Abstract
We have used phospho-specific antibodies to re-examine the multisite phosphorylation of c-Jun in murine RAW macrophages and embryonic fibroblasts. Our results indicate that JNK isoforms are required and sufficient for the phosphorylation of Thr91 and Thr93, as well as the phosphorylation of Ser63 and Ser73, in response to LPS or anisomycin in macrophages and TNFα or anisomycin in fibroblasts. However, the phorbol ester (TPA) and EGF-induced phosphorylation of Ser63 and Ser73 is mediated by ERK1/ERK2, as well as JNK1/JNK2, in fibroblasts from wild-type mice and by ERK1/ERK2 alone in fibroblasts from JNK-deficient mice. The phosphorylation of Thr239 is catalysed by GSK3 and the phosphorylation of Ser243 by an as yet unidentified protein kinase. The inhibition of GSK3 is not required for the dephosphorylation of Thr239 in response to LPS, and nor is the phosphorylation of Thr91 and Thr93 required for the TPA- or EGF-induced dephosphorylation of Thr239 in fibroblasts. The agonist-induced dephosphorylation of Thr239 may involve a conformational change that exposes Thr239 to dephosphorylation and/or the activation of a Thr239 phosphatase.
Keywords: c-Jun/ERK/GSK3/JNK/LPS
Introduction
The proto-oncogene c-Jun is one of the components of AP-1, a transcription factor complex believed to play key roles in cell proliferation, survival and death (reviewed in Angel and Karin, 1991; Shaulian and Karin, 2001). The activity of c-Jun is regulated at the transcriptional level, as well as post-translationally. Thus, c-Jun is an immediate early gene whose level can increase within an hour of exposure to a variety of extracellular stimuli. However, changes in the phosphorylation state of c-Jun are also required to generate transactivation potential. Thus, the same stimuli that induce c-Jun expression also trigger its phosphorylation at Ser63 and Ser73 in the N-terminal domain, which is required for it to become transcriptionally active (Pulverer et al., 1991; Smeal et al., 1991). The phosphorylation of these residues is thought to be mediated by the isoforms of JNK (Davis, 2000). c-Jun has also been reported to become phosphorylated at Thr231, Thr239, Ser243 and Ser249, which are located proximal to the DNA-binding domain (Boyle et al., 1991; Lin et al., 1992). Thr239, Ser243 and Ser249 were reported to be phosphorylated by GSK3 (Boyle et al., 1991) and Thr231 and Ser249 to be phosphorylated by CK2 (Lin et al., 1992) in vitro, and to inhibit the binding of c-Jun to DNA (Boyle et al., 1991). All four residues are situated within the same tryptic peptide whose phosphorylation was decreased when either HeLa or human osteosarcoma MG63 cells were stimulated with the tumour-promoting phorbol ester TPA. Thus, it has become generally accepted that the activation of c-Jun requires the phosphorylation of Ser63/Ser73, as well as the dephosphorylation of one or more of the C-terminal sites.
The tryptic peptide containing the C-terminal phosphorylation sites was initially resolved into three spots, which were suggested to represent the triply, doubly and singly phosphorylated forms of this peptide (Boyle et al., 1991). However, later work showed that one phosphopeptide resulted from incomplete tryptic digestion, and that c-Jun was actually phosphorylated at two C-terminal sites, namely Ser243 and either Thr231 or Thr239, and not at Ser249 (Alvarez et al., 1991; Chou et al., 1992). These investigators also showed that c-Jun could be phosphorylated at Ser243 in vitro (equivalent to Ser246 in rat c-Jun) by ERK1/ERK2.
More recently, a further peptide in c-Jun was reported to become phosphorylated at a threonine residue(s) when HeLa cells were exposed to ultraviolet radiation or when Jurkat cells were stimulated with TPA (Hibi et al., 1993). The TPA-induced phosphorylation of the phospho-threonine-containing peptides did not occur in 293 cells transfected with a mutant of c-Jun in which Thr91 and Thr93 had been changed to Ala, suggesting that these residues were the sites of phosphorylation (Papavassiliou et al., 1995). The TPA-induced binding of c-Jun to DNA was also abolished in the c-Jun(T91A,T93A) mutant, while the substitution of these residues by aspartic acid generated a gain-of-function mutant that bound to DNA, even in the absence of TPA. These findings, together with the observation that the mutation of Thr91 and Thr93 to Asp promoted dephosphorylation of the tryptic peptide containing the C-terminal sites, led to the conclusion that the phosphorylation of Thr91 and Thr93 induces a change in the conformation of c-Jun that enhances accessibility of the C-terminal sites to a protein phosphatase(s) (Papavassiliou et al., 1995). The identity of the protein kinase that phosphorylates Thr91 and Thr93 in vivo is unknown.
The studies on c-Jun phosphorylation reviewed above were carried out before the advent of phospho-specific antibodies, which have greatly facilitated analysis of proteins that are regulated by multisite phosphorylation, and before the advent of relatively specific cell-permeant inhibitors of some protein kinases. In this paper, we have therefore generated appropriate phospho-specific antibodies and reinvestigated the phosphorylation states of six sites on c-Jun in response to several stimuli and in the presence and absence of inhibitors of several protein kinases.
Results
Characterization of phospho-specific antibodies
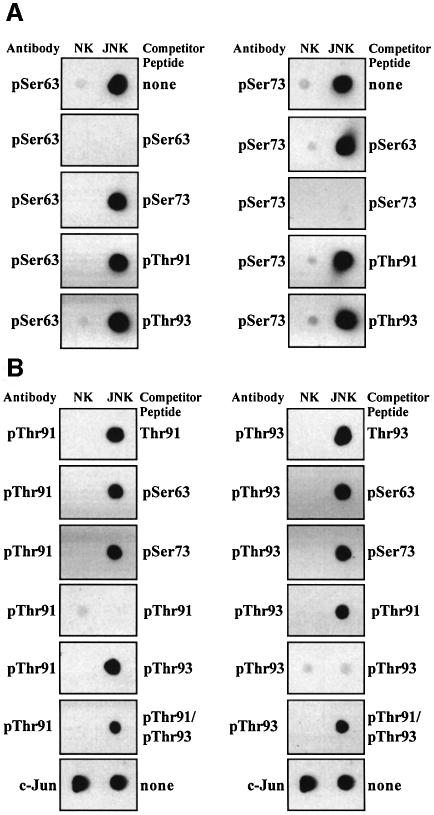
In order to investigate the multisite phosphorylation of c-Jun, we raised phospho-specific antibodies capable of recognizing c-Jun only when it was phosphorylated at Thr91, Thr93, Thr239 and Ser243, and purchased two other phospho-specific antibodies that recognize c-Jun phosphorylated at Ser63 or Ser73 (Figure 1). The specificity of the antibodies that recognize phosphorylated Ser63, Ser73, Thr91 or Thr93 was verified by the demonstration that recognition was prevented by preincubation of the antibody with the phosphopeptide immunogen, but not by incubation with phosphopeptides corresponding to any of the other three N-terminal phosphorylation sites (Figure 1A and B). The specificity of the antibodies that recognize phosphorylated Thr91 or Thr93 was also verified by the demonstration that when preincubated with the unphosphorylated form of the peptide immunogen, they only recognized phosphorylated c-Jun (Figure 1). The signal obtained with the antibodies that recognize phosphorylated Thr91 or Thr93 was only suppressed slightly by a synthetic peptide containing phosphothreonine at both Thr91 and Thr93 (Figure 1B). Thus, these antibodies predominantly recognize c-Jun phosphorylated at either Thr91 or Thr93.
Fig. 1. Characterization of phospho-specific antibodies that recognize particular phosphorylation sites on c-Jun. Bacterially expressed c-Jun was left unphosphorylated (no kinase, NK) or maximally phosphorylated with either JNK, GSK3β or ERK2, and 100 ng aliquots spotted on to nitrocellulose membranes and immunoblotted using the phospho-specific antibodies indicated, or an antibody that recognizes phosphorylated and unphosphorylated c-Jun equally well, in the presence or absence (none) of the competitor peptides shown. The sequences of these peptides are given in Materials and methods. The prefix ‘p’ denotes the phosphorylated residue. (A) Examination of the specificity of the antibodies that recognize c-Jun phosphorylated at Ser63 or Ser73. (B) Specificity of the antibodies that recognize c-Jun phosphorylated at Thr91 or Thr93. (C and D) Specificity of the antibodies that recognize c-Jun phosphorylated at Thr239 or Ser243.
The specificities of the antibodies that recognize c-Jun phosphorylated at Thr239 or Ser243 were established by analogous experiments (Figure 1C and D). In particular, the signal from the antibody that recognizes c-Jun phosphorylated at Thr239 was not suppressed by phosphopeptides corresponding to the sequences surrounding Thr231, Ser243 or Ser249, while the signal from the antibody that recognizes c-Jun phosphorylated at Ser243 was not suppressed by phosphopeptides corresponding to the sequences surrounding Thr231, Thr239 or Ser249. These experiments also showed that GSK3 was capable of phosphorylating c-Jun at Thr239 and more weakly at Ser243 in vitro, and that ERK2 was able to phosphorylate c-Jun at Ser243 but not Thr239 in vitro (Figure 1).
Most importantly, the signal from the antibody that recognizes c-Jun phosphorylated at Thr239 was completely suppressed by a synthetic peptide with phosphothreonine at Thr239 and phosphoserine at Ser243 (Figure 1D), as well as by the antigen with phosphothreonine at position 239 alone (Figure 1C). Thus, this phospho-specific antibody recognizes c-Jun phosphorylated at Thr239, irrespective of whether Ser243 is phosphorylated. However, the antibody that recognizes c-Jun phosphorylated at Ser243 was not suppressed by the peptide with phosphate at both Thr239 and Ser243 (Figure 1D). Thus, this phospho-specific antibody recognizes c-Jun phosphorylated at Ser243 alone more efficiently than c-Jun phosphorylated at both Thr239 and Ser243.
These six phospho-specific antibodies were then used to study the phosphorylation of c-Jun at all six sites in cells.
The phosphorylation of c-Jun in RAW264.7 macrophages
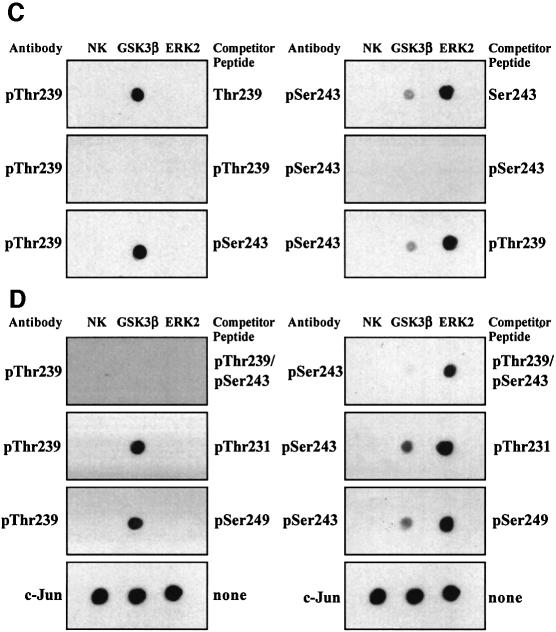
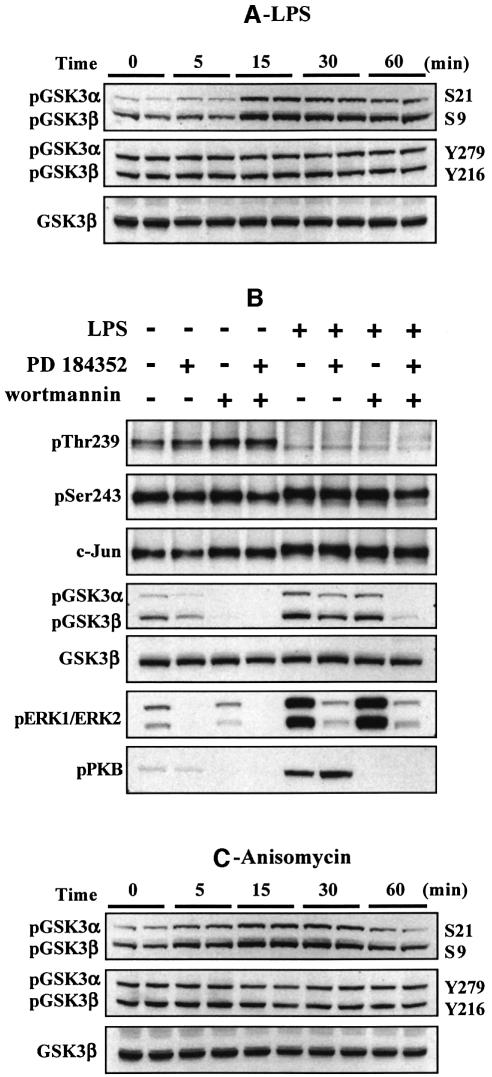
In RAW264.7 cells grown in 10% serum, c-Jun was not phosphorylated at Ser63, slightly phosphorylated at Ser73, not phosphorylated at Thr91 and Thr93, and phosphorylated at Thr239 and Ser243. The macrophages were then stimulated with two agonists known to induce the activation of MAP kinase cascades in these and other cells, namely bacterial LPS and anisomycin (Figure 2). Both agonists induced the phosphorylation of c-Jun at Ser63, Ser73, Thr91 and Thr93, but had no effect on the state of phosphorylation of Ser243 (Figure 2A and B). The apparent increase in phosphorylation of Ser243 in response to LPS is explained by the induction of the c-Jun protein between 45 and 90 min, as judged by immunoblotting with an antibody that recognizes the phosphorylated and unphosphorylated forms of c-Jun equally well (Figure 2A). This was confirmed by a quantitative analysis after densitometric analysis of the gels from several different experiments (data not shown). c-Jun is not induced by anisomycin (Figure 2B) because, at the concentration tested (10 µg/ml), this compound inhibits protein synthesis.
Fig. 2. Effect of LPS and anisomycin on the phosphorylation of six sites on c-Jun in RAW macrophages. Macrophages were stimulated with LPS (100 ng/ml) or anisomycin (10 µg/ml) for the times indicated and lysed. c-Jun was immunoprecipitated from the lysates denatured in SDS, subjected to SDS–PAGE and transferred to nitrocellulose. The membranes were immunoblotted with antibodies that recognize each of the six phosphorylation sites on c-Jun, as well as with an antibody that recognizes the phosphorylated and unphosphorylated forms of c-Jun equally well (A and B). Further aliquots of the lysates were immunoblotted with antibodies that recognize the active phosphorylated forms of several MAP kinases, namely JNK isoforms, p38α and ERK1 and ERK2, as well as with antibodies that recognize the phosphorylated and dephosphorylated forms of these kinases equally well (C and D). The prefix ‘p’ denotes the phosphorylated residue. Similar results were obtained in several independent experiments.
The LPS-induced dephosphorylation of Thr239 reached a plateau between 30 and 45 min and increased thereafter. The transient nature of the response is consistent with the transient effect of LPS on the activation of several MAP kinase cascades. The effect was quantitated by densitometric analysis of the gels from several experiments. This showed that the level of phosphorylation of Thr239 (normalized for the level of c-Jun protein) decreased by 66 ± 8% after 30–45 min but only by 42 ± 2% after 60 min.
Anisomycin (at 10 µg/ml) induced a partial dephosphorylation of Thr239 after 30 min, which was sustained for up to 90 min (confirmed by densitometric analysis of the gels). This is consistent with the sustained activation of several MAP kinase cascades under these conditions. We also carried out experiments after stimulating the macrophages with a concentration of anisomycin that does not inhibit protein synthesis (50 ng/ml). Under these conditions, the increase in phosphorylation of Ser63, Ser73, Thr91 and Th93 and decrease in the phosphorylation of Thr239 was similar to that observed at high anisomycin after normalizing for the induction of the c-Jun protein after 30 min (data not shown). However, the decrease in the phosphorylation of Thr239 was smaller than that observed at high anisomycin (data not shown).
The effect of LPS and anisomycin on each site was maximal after 30–45 min, the same time at which the activation of several different MAP kinases (JNK1/JNK2, p38α MAP kinase and ERK1/ERK2) was maximal (Figure 2C and D). Subsequent experiments were therefore performed after stimulation for 30 min.
Role of GSK3 in regulating the phosphorylation states of Thr239 and Ser243
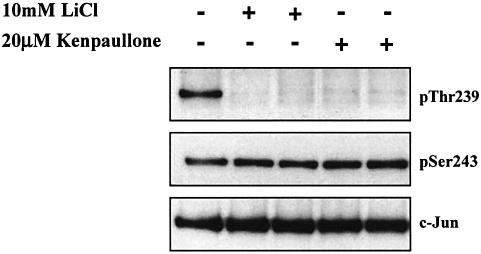
In order to obtain information about the nature of the protein kinases that phosphorylate c-Jun at Thr239 and Ser243, we incubated the unstimulated RAW macrophages with or without lithium chloride (LiCl) (Klein and Melton, 1996; Stambolic et al., 1996) or Kenpaullone (Leost et al., 2000). These compounds not only inhibit GSK3 relatively specifically, but complement one another in as much as the few other enzymes that they inhibit are quite different (Bain et al., 2003). Both compounds triggered the dephosphorylation of c-Jun at Thr239, but had no effect on the dephosphorylation of Ser243 (Figure 3).
Fig. 3. Inhibitors of GSK3 induce the dephosphorylation of c-Jun at Thr239 without affecting the phosphorylation of Ser243. RAW cells were incubated for 1 h with or without LiCl (10 mM) or Kenpaullone (20 µM) and lysed. c-Jun was immunoprecipitated from the lysates, denatured in SDS and immunoblotted using antibodies that recognize c-Jun phosphorylated at Thr239 or Ser243 or with an antibody that recognizes the phosphorylated and unphosphorylated forms of c-Jun equally well (see legend to Figure 2). The prefix ‘p’ denotes the phosphorylated residue. Similar results were obtained in several independent experiments.
The experiments with LiCl and Kenpaullone indicated that GSK3 was likely to be the protein kinase responsible for the phosphorylation of c-Jun at Thr239. Since GSK3 can itself be inactivated via the phosphorylation of Ser21 (GSK3α) and Ser9 (GSK3β), we wondered whether the agonist-induced dephosphorylation of Thr239 resulted from the inhibition of GSK3. Consistent with this possibility, LPS increased the phosphorylation of both GSK3 isoforms at Ser21/Ser9 and phosphorylation was maximal after 15–30 min (Figure 4A), the time at which dephosphorylation of Thr239 occurred. The phosphorylation of GSK3 at Ser21/Ser9 is known to be catalysed in cells by protein kinase B (PKB; also called Akt), which lies downstream of phosphatidylinositol (PI) 3-kinase, as well as by MAP kinase-activated protein kinase-1 (MAPKAP-K1; also known as RSK), a protein kinase activated by ERK1/ERK2 (reviewed in Frame and Cohen, 2001). We therefore examined the effects of wortmannin (an inhibitor of PI 3-kinase) with and without PD 184352 (an inhibitor of the classical MAP kinase cascade, which prevents the activation of ERK1/ERK2; Sebolt-Leopold et al., 1999; Davies et al., 2000), on the LPS-induced phosphorylation of GSK3 and dephosphorylation of c-Jun at Thr239. These experiments showed that the LPS-induced phosphorylation of GSK3 was partially reduced by either wortmannin or PD 184352, but abolished in the presence of both compounds, while the basal phosphorylation of GSK3 in the absence of LPS was suppressed by wortmannin alone. In contrast, wortmannin, with or without PD 184352, did not prevent the LPS induced dephosphorylation of Thr239 (Figure 4B). These results indicate that the inhibition of GSK3 resulting from the phosphorylation of Ser21/Ser9 is not required for the LPS-induced dephosphorylation of c-Jun at Thr239. These observations, which suggest that it is the dephosphorylation of Thr239 that is regulated, are considered further in the Discussion.
Fig. 4. The inhibition of GSK3 is not required for the LPS-induced phosphorylation of c-Jun. RAW cells were stimulated for 30 min with 100 ng/ml LPS (A and B) or 10 µg/ml anisomycin (C) and lysed. (A and C) Lysates were subjected to SDS–PAGE and immunoblotted using antibodies that recognize GSK3 phosphorylated at Ser21 (GSK3α) and Ser9 (GSK3β), or GSK3 phosphorylated at Tyr279 (GSK3α) and Tyr216 (GSK3β) or with an antibody that recognizes the unphosphorylated and phosphorylated forms of GSK3β equally well. (B) The cells were incubated with or without 2 µM PD 184352 and/or 100 nM wortmannin prior to stimulation for 30 min with 100 ng/ml LPS and then lysed. c-Jun was immunoprecipitated from the lysates, denatured in SDS and immunoblotted using antibodies that recognize c-Jun phosphorylated at Thr239 or Ser243. The lysates were also immunoblotted with the anti-GSK3 antibodies in (A and C), as well as with antibodies that recognize the phosphorylated forms of ERK1 and ERK2 or PKB phosphorylated at Ser473. The prefix ‘p’ denotes the phosphorylated residue. Similar results were obtained in several independent experiments.
GSK3 is also known to be phosphorylated on a tyrosine residue (Tyr279 in GSK3α and Tyr216 in GSK3β), and the phosphorylation of this residue is critical for GSK3 activity (Hughes et al., 1993; Wang et al., 1994). Neither LPS (Figure 4A) nor anisomycin (Figure 4C) changed the phosphorylation of Tyr279/Tyr216, excluding the possibility that GSK3 isoforms become inactivated as a result of tyrosine dephosphorylation.
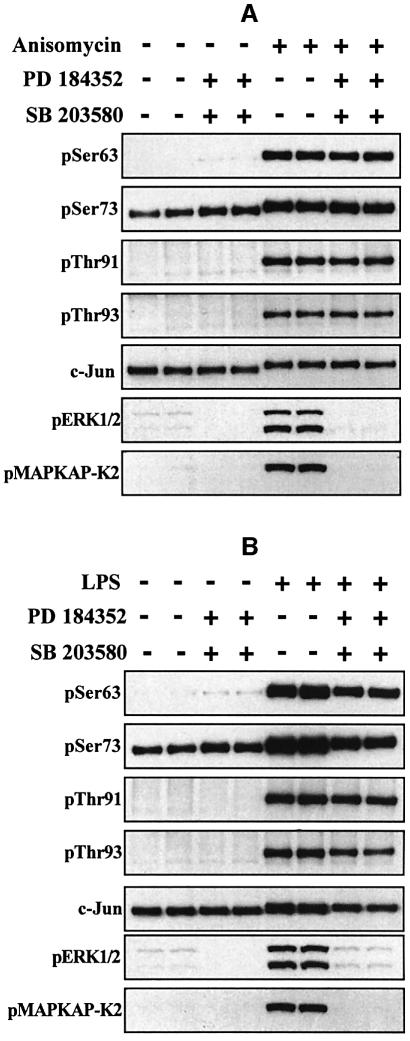
Effect of SB 203580 and PD 184352 on the LPS- and anisomycin-induced phosphorylation of c-Jun at Ser63, Ser73, Thr91 and Thr93
In order to obtain information about the protein kinases acting on Ser63, Ser73, Thr91 and Thr93, we incubated the cells in the presence of both SB 203580 (to inhibit p38α and p38β MAP kinases) (Cuenda et al., 1995) and PD 184352. These compounds prevented the phosphorylation of MAPKAP-K2 (a substrate of p38α MAP kinase) (Cuenda et al., 1995) and ERK1/ERK2, as expected, but had no effect on the anisomycin- or LPS-induced phosphorylation of the four N-terminal sites (Figure 5). The combined addition of SB 203580 and PD 184352 suppressed the LPS-stimulated induction of the c-Jun protein, but the phosphorylation of the four N-terminal sites relative to the amount of c-Jun protein was similar in the absence or presence of SB 203580 and PD 184352 (Figure 5B). Quantitation of the data by densitometric analysis of the gels showed that, after normalization for the level of c-Jun protein, the phosphorylation of Ser63 and Ser73 actually increased slightly (10–15%) in the presence of SB 203580 plus PD 184352.
Fig. 5. SB 203580 and PD 184352 do not prevent the LPS- or anisomycin-induced phosphorylation of c-Jun at Ser63, Ser73, Thr91 or Thr93. RAW cells were stimulated for 30 min with 10 µg/ml anisomycin (A) or 100 ng/ml LPS (B) and lysed. c-Jun was immunoprecipitated from the lysates, denatured in SDS and immunoblotted using antibodies that recognize c-Jun phosphorylated at Ser63, Ser73, Thr91 or Thr93 as described in the legend to Figure 2. Further aliquots of the cell lysates were immunoblotted (without immunoprecipitation) using antibodies that recognize the active phosphorylated forms of ERK1 and ERK2, and MAPKAP-K2 phosphorylated at Thr334. The prefix ‘p’ denotes the phosphorylated residue. Similar results were obtained in several independent experiments.
In order to examine whether the effect of SB 203580 plus PD 184352 to suppress the LPS-induced increase in the c-Jun protein was caused by inhibition of p38α or inhibition of the classical MAP kinase cascade, we performed additional experiments using each inhibitor individually. These experiments showed that neither inhibitor alone suppressed the induction of c-Jun (data not shown). It would therefore appear that both signalling pathways contribute to the induction of c-Jun under these conditions.
In summary, our results indicate that the activity of JNK and/or other PD 184352 and SB 203580-insensitive kinases, such as p38γ and p38δ MAP kinases, is sufficient for anisomycin and LPS to induce maximal phosphorylation of the four N-terminal sites on c-Jun.
The phosphorylation of c-Jun in murine embryonic fibroblasts that do not express JNK1 and JNK2
Compounds that inhibit JNK with sufficient potency and specificity to be useful in cell-based assays are not yet available (Bain et al., 2003). In order to study the role of this protein kinase in the phosphorylation of c-Jun, we therefore used transformed embryonic fibroblasts from wild-type (WT) mice and mice that do not express either JNK1 and JNK2 (termed JNK–/– cells) (Tournier et al., 2000). The third isoform JNK3 is not expressed in fibroblasts.
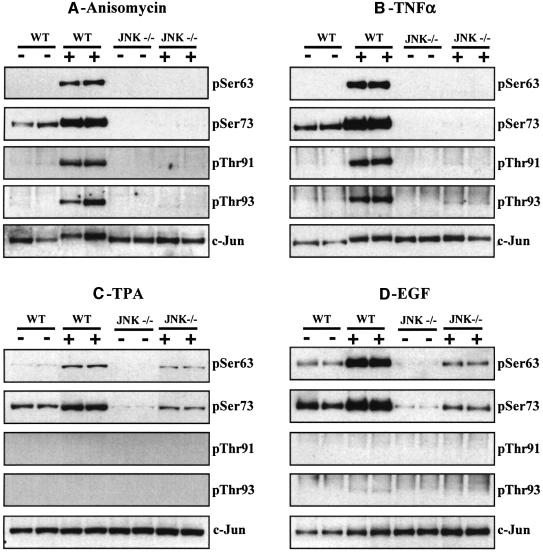
We studied the effect of anisomycin, TNFα, TPA and EGF on the phosphorylation of the four N-terminal sites. Anisomycin and TNFα both stimulated the phosphorylation of c-Jun at Ser63, Ser73, Thr91 and Thr93 and the phosphorylation of all four sites was abolished in the JNK–/– cells (Figure 6A and B), demonstrating that JNK1 and/or JNK2 either phosphorylate these sites directly, or are required for the synthesis of another protein kinase(s) that phosphorylates these sites. The anisomycin-induced activation of p38α MAP kinase was similar in both the WT and JNK–/– fibroblasts (data not shown), showing that JNK isoforms do not control the expression of this MAP kinase family member.
Fig. 6. Phosphorylation of the four N-terminal sites in c-Jun in WT and JNK-deficient fibroblasts. Fibroblasts from WT (WT) and JNK-deficient (JNK–/–) mice were stimulated (A) for 30 min with anisomycin (10 µg/ml), (B) for 15 min with TNFα (10 ng/ml), (C) for 30 min with TPA (100 ng/ml) and (D) for 15 min with EGF (100 ng/ml). Following cell lysis, c-Jun was immunoprecipitated from the lysates, denatured in SDS, subjected to SDS–PAGE and transferred to nitrocellulose. The membranes were immunoblotted with antibodies that recognize c-Jun phosphorylated at Ser63, Ser73, Thr91 and Thr93, as well as with an antibody that recognizes the phosphorylated and unphosphorylated forms of c-Jun equally well. The prefix ‘p’ denotes the phosphorylated residue. Similar results were obtained in several independent experiments.
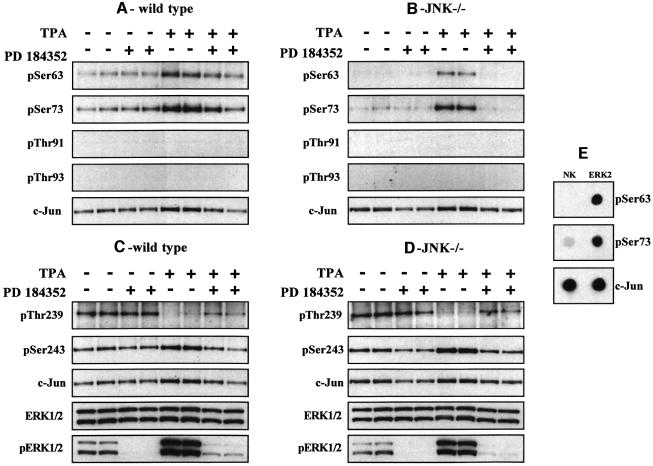
In contrast, TPA or EGF induced some phosphorylation of Ser63 and Ser73 in the JNK–/– cells. Neither TPA nor EGF induced the phosphorylation of Thr91 or Thr93 in either the WT or JNK-deficient cells (Figure 6C and D). TPA and EGF are strong activators of the classical MAP kinase cascade, in fibroblasts. In order to investigate whether this pathway plays a role in the TPA- and EGF-induced phosphorylation of c-Jun at Ser63 and Ser73 in JNK–/– cells, we therefore examined the effect of PD 184352. These studies demonstrated that PD 184352 reduced the TPA-stimulated phosphorylation of Ser63 and Ser73 in WT cells (Figure 7A) and abolished the phosphorylation of these sites in JNK–/– cells (Figure 7B). Similar results were obtained after stimulation with EGF (data not shown).
Fig. 7. Effect of PD 184352 on the TPA-induced phosphorylation of the c-Jun in WT and JNK-deficient fibroblasts. Fibroblasts from WT (WT) (A and C) and JNK-deficient (JNK–/–) (B and D) mice were incubated for 1 h with or without 2 µM PD 184352 and then stimulated for 30 min with TPA (100 ng/ml). The cells were lysed and immunoblotting carried out as described in the legend to Figure 6. Similar results were obtained in several independent experiments. (E) Bacterially expressed c-Jun was left unphosphorylated (no kinase, NK) or phosphorylated with ERK2 and aliquots spotted onto a nitrocellulose membrane. They were then immunoblotted using the phospho-specific antibodies indicated or an antibody that recognizes phosphorylated and unphosphorylated c-Jun equally well. The prefix ‘p’ denotes the phosphorylated residue. Similar results were obtained in several independent experiments.
One interpretation of these results was that the MAP kinases JNK1/2 and ERK1/2 both contribute to the TPA- and EGF-induced phosphorylation of Ser63 and Ser73 in WT fibroblasts, while ERK1/ERK2 are the major protein kinases that phosphorylate Ser63 and Ser73 in JNK-deficient fibroblasts. Consistent with this possibility, we found that ERK2 was capable of phosphorylating c-Jun at Ser63 and Ser73 in vitro (Figure 7E).
The phosphorylation of Thr239 and Ser243 in TPA-stimulated WT and JNK–/– fibroblasts
TPA stimulated the dephosphorylation of Thr239 in embryonic fibroblasts from WT (Figure 7C) or JNK–/– (Figure 7D) mice and this was partially reversed by prior incubation of the cells with PD 184352. TPA had little effect in the phosphorylation of Ser243, in both WT (Figure 7C) and JNK–/– (Figure 7D) mice. Similar results were obtained with EGF (data not shown).
Discussion
Prior to the present study, it had been reported that several agonists induce the phosphorylation of c-Jun at Ser63 and Ser73 and threonine residues thought to be Thr91 and Thr93. It had also been reported that the C-terminal region of c-Jun was phosphorylated at two sites, namely Ser243 and either Thr231 or Thr239 and that this region underwent dephosphorylation in response to TPA (see Introduction). In the present study, we used phospho-specific antibodies that recognize six of these sites to make a detailed analysis of the multisite phosphorylation of c-Jun. Our results establish that the C-terminal region of c-Jun is phosphorylated at Thr239 and Ser243. We did not raise antibodies against Thr231 or Ser249 (previously reported to be phosphorylated by CK2; Lin et al., 1992) because we found c-Jun to be a very poor substrate for CK2 in vitro, consistent with the lack of good CK2 consensus sequence around either of these sites. However, our data does not exclude the possibility that these and other sites become phosphorylated in response to stimuli that we have not examined.
In this paper, we have found that TNFα and anisomycin induce the phosphorylation of c-Jun at Thr91 and Thr93, as well as the phosphorylation of Ser63 and Ser73 in embryonic fibroblasts from WT mice, but not in JNK–/– fibroblasts (Figure 6). The insulin-induced phosphorylation of c-Jun at Ser63 is also abolished in JNK–/– fibroblasts (Lee et al., 2003). It is therefore likely that JNK isoforms mediate the phosphorylation of all four sites in TNFα- and anisomycin-stimulated fibroblasts, although the possibility that phosphorylation is catalysed by another protein kinase(s) whose synthesis is dependent on JNK activity cannot yet be excluded. A specific cell permeant compound that inhibited JNK isoforms within minutes would be useful to resolve this issue, but compounds with the requisite potency and specificity are not yet available commercially (Bain et al., 2003).
In contrast to the effects of TNFα or anisomycin, the TPA- or EGF-induced phosphorylation of Ser63 and Ser73 was partially inhibited by PD 184352 in embryonic fibroblasts from WT mice and completely blocked by PD 184352 in JNK-deficient fibroblasts (Figure 7A and B). Since ERK2 is capable of phosphorylating c-Jun at Ser63 and Ser73 in vitro (Figure 7E), the simplest interpretation of these results is that ERK1/ERK2, as well as JNK1/JNK2, mediate the TPA- or EGF-induced phosphorylation of these sites in WT fibroblasts, while ERK1/ERK2 are the dominant Ser63 and Ser73 kinases in TPA- or EGF-stimulated JNK–/– cells. TPA and EGF are strong activators of the classical MAP kinase cascade, but rather weak activators of JNK. This explains why the effects of ERK1/ERK2 on the phosphorylation of Ser63 and Ser73 can easily be detected with these agonists, in contrast to TNFα, for example, which is a very powerful activator of JNK, and a weaker activator of ERK1/ERK2. Similarly, although LPS stimulates both the JNK pathway and the classical MAP kinase cascade, blocking the latter pathway does not decrease the LPS-stimulated phosphorylation of Ser63 and Ser73 (Figure 5B). We therefore conclude that, although ERK1/ERK2 may contribute to the LPS-stimulated phosphorylation of Ser63 and Ser73 in macrophages, the activity of JNK is sufficient for maximal phosphorylation of these sites, even when the classical MAP kinase cascade is blocked.
We have confirmed that the C-terminal domain of c-Jun is phosphorylated at Thr239 and Ser243 (Figure 2). However, in contrast to earlier reports, our data indicates that only Thr239 is phosphorylated by GSK3, since the phosphorylation of Ser243 in RAW macrophages was not decreased by inhibitors of GSK3 (Figure 3). These observations are consistent with the known specificity requirements of GSK3 and the situation that prevails in other substrates of this enzyme, such as glycogen synthase and eIF2B. Thus, it is well established that GSK3 phosphorylates serine and threonine residues that lie in Ser/Thr–Xaa–Xaa–Xaa–pSer sequences (where pSer is phosphoserine). The pSer residue, termed the ‘priming phosphate’, binds to a specific site in GSK3 and phosphorylation is catalysed by a distinct ‘priming’ kinase that varies from substrate to substrate (reviewed in Frame and Cohen, 2001). The priming kinase is believed to be CK2 in the case of glycogen synthase (Picton et al., 1982) and DYRK (dual tyrosine regulated kinase) in the case of eIF2B (Woods et al., 2001).
Like Ser63, Ser73, Thr91, Thr93 and Thr239, Ser243 is followed by a proline residue. However, the level of phosphorylation of Ser243 is unaffected by SB 203580 and PD 184352 and, in contrast to the four most N-terminal sites, it is also not decreased in JNK-deficient fibroblasts. Moreover, the phosphorylation of Ser243 is not decreased in primary fibroblasts from mice that do not express the SB 203580-insensitive protein kinases p38γ and p38δ MAP kinases (S.Morton, P.Cohen, S.Arthur and A.Cuenda, unpublished data). Therefore, Ser243 must be phosphorylated by a ‘proline-directed’ protein kinase(s) distinct from GSK3, p38α, p38β, p38γ and p38δ MAP kinases, ERK1/ERK2 and JNK isoforms that has yet to be identified. In eIF2B, the ‘priming phosphate’ for GSK3, which can be introduced by DYRK isoforms in vitro, is also followed by a proline residue (Woods et al., 2001) and we have found that DYRK1A and DYRK2 are capable of phosphorylating c-Jun at Ser243 in vitro (S.Morton and P.Cohen, unpublished data). However, whether Ser243 is phosphorylated by a DYRK isoform in vivo is unknown.
Interestingly, Ser243 is mutated to a phenylalanine residue in v-Jun, the viral oncogenic form of this protein (Maki et al., 1987). The lack of a ‘priming phosphate’ implies that v-Jun would also not be phosphorylated at Thr239 by GSK3. This would result in a protein that is dephosphorylated at its C-terminus and may bind DNA constitutively, contributing to the oncogenicity of v-Jun. We therefore speculate that the Ser243 kinase may be a tumour suppressor and its identity is clearly of considerable interest.
The agonists we studied all induced a striking decrease in the phosphorylation of Thr239, raising the question of how this takes place. In RAW macrophages, pretreatment with wortmannin plus PD 184352 did not affect the LPS-induced dephosphorylation of Thr239, although the inhibition of GSK3 resulting from the phosphorylation of Ser21 (GSK3α) or Ser9 (GSK3β) did not occur under these conditions; nor did LPS decrease the phosphorylation of the essential phosphotyrosine residue in GSK3 (Figure 4). Thus, assuming that GSK3 activity is not decreased by a novel mechanism, the inhibition of GSK3 does not appear to be required for the LPS- or anisomycin-induced dephosphorylation of c-Jun at Thr239 in these cells.
Based on site-directed mutagenesis studies, it was reported that the phosphorylation of c-Jun at Thr91/Thr93 is required for dephosphorylation of the C-terminus of c-Jun (presumably Thr239) (see Introduction). Although, the phosphorylation of Thr91 and/or Thr93 alone may be sufficient to induce the conformational change that makes Thr239 susceptible to dephosphorylation, we found that TPA or EGF both induced the dephosphorylation of Thr239 in embryonic fibroblasts from either WT or JNK-deficient mice, without causing any phosphorylation of Thr91 or Thr93. Thus, under these conditions, the dephosphorylation of Thr239 cannot be triggered by the phosphorylation of Thr91/Thr93, and it may be the phosphorylation of Ser63 and/or Ser73 which triggers the conformational change that exposes Thr239 to dephosphorylation. Consistent with this possibility, PD 184352, which prevented the phosphorylation of Ser63 and Ser73 in JNK-deficient fibroblasts, partially suppressed the TPA-induced (Figure 7) and EGF-induced (data not shown) dephosphorylation of Thr239. A slightly smaller effect of PD 184352 to suppress the dephosphorylation of Thr239 was observed in WT fibroblasts in which the dephosphorylation of Ser63 and Ser73 was only inhibited partially (Figure 7). However, as PD 184352 only inhibits the TPA- or EGF-induced dephosphorylation of Thr239 partially, it would appear that another mechanism must also operate. For example, phosphorylation of another component of the AP-1 complex might induce a conformational change that makes Thr239 more susceptible to dephosphorylation by TPA. Alternatively, TPA and other agonists could activate a protein phosphatase(s) that dephosphorylates Thr239. The identity of the Thr239 phosphatase(s) is of interest since, like the protein kinase that phosphorylates Ser243, it plays an important role in preventing c-Jun from stimulating transcription in an uncontrolled way.
Materials and methods
Materials
PD 184352 (Sebolt-Leopold et al., 1999) was obtained by chemical synthesis. Kenpaullone and SB 203580 were purchased from Calbiochem (Nottingham, UK). TPA, Escherichia coli LPS, dimethylsulfoxide (DMSO), anisomycin and LiCl were from Sigma (Poole, UK) and protein G-HRP from Bio-Rad (Hemel Hempstead, Herts, UK). Microcystin-LR was obtained from Dr Linda Lawton (Robert Gordons University, Aberdeen, UK), ‘Complete’ Protease Inhibitor tablets from Roche (Lewes, Sussex, UK), cell culture media from Gibco (Paisley, UK), precast Bis–Tris gradient SDS–polyacrylamide gels, running buffer and transfer buffer from Invitrogen (Paisley, UK), protein G–Sepharose and enhanced chemiluminescence (ECL) reagent from Amersham (Bucks, UK). All the peptides utilized in this study were synthesized by Dr Graham Bloomberg (University of Bristol, UK).
Proteins
JNK1α1, ERK2 and GSK3β were expressed, purified, activated and assayed as described previously (Davies et al., 2000). Bacterially expressed c-Jun was produced using the inducible pRK172 c-Jun expression vector in pLysS E.coli (Pulverer et al., 1991; Black et al., 1994). Briefly, induced cultures were lysed and inclusion bodies containing insoluble c-Jun protein purified and subsequently resuspended in 20 mM Tris–HCl pH 7.5, 1 mM EDTA, 2 mM dithiothreitol and 0.1% (w/v) Triton X-100. Solid urea was added to a final concentration of 8 M, dissolved at 37°C and the solubilized c-Jun protein dialysed overnight against three successive changes of 20 mM Tris–HCl pH 7.5, 1 mM EDTA, 1 mM dithiothreitol, 0.1% Triton X-100 and 50% (v/v) glycerol. Purified c-Jun protein was stored at –70°C. EGF was from Invitrogen and TNFα from Sigma.
Antibodies
Antibodies that recognize ERK1 and ERK2, p38 and JNK isoforms, and phospho-specific antibodies that recognize the activated forms of ERK1/ERK2, p38, c-Jun phosphorylated at Ser63, c-Jun phosphorylated at Ser73, MAPKAP-K2 phosphorylated at Thr334, GSK3α phosphorylated at Ser21 and GSK3β phosphorylated at Ser9 were obtained from Cell Signaling Technologies (Hitchin, UK). Phospho-specific antibodies that recognize the activated forms of JNK1 and JNK2 were purchased from Biosource (Nivelles, Belgium), while an antibody that recognizes GSK3α phosphorylated at Tyr279 and GSK3β phosphorylated at Tyr216 was purchased from Upstate Inc. (Milton Keynes, UK). Horseradish peroxidase-conjugated secondary antibodies were from Pierce (Cheshire, UK).
All other antibodies were raised in sheep at Diagnostics Scotland (Edinburgh, UK). An antibody capable of immunoprecipitating c-Jun was raised against GST–c-Jun(1–191) and the antisera affinity purified on CH-Sepharose to which GST–c-Jun(1–191) had been coupled covalently. Synthetic phosphopeptides were coupled separately to bovine serum albumin (BSA) and keyhole limpet haemocyanin before being mixed and injected into a sheep. The antisera were affinity purified on CH-Sepharose to which the relevant phosphorylated peptide had been coupled covalently. These antibodies were used for immunoblotting in the presence of the dephosphorylated peptide antigen (10 µg/ml) to neutralize any antibodies that recognized the dephosphorylated form of c-Jun. Phospho-specific antibodies were raised against the following peptides: Thr91 (NGHITTT*PTPTQFLCPK, residues 85–101 of human c-Jun), Thr93 (NGHITTTPT*PTQFLCPK, residues 85–101 of human c-Jun), Thr239 (PEMPGET*PPLSPI, residues 233–245 of human c-Jun) and Ser243 (GETPPLS*PIDMES, residues 237–249 of human c-Jun). Synthetic phosphopeptides corresponding to the sequences surrounding Thr231 (KEEPQT*VPEMPG) and Ser249 (SPIDMES*QERIKA) were also synthesized and used in the control experiments detailed under Results. Further phosphopeptides were synthesized with phosphothreonine at both Thr91 and Thr93 (NGHITTT*PT*PTQFLCPK) or with phosphothreonine at Thr239 and phosphoserine at Ser243 (PEMPG ET*PPLS*PIDMES). These two peptides were also used to assess the specificities of the antibodies (see Results). In each peptide, the asterisks denote the phosphorylated residues.
Cell culture and stimulation
Murine RAW 264.7 macrophages (purchased from the European Tissue Culture Collection) and immortalized embryonic fibroblasts deficient in JNK1 and JNK2 (JNK–/– cells) (Tournier et al., 2000) were maintained in a 95% air/5% CO2 37°C atmosphere. RAW macrophages were cultured in Dulbecco’s modified Eagle’s medium containing 10% heat-inactivated fetal calf serum (FCS), 100 U/ml penicillin, 100 µg/ml streptomycin and 2 mM l-glutamine. WT and JNK–/– fibroblasts were cultured in the same manner, except that the FCS was not heat inactivated. Cells were stimulated for various times with TPA, EGF, LPS, TNFα or anisomycin at the concentrations specified in the figure legends. Where indicated, the cells were incubated for 1 h prior to stimulation with PD 184352 (2 µM), SB 203580 (10 µM), LiCl (10 mM) or Kenpaullone (20 µM). Where added, wortmannin (100 nM) was included 10 min before stimulation. All inhibitors were dissolved at 10–20 mM in DMSO, except for LiCl, which was dissolved in water. An equivalent volume of DMSO or water was therefore added to the culture medium as a control. The final concentration of DMSO was never greater than 0.2% (v/v).
Cell lysis
After stimulation, the medium was aspirated, the cells washed with 5 ml of ice-cold PBS and then lysed in 50 mM Tris–HCl pH 7.5, 1 mM EDTA, 1 mM EGTA, 1 mM sodium orthovanadate, 10 mM sodium β-glycerophosphate, 50 mM sodium fluoride, 5 mM sodium pyrophosphate, 0.27 M sucrose, 1% (v/v) Triton X-100, 0.1% (v/v) 2-mercaptoethanol, 1 µM microcystin-LR and ‘Complete’ Protease Inhibitor cocktail (one tablet/50 ml). The lysates were centrifuged at 14 000 g for 10 min at 4°C and the supernatants frozen in liquid nitrogen and stored at –80°C. Protein concentrations were determined according to Bradford (Bradford, 1976).
Immunoprecipitation of c-Jun
Five micrograms of affinity-purified antibody were added to10 µl of packed protein G–Sepharose, which had been prewashed with PBS. After incubation for 1 h at 4°C with shaking, the antibody–Sepharose suspension was centrifuged briefly, and the pellet washed once with cell lysis buffer containing 0.5 M NaCl and 0.1% (v/v) 2-mercaptoethanol and twice with cell lysis buffer containing 0.1% (v/v) 2-mercaptoethanol. The antibody–Sepharose conjugate was either used immediately or stored until use at 4°C in PBS containing 0.02% (w/v) sodium azide. Cell lysate (1–2 mg protein) was incubated with the antibody–Sepharose conjugate for 2 h at 4°C with continuous agitation. After centrifugation for 1 min at 13 000 g, the Sepharose pellet was washed once with cell lysis buffer plus 0.5 M NaCl and 0.1% (v/v) 2-mercaptoethanol, followed by two washes with cell lysis buffer containing 0.1% (v/v) 2-mercaptoethanol. The Sepharose pellet was then incubated in SDS at 95°C to dissociate the antibody–antigen conjugate and, after centrifugation, the supernatant was subjected to SDS–PAGE.
Immunoblotting
Samples were denatured in SDS, run on polyacylamide gels and transferred to nitrocellulose membranes. The membranes were incubated for 1 h at room temperature in 50 mM Tris–HCl pH 7.5, 150 mM NaCl, 0.2% Tween and 5% (w/v) skimmed milk powder. Antibodies were then added to 10 ml of the same buffer, except for antibodies from Cell Signalling Technologies where the skimmed milk powder was replaced by 5% (w/v) BSA. Antibodies were then incubated with the membranes on a shaker at 4°C overnight. The membranes were then washed four times with buffer (5 min per wash) to remove the primary antibodies, and either secondary antibodies or protein G–HRP were incubated with the membranes for 1 h at room temperature. After washing six times with buffer to remove the secondary antibodies (5 min per wash), immunoreactive proteins were visualized with the ECL reagent according to the manufacturer’s instructions.
Acknowledgments
Acknowledgements
This paper is dedicated to the memory of Eraldo Antonini, eminent biochemist, who died prematurely 20 years ago on March 19, 1983. We thank Jane Leitch and Moustapha Aoubala for antibody production, Juan Jose Ventura (University of Massachusetts, Worcester, USA) for generating the immortalized embryonic fibroblasts from WT and JNK–/– mice and David Gillespie for helpful discussions. We are also indebted to the UK Medical Research Council for providing a studentship (to S.M.) and the UK Medical Research Council, the Royal Society, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, NovoNordisk and Pfizer for the financial support that made this study possible.
References
- Alvarez E., Northwood,I.C., Gonzalez,F.A., Latour,D.A., Seth,A., Abate,C., Curran,T. and Davis,R.J. (1991) Pro–Leu–Ser/Thr–Pro is a consensus primary sequence for substrate protein phosphorylation. J. Biol. Chem., 266, 15277–15285. [PubMed] [Google Scholar]
- Angel P. and Karin,M. (1991) The role of Jun, Fos and the AP-1 complex in cell proliferation and transformation. Biochim. Biophys. Acta, 1072, 129–157. [DOI] [PubMed] [Google Scholar]
- Bain J., McLauchlan,H., Elliott,M. and Cohen,P. (2003) The specificities of protein kinase inhibitors; an update. Biochem. J., 371, 199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black E.J., Catling,A.D., Woodgett,J.R., Kilbey,A. and Gillespie,D.A.F. (1994) Transcriptional activation by the v-Jun oncoprotein is independent of positive regulatory phosphorylation. Oncogene, 9, 2363–2368. [PubMed] [Google Scholar]
- Boyle W.J., Smeal,T., Defize,L.H.K., Angel,P., Woodgett,J., Karin,M. and Hunter,T. (1991) Activation of protein kinase C decreases phosphorylation of c-Jun at sites that negatively regulate its DNA-binding activity. Cell, 64, 573–584. [DOI] [PubMed] [Google Scholar]
- Bradford M.M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilising the principle of protein-dye binding. Anal. Biochem., 72, 248–254. [DOI] [PubMed] [Google Scholar]
- Chou S., Baichwal,V. and Ferrell,J.E. (1992) Inhibition of c-Jun DNA-binding by mitogen-activated protein kinase. Mol. Biol. Cell, 3, 1117–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuenda A., Rouse,J., Doza,Y.N., Meir,R., Cohen,P., Gallagher,T.F., Young,P.R. and Lee,J.C. (1995) SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett., 364, 229–233. [DOI] [PubMed] [Google Scholar]
- Davies S.P., Reddy,H., Caivano,M. and Cohen,P. (2000) Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J., 351, 95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R.J. (2000) Signal transduction by the JNK Group of MAP kinases. Cell, 103, 239–252. [DOI] [PubMed] [Google Scholar]
- Frame S. and Cohen,P. (2001) GSK3 takes centre stage more than 20 years after its discovery. Biochem. J., 359, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibi M., Lin,A., Smeal,T., Minden,A. and Karin,M. (1993) Identification of an oncoprotein and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev., 7, 2135–2148. [DOI] [PubMed] [Google Scholar]
- Hughes K., Nikolakaki,E., Plyte,S.E., Totty,N.F. and Woodgett,J.R.(1993) Modulation of the glycogen synthase kinase-3 family by tyrosine phosphorylation. EMBO J., 12, 803–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein P.S. and Melton,D.A. (1996) A molecular mechanism for the effect of lithium on development. Proc. Natl Acad. Sci. USA, 93, 8455–8459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.H., Giraud,J., Davis,R.J. and White,M.F. (2003) c-Jun N-terminal kinase (JNK) mediates feedback inhibition of the insulin signalling cascade J. Biol. Chem., 278, 2896–2902. [DOI] [PubMed] [Google Scholar]
- Leost M. et al. (2000) Paullones are potent inhibitors of glycogen synthase kinase-3β and cyclin-dependent kinase 5/p25. Eur. J. Biochem., 267, 5983–5994. [DOI] [PubMed] [Google Scholar]
- Lin A., Frost,J., Deng,T., Smeal,T., Al-Alawi,N., Kikkawa,U., Hunter,T., Brenner,D. and Karin,M. (1992) Casein kinase II is a negative regulator of c-Jun DNA binding and AP-1 activity. Cell, 70, 777–789. [DOI] [PubMed] [Google Scholar]
- Maki Y., Bos,T.J. and Davis,C., Starbuck,M. and Vogt,P.K. (1987) Avian sarcoma virus 17 carries the jun oncogene. Proc. Natl Acad. Sci. USA, 84, 2848–2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papavassiliou A.G., Treier,M. and Bohmann,D. (1995) Intramolecular signal transduction in c-Jun. EMBO J., 14, 2014–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picton C., Woodgett,J.R., Hemmings,B. and Cohen,P. (1982) Multisite phosphorylation of glycogen synthase from rabbit skeletal muscle. Phosphorylation of site 5 by glycogen synthase kinase-5 (casein kinase-II) is a prerequisite for phosphorylation of site 3 by glycogen synthase kinase-3. FEBS Lett., 150, 191–196. [DOI] [PubMed] [Google Scholar]
- Pulverer B.J., Kyriakis,J.M., Avruch,J., Nikolakaki,E. and Woodgett,J.R. (1991) Phosphorylation of c-Jun mediated by MAP kinases. Nature, 353, 670–674. [DOI] [PubMed] [Google Scholar]
- Sebolt-Leopold J.S. et al. (1999) Blockade of the MAP kinase pathway suppresses growth of colon tumours in vivo. Nat. Med., 5, 810–816. [DOI] [PubMed] [Google Scholar]
- Shaulian E. and Karin,M. (2001) AP-1 in cell proliferation and survival. Oncogene, 20, 2390–2400. [DOI] [PubMed] [Google Scholar]
- Smeal T., Binetruy,B., Mercola,D.A., Birrer,M. and Karin,M. (1991) Oncogenic and transcriptional cooperation with Ha-Ras requires phosphorylation of c-Jun on serines 63 and 73. Nature, 354, 494–496. [DOI] [PubMed] [Google Scholar]
- Stambolic V, Ruel,L. and Woodgett,J.R. (1996) Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr. Biol., 6, 1664–1668. [DOI] [PubMed] [Google Scholar]
- Tournier C. et al. (2000) Requirement of JNK for stress induced activation of the cytochrome-c mediated death pathway. Science, 288, 870–874. [DOI] [PubMed] [Google Scholar]
- Wang Q.M., Fiol,C.J., DePaoli-Roach,A.A. and Roach,P.J. (1994) Glycogen synthase kinase-3β is a dual specificity kinase differentially regulated by tyrosine and serine/threonine phosphorylation. J. Biol. Chem., 269, 14566–14574. [PubMed] [Google Scholar]
- Woods Y.L., Cohen,P., Becker,W., Jakes,R., Goedert,M., Wang,X. and Proud,C.G. (2001) The kinase DYRK phosphorylates protein-synthesis initiation factor eIF2Bε at Ser359 and the microtubule-associated protein tau at Thr212: potential role for DYRK as a glycogen synthase kinase 3-priming kinase. Biochem. J., 355, 609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]