in vivo requires specific cleavage of fibrillar type I collagen. Blood, 97, 2323–2332. [DOI] [PubMed] [Google Scholar]
- Silletti S., Kessler,T., Goldberg,J., Boger,D.L. and Cheresh,D.A. (2001) Disruption of matrix metalloproteinase 2 binding to integrin alpha vβ3 by an organic molecule inhibits angiogenesis and tumor growth in vivo. Proc. Natl Acad. Sci. USA, 98, 119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetler-Stevenson W.G. (1999) Matrix metalloproteinases in angiogenesis: a moving target for therapeutic intervention. J. Clin. Invest., 103, 1237–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stürzl M. et al. (1999) Expression of K13/v-FLIP gene of human herpesvirus 8 and apoptosis in Kaposi’s sarcoma spindle cells. J. Natl Cancer Inst., 91, 1725–1733. [DOI] [PubMed] [Google Scholar]
- Stürzl M., Zietz,C., Monini,P. and Ensoli,B. (2001) Human herpesvirus-8 and Kaposi’s sarcoma: relationship with the multistep concept of tumorigenesis. Adv. Cancer Res., 81, 125–159. [DOI] [PubMed] [Google Scholar]
- Torisu H., Ono,M., Kiryu,H., Furue,M., Ohmoto,Y., Nakayama,J., Nishioka,Y., Sone,S. and Kuwano,M. (2000) Macrophage infiltration correlates with tumor stage and angiogenesis in human malignant melanoma: possible involvement of TNFα and IL-1α. Int. J. Cancer, 85, 182–188. [PubMed] [Google Scholar]
- Unemori E.N., Ferrara,N., Bauer,E.A. and Amento,E.P. (1992) Vascular endothelial growth factor induces interstitial collagenase expression in human endothelial cells. J. Cell. Physiol., 153, 557–562. [DOI] [PubMed] [Google Scholar]
- Vacca A. et al. (1997) Progression of mycosis fungoides is associated with changes in angiogenesis and expression of the matrix metalloproteinases 2 and 9. Eur. J. Cancer, 33, 1685–1692. [DOI] [PubMed] [Google Scholar]
- van der Bliek A.M. (1999) Functional diversity in the dynamin family. Trends Cell Biol., 9, 96–102. [DOI] [PubMed] [Google Scholar]
- Vihinen P. and Kahari,V.M. (2002) Matrix metalloproteinases in cancer: prognostic markers and therapeutic targets. Int. J. Cancer, 99, 157–166. [DOI] [PubMed] [Google Scholar]
- Vincenti M.P. and Brinckerhoff,C.E. (2002) Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in arthritis: integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res., 4, 157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virolle T., Krones-Herzig,A., Baron,V., De Gregorio,G., Adamson,E.D. and Mercola,D. (2003) Egr1 promotes growth and survival of prostate cancer cells. Identification of novel Egr1 target genes. J. Biol. Chem., 278, 11802–11810. [DOI] [PubMed] [Google Scholar]
- Westermarck J. and Kahari,V.M. (1999) Regulation of matrix metalloproteinase expression in tumor invasion. FASEB J., 13, 781–792. [PubMed] [Google Scholar]
- Zucker S., Mirza,H., Conner,C.E., Lorenz,A.F., Drews,M.H., Bahou,W.F. and Jesty,J. (1998) Vascular endothelial growth factor induces tissue factor and matrix metalloproteinase production in endothelial cells: conversion of prothrombin to thrombin results in progelatinase A activation and cell proliferation. Int. J. Cancer, 75, 780–786. [DOI] [PubMed] [Google Scholar]