Abstract
The ability of phages to survive processing is an important aspect of their potential use in the biocontrol of Campylobacter in poultry production. To this end, we have developed a procedure to recover Campylobacter bacteriophages from chilled and frozen retail poultry and have validated the sensitivity of the method by using a characterized Campylobacter phage (i.e., NCTC 12674). By using this method, we have shown that Campylobacter phages can survive on retail chicken under commercial storage conditions. Retail chicken portions purchased in the United Kingdom were screened for the presence of endogenous Campylobacter phages. Thirty-four Campylobacter bacteriophages were isolated from 300 chilled retail chicken portions, but none could be recovered from 150 frozen chicken portions. The phage isolates were characterized according to their lytic profiles, morphology, and genome size. The free-range products were significantly more likely to harbor phages (P < 0.001 by single-factor analysis of variance) than were standard or economy products. This study demonstrates that Campylobacter bacteriophages, along with their hosts, can survive commercial poultry processing procedures and that the phages exhibited a wide range of recovery rates from chicken skin stored at 4°C.
Campylobacter jejuni is a major cause of food-borne gastrointestinal disease (10). There were over 56,000 reported cases of Campylobacter enteritis in England and Wales in the year 2001 (http://www.phls.co.uk/topics_az/campylo/data_faecal_ew.htm), and estimates suggest that the real incidence may be up to 10 times this figure. This level of disease and the association of Campylobacter infections with chronic and potentially life-threatening autoimmune conditions such as Guillain-Barré syndrome (24) have served to heighten concern over this organism since its isolation from human stools in the 1970s (29). Development of routine culturing procedures for the detection of campylobacters has no doubt had an impact on the annual recorded incidence of campylobacteriosis by the Public Health surveillance services (32). At the same time, there has been an increase in the awareness of food-borne disease by health professionals and the public at large—which will also contribute to the increase in reported incidence (29). However, these factors alone cannot explain the increase in Campylobacter enteritis over the past decade. In contrast, the total number of Salmonella infections over the same period has fallen, with a reduced number of subtypes being responsible for the disease burden (12).
Studies of several Campylobacter outbreaks have shown undercooked poultry—specifically chicken—to be the source of infection (6, 15, 20). However, despite these documented outbreaks, Campylobacter continues to be a primarily sporadic disease (23). Campylobacters readily colonize poultry (9) and seem well adapted to the avian gut (4). This may partly explain the uphill battle of poultry farmers in the prevention of Campylobacter colonization of their flocks. Even when implementing and maintaining strict hygiene procedures, preventing Campylobacter colonization is difficult (22). Once present in a flock, horizontal transfer between birds occurs rapidly, with one study reporting 100% colonization in just 2 days (12).
The problem of Campylobacter colonization of poultry in the broiler house is exacerbated when the birds are slaughtered, as negative flocks are often mixed with positive flocks, thereby leading to large-scale cross-contamination (21). As up to 76% of flocks slaughtered are Campylobacter positive (13), control of contamination remains a major challenge to the industry. While postprocessing treatment and storage conditions may reduce Campylobacter contamination on broiler carcasses, in some cases (30) these treatments are considered to have a negligible impact on human exposure to this organism (16). A recent survey in the United Kingdom demonstrated that 83.3% of chicken samples were Campylobacter positive (18). In addition, Campylobacter is able to survive on kitchen surfaces for several hours after contamination (5), thus leading to cross-contamination events in the home.
General concerns regarding the use of chemical additives in food production have led the European Union to ban many antibiotics and growth promoters used in the rearing of broiler chickens. This includes virginiamycin, spiramycin, and tylosin phosphate, which were banned in 1998. Concern regarding reports of increasing antibiotic resistance in pathogens harbored by farm animals has only strengthened the European Union's resolve to phase out the use of antibiotics in food production altogether. The use of antibiotics in the United States has also come under scrutiny, with the Food and Drug Administration proposing the discontinuation of the use of fluoroquinolones in poultry production. The therapeutic use of specific bacteriophage may help fill the void left by the abandonment of antibiotics. Bacteriophages have unique advantages over antibiotics in that they are both self-replicating and self-limiting. Their host specificity also avoids the dysbiosis that is often observed when using broad-spectrum antibiotics.
Campylobacter bacteriophages have been isolated from sewage and abattoir effluent (27). These phages were found to possess double-stranded DNA genomes and have been ascribed to the Myoviridae and Siphoviridae families (25). Phage isolates have been used to form typing schemes and complement other typing methods (7, 8, 26). The lytic capability and host specificity of phages also present the opportunity of using them to reduce the numbers of campylobacters emanating from animal sources and subsequently reducing cross contamination during processing. Generally, a reduction in the numbers of campylobacters entering the human food chain is likely to have a beneficial effect on the disease burden. In particular, the rearing of broiler chickens is a prime target for intervention with bacteriophages, given the reported scale of Campylobacter colonization of broilers (12) and the relatively large numbers of campylobacters present in the gastrointestinal tract and secreted by colonized birds (31). As a first step toward implementing these strategies, we sought to establish whether bacteriophages were already present on retail poultry in order to gauge the possible impact of intervention and to establish whether these strategies would introduce any new biological elements into the human food chain.
To date, there have been no reports of the isolation of Campylobacter phages from retail chicken products. The aims of this study were to establish recovery methods for Campylobacter bacteriophages from poultry meat and to determine the incidence and characteristics of phages from fresh and frozen retail poultry in the United Kingdom. Here we report the isolation of Campylobacter phages from retail poultry meat and the characterization of these phages in terms of their genome size, morphology, and lytic profiles by using National Collection of Type Cultures (NCTC) and contemporary fla-typed poultry Campylobacter isolates.
MATERIALS AND METHODS
Bacterial strains and bacteriophage.
Campylobacter jejuni NCTC 12662 phage type 14 (PT14) was kindly supplied by the Central Public Health Laboratory (CPHL; Colindale, London, United Kingdom) and used for all primary phage isolation. PT14 is sensitive to all the phages employed in the current typing scheme (7) and was considered the most suitable strain for use in phage recovery. Bacteriophage φ2 (NCTC 12674, ATCC 35922-B2) was also supplied by the Central Public Health Laboratory and is a constituent of the phage-typing scheme (7). Bacteriophage φ2 was used as a positive control to estimate the recovery efficiency from chicken skin. This phage consistently gives clear, distinct plaques on the C. jejuni PT14 propagating strain.
Isolation of Campylobacter from retail poultry.
Retail chicken portions were purchased from a United Kingdom supermarket selected on the basis of different product bar code and processing unit numbers to give a cross section of samples from United Kingdom producers. The portions were from six different producers, all based in the United Kingdom. Swabs dipped in Maximum Recovery Diluent (Oxoid code CM733; Basingstoke, United Kingdom) were used to wipe a 10-cm2 area of chicken skin, and these were then used to inoculate plates of modified Cefoperazone Charcoal Deoxycholate Agar selective medium (Oxoid code CM739; selective supplement code SR155). The plates were then incubated at 42°C for 48 h in microaerobic conditions (5% O2, 5% H2, 10% CO2, 80% N2) before being examined for typical Campylobacter colonies. Colonies were examined by Gram stain and wet mount for typical Campylobacter morphology and motility, respectively. After subculturing the isolates on Columbia Blood Agar plates (Oxoid code CM331), oxidase, catalase, and hippurate tests were applied for further confirmation and speciation.
Bacteriophage propagation.
The propagating Campylobacter strain (C. jejuni PT14) was subcultured on Columbia Blood Agar for 18 h at 42°C under microaerobic conditions, and the cells were harvested in 20 ml of 10 mM MgSO4 solution. Cell density was adjusted to that of a MacFarland no. 3 standard (approximately 109 CFU per ml) by using freshly prepared standards, and the suspension was kept on ice until required. By using these cells, the bacteriophages were propagated as described by Frost and colleagues (7), amplified by using plate lysates, and resuspended in SM buffer (50 mM Tris-HCl [pH 7.5], 0.1 M NaCl, 8 mM MgSO4, and 0.01% gelatin [Sigma G-1393]).
Isolation of phages from chicken skin.
Sections of chicken skin (10 cm2) were removed and stomached in 10 ml of SM buffer in a Seward Lab Blender 400 for 5 min by using filter stomacher bags (BA6041/STR; Seward). The stomachate was then centrifuged at 2.5 × g for 10 min at 20°C, and the supernatant was filtered through a 0.45-μm nitrocellulose membrane (Sartorius, Gottingen, Germany). One hundred microliters of this filtrate was added to 400 μl of PT14 suspension and incubated aerobically for 30 min at 42°C. After incubation, the suspension was added to 5 ml of NZCYM overlay agar (NZCYM broth [Difco, Oxford, United Kingdom] with 0.6% bacteriological agar [Oxoid Agar No. 1, code L13]), gently shaken, and added to prewarmed (42°C, 30 min) NZCYM base agar (NZCYM broth with 1% bacteriological agar). Plates were incubated microaerobically at 42°C for 24 h and examined for the presence of plaques.
Phage recovery and survival.
The efficiency of phage recovery was examined by spotting out serial dilutions of a φ2 suspension onto a 10-cm2 area of skin from chicken portions in triplicate and left to dry at room temperature for 1 h. The skins were then removed and processed as described above. To examine the survival of bacteriophage φ2, ∼108 PFU were spotted on each of 11 chicken portions in triplicate and left to dry. Frozen chicken portions were defrosted for 24 h at 4°C prior to inoculation. After inoculation, portions were individually sealed in sterile containers and stored at 4°C (fresh chicken) or refrozen at −20°C (frozen chicken). In parallel, uninoculated samples were used as a negative control. Samples were initially taken 1 h after inoculation and then at 24-h intervals thereafter. Serial dilutions of the recovery medium were plated in NZCYM overlay medium for phage enumeration. Phage recovery data were recorded as the mean percentage recovery ± standard deviation (SD) over a 10-cm2 area of chicken skin. This procedure was repeated for the phages isolated from the retail chicken portions, representing five different lytic spectra classes.
Phage genome size determination using PFGE.
For preparation of phage genomic DNA, 10 μl of a 1010 PFU/ml suspension of phages, prepared using the centrifuge concentration method described by Sambrook et al. (28), was diluted in 40 μl of TE buffer (10 mM Tris, 1 mM EDTA [pH 7.5]). This was mixed with an equal volume of 1.4% molten agarose (pulsed-field gel electrophoresis [PFGE] grade) in TE buffer and dispensed into plug molds (Bio-Rad). The plugs were allowed to set at room temperature and were then transferred to 60-mm-diameter petri dishes containing 5 ml of lysis buffer (100 mM EDTA, 10 mM Tris [pH 7.2], 1% Sarkosyl [wt/vol], 0.1 mg of proteinase K per ml; reagents from Sigma). The plates were incubated at 55°C for 18 h with gentle shaking to lyse the phage capsids and digest the protein components. The lysis solution was discarded and proteinase K was inactivated by the addition of 5 ml of 1 mM phenylmethylsulfonyl fluoride in wash buffer (50 mM EDTA, 20 mM Tris [pH 7.2]) and incubated for 1 h at room temperature with gentle shaking. The plugs were then washed three times for 20 min each with successive changes of wash buffer at room temperature with gentle shaking. A 2-mm slice of each plug was then inserted into the wells of a 1% agarose gel. The gel was run using a Bio-Rad CHEF DRII system in 0.5 TBE for 18 h at 200 V with a switch time of 30 to 60 s. Lambda concatemers (Bio-Rad) were used as markers.
For restriction endonuclease digests, a 2-mm slice of each plug was incubated at 37°C overnight with 10 U of restriction enzyme in 100 μl of digestion buffer prepared according to the manufacturer's instructions (Promega Ltd., Southampton, United Kingdom). Enzymes used were TaqI, DpnI, SspI, MseI, HaeIII, DraI, MboI, HindIII, PstI, EcoRI, and EcoRV. The plug digests were then transferred to a 1% agarose gel and run using the Bio-Rad CHEF DRII system as described above but with a switch time of 2 to 10 s for better resolution of the smaller DNA bands.
Examination of phage morphology (electron microscopy).
Eight microliters of a 108 PFU/ml suspension of phage was added to the surface of a glow-discharged carbon-coated Pioloform grid and fixed for 2 min using glutaraldehyde vapor. Excess sample was removed, and the grid was washed with a drop of double-distilled water. Negative staining was performed by adding 1 drop of 0.5% uranyl acetate to the grid surface, thereby removing excess stain immediately. The grids were allowed to air dry for 20 min and were then observed with a JEOL 100CX transmission electron microscope.
Lytic spectra.
Isolated phages were plaque purified and propagated to a titer of approximately 108 PFU/ml (titer determined on PT14). Serial dilutions of these phage stocks were then prepared and screened by using the method of Miles-Misra (19) for their ability to infect 11 NCTC Campylobacter strains representing the phage types present in the United Kingdom typing scheme (7) and 18 campylobacters exhibiting different fla types isolated from retail chicken in this study. The Campylobacter fla typing was performed in accordance with the protocol described by Alm et al. (3) using forward primer pg50 (5′-ATG GGATTTCGTATTAAC-3′) for flaA, forward primer RAA9 (5′-AAGGATTTAAAATGG GTTTTAGAAT AAACACC-3′) for flaB, and primer RAA19 (5′-GCACC(CT)TTAAG(AT)GT (A G)GTTACACCTGC-3′) as a universal reverse primer for both genes.
RESULTS
Development and validation of phage recovery method.
In order to estimate the limit of detection on chicken skin, we examined the recovery efficiency of a Campylobacter phage propagated in the laboratory (φ2). Table 1 shows the detection limit determined for the recovery of phage φ2 following the addition of serial dilutions of the phage onto retail chicken at 4°C. The method will reliably detect at least 2 × 103 PFU per 10-cm2 area of chicken skin. These results are based on three replicates.
TABLE 1.
Validation of the bacteriophage recovery methoda
| Inoculum (PFU) | Mean PFU recovery | Avg % recovery |
|---|---|---|
| 2.2 × 107 | 4.1 × 106 | 18.7 ± 4.0 |
| 2.2 × 106 | 9.3 × 105 | 42.3 ± 7.8 |
| 2.2 × 105 | 9.5 × 104 | 43.3 ± 4.5 |
| 2.2 × 104 | 9.1 × 103 | 20.3 ± 4.1 |
| 2.2 × 103 | 3.8 × 102 | 17.3 ± 2.5 |
| 2.2 × 102 | 9.6 × 101 | 9.6 ± 4.1 |
| 2.2 × 101 | 0.67 × 100 | 3 ± 5.2 |
The left column shows the number of phage inoculated onto the surface of retail chicken thighs. The central column shows the mean number of phages recovered from the surface of the inoculated chicken skin that is also given as a percentage ± standard deviation in the right column.
Recovery of phage φ2 from surface of chicken skin over time.
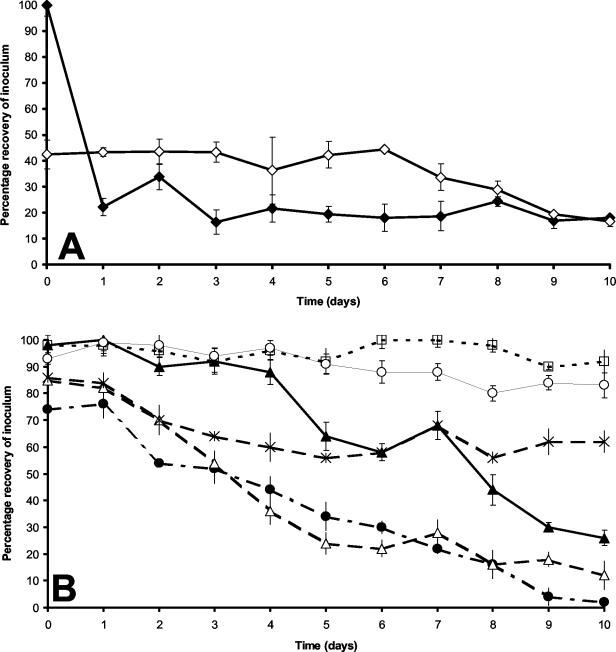
As a prerequisite to a survey of retail chicken for the presence of Campylobacter phage, we sought to establish that phages could survive in situ on chicken skin under retail storage conditions. To ensure that any incumbent Campylobacter phages would not influence our recovery results, we examined uninoculated samples for environmental phage; if these proved positive, the test was discarded so that our results would not be influenced by the presence of natural phages. Data were only recorded from batches for which the controls proved negative. Data showing the recovery of the inoculated Campylobacter phages from the surface of fresh and frozen chicken thighs over a 10-day period at 4°C are presented in Fig. 1A. Phage recovery remained constant at 42 to 44% of the initial inoculated titer over a 6-day period. At day 7, the titer fell from 44% ± 0.8% to 34% ± 7.0% and continued to fall for the duration of the experiment until reaching 17% ± 1.8% at day 10. In contrast, the recovery of phages from thawed frozen chicken was initially close to 100% (1 h), but following refreezing and subsequent thaw the titer fell to 22% ± 0.1%. Phage recovery from chicken portions thawed at 24-h intervals thereafter were in the range of 17 to 34% over a 10-day period. The level of phage recovery did not appear to be influenced by the presence of Campylobacter cells on the chicken surface.
FIG. 1.
(A) Recovery of phage φ2 (NCTC 12674) from chicken skin stored for 10 days under fresh (4°C; ⋄) and frozen (−20°C; ♦) conditions. Triplicate 10-cm2 sections of chicken thigh skin were inoculated with 108 PFU of φ2, and then percent recovery of this inoculum (± SD) was recorded at 24-h intervals. (B) Recovery of six Campylobacter bacteriophage chicken skin isolates exhibiting different lytic spectra. 108 PFU of each phage were applied to fresh chicken thigh skin in triplicate and stored for 10 days at 4°C. The percent recovery of the initial inoculum (± SD) was recorded at 24-h intervals. Key: ▴, W2; •, W3; ▵, W4; □, W5; X, W8; and ○, W10. In all cases, the initial samples (day 1) were collected 1 h after inoculation.
Bacteriophage isolation from supermarket chickens.
Three different classes (free-range, standard, and economy) of chilled (4°C) chicken thighs were purchased from a single supermarket over a 2-week period. Two different classes (standard and economy) of frozen (−20°C) chicken thighs were purchased from the same supermarket over a 1-week period. One hundred samples of each class of fresh meat and 75 portions of each class of frozen meat—representing a range of poultry producers—were screened for the presence of Campylobacter phages.
Campylobacter phages were recovered from the skin of 34 fresh chicken thighs out of the 300 tested (11%). Twenty-seven (79%) of the phage-positive chicken thighs were from free-range chicken products, compared with five (15%) and two (6%) from standard and economy products, respectively. A single-factor analysis of variance demonstrated that the observed incidence on the free-range product is significantly different (P < 0.001) from that of the other products. The mean number of phages isolated from the whole-chicken skin surface was 4.59 × 105 PFU (recovery ranged from 1 × 102 to 4 × 106 PFU). Phage recovery from frozen chicken thighs was unsuccessful. Given that we found an 80% reduction in the recovery of phage φ2 from frozen samples, this result may not be unexpected. It was also noted that the presence of the host was reduced on frozen samples.
fla typing of Campylobacter isolates and lytic spectra of phage isolates.
In parallel with the phage isolation, campylobacters were also isolated from the same retail chicken samples. The majority of isolates (96%) were C. jejuni, and based on published findings, the rest were presumed to be C. coli or C. lari (17, 18). Campylobacter was isolated from 100% of free-range chicken portions compared with 75 and 62% for standard and economy products, respectively. Campylobacter was isolated at a lower frequency from frozen chicken samples, with 9% of standard and 12% of economy products being positive. Sixty of the Campylobacter strains contemporaneously isolated from fresh chicken samples were fla typed into 18 classes. Strains of each fla type were selected for use in the investigation of the lytic spectrum of the bacteriophage isolates, along with 11 NCTC strains that represent the various Campylobacter classes discriminated by the phage-typing scheme adopted in the United Kingdom (7). The lytic spectra of the phage are presented in Table 2. The 34 independent phage isolates could be assigned to eight classes based on their host range. The bacteriophage isolates assigned to each class are as follows: W1, W2, W6, and W7 (Class I); W3, W12, W14, W16, and W18 (Class II); W4, W13, W15, and W17 (Class III); W5, W9, and W11 (Class IV); W8 (Class V); W10 (Class VI); W19, W25, W26, W27, W31, and W34 (Class VII); and W20, W21, W22, W23, W24, W28, W29, W30, W32, and W33 (Class VIII). The phage within classes containing multiple isolates arose from independent chicken portions.
TABLE 2.
Lytic spectra defining eight different classes of bacteriophage isolated from fresh retail chickena
| Bacteriophage class
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | VI | VII | VIII | φ2 | |
| CPHL PT (ref) | |||||||||
| PT1 (C605) | − | − | − | − | − | − | − | − | − |
| PT2 (C682) | + | + | + | + | + | − | − | − | − |
| PT5 (C856) | − | + | − | − | − | − | − | + | − |
| PT6 (C594) | − | + | − | − | − | − | − | − | + |
| PT14 (NCTC 12661) | + | + | + | + | + | + | + | + | + |
| PT14 (NCTC 12662) | + | + | + | + | + | + | + | + | + |
| PT19 (C11288) | − | + | + | + | + | (+) | − | − | − |
| PT33 (C1312) | − | − | − | + | − | − | − | − | − |
| PT35 (C13553) | − | − | − | − | − | − | − | − | − |
| PT44 (C10131) | − | − | − | − | − | − | − | − | + |
| NCTC 11168 | − | − | − | − | − | − | − | + | (+) |
| fla-typed isolates | |||||||||
| 1 | − | + | + | + | + | + | − | − | − |
| 2 | − | + | + | + | + | + | − | − | − |
| 7 | − | + | + | + | + | + | − | − | − |
| 12 | + | − | − | − | − | − | − | − | − |
| 17 | − | − | − | − | − | − | − | − | − |
| 19 | − | + | + | + | + | + | − | − | − |
| 29 | − | − | − | − | + | (+) | − | − | + |
| 31 | − | − | − | − | − | − | − | − | − |
| 33 | − | − | + | − | − | + | − | − | − |
| 34 | − | − | − | − | − | − | − | − | − |
| 35 | − | − | − | − | − | − | − | − | − |
| 39 | − | − | − | − | − | − | − | − | (+) |
| 77 | − | + | + | + | + | + | − | − | − |
| 190 | − | − | (+) | − | − | − | − | − | − |
| 191 | − | − | − | − | − | − | − | − | − |
| 192 | − | − | − | − | − | − | − | − | − |
| 193 | − | − | − | − | − | − | − | − | − |
| 194 | − | − | − | − | − | − | − | − | − |
| Total strains infected | 4 | 11 | 11 | 10 | 10 | 10 | 2 | 4 | 7 |
All 34 phage isolates were screened against 11 Campylobacter phage types from the typing scheme adopted in the United Kingdom (top half of the table) and 18 contemporary Campylobacter retail chicken isolates with different fla types (bottom half of the table). +, lysis; −, no lysis; (+), opalescent lysis. The total number of Campylobacter strains lysed by each bacteriophage class is given at the bottom of the table.
Bacteriophage genome size and morphological properties.
A representative of each class was chosen for further characterization (W2, W3, W4, W5, W8, W10, W19, and W20). Despite all being propagated on C. jejuni NCTC 12662 PT14, phages W19 and W20 did not produce observable bands by PFGE. A summary of the genome size and morphological properties of the retail chicken phage isolates is presented in Table 3. The phages all possessed double-stranded DNA genomes that ranged in size from 110 to 190 kb on the basis of their migration following PFGE. Phage W5 exhibited two DNA bands of 110 and 150 kb. Phage W10 also exhibited the smaller DNA band, but the upper band of W10 (190 kb) was larger than that of W5. W5 and W10 samples all retained the two DNA bands present in the parental phage, even following six successive rounds of plaque purification.
TABLE 3.
Morphological and genetic characteristics of phage grouped according to their lytic spectraa
| Lytic class | Genome size (kb) | Restriction pattern | Head diameter, nm ± SD | Tail
|
n | |
|---|---|---|---|---|---|---|
| Diameter, nm ± SD | Length, nm ± SD | |||||
| I | 110 | 1 | 85 ± 1.8 | 16 ± 3.6 | 103 ± 1.9 | 6 |
| II | 180 | Uncut | 83 ± 1.3 | 20 ± 2.8 | 124 ± 2.9 | 5 |
| III | 170 | Uncut | 92 ± 4.3 | 19 ± 2.4 | 116 ± 5.8 | 5 |
| IV | 110 + 150 | 2 | 91 ± 1.9 | 20 ± 2.8 | 112 ± 3.8 | 6 |
| V | 190 | Uncut | 89 ± 4.0 | 21 ± 2.4 | 130 ± 3.3 | 6 |
| VI | 190 + 150 | 3 | 88 ± 4.5 | 21 ± 2.4 | 125 ± 2.1 | 7 |
| VII | ND | ND | 90 ± 2.4 | 19 ± 1.5 | 115 ± 3.6 | 6 |
| VIII | ND | ND | 91 ± 2.6 | 19 ± 2.1 | 116 ± 2.7 | 5 |
Genome sizes were ascertained by pulsed field gel electrophoresis (PFGE). Genomes of phages that failed to yield defined bands by PFGE are labeled ND (not determined). Digestion of the phage genomes with endonuclease HhaI yielded three banding patterns. Genomes refractory to digestion with HhaI are designated “uncut.” Morphological features were observed by negative-staining electron-microscopy. Measurements of phage structure dimensions are recorded as the mean (nm) ± SD for n numbers of phage examined from each lytic class.
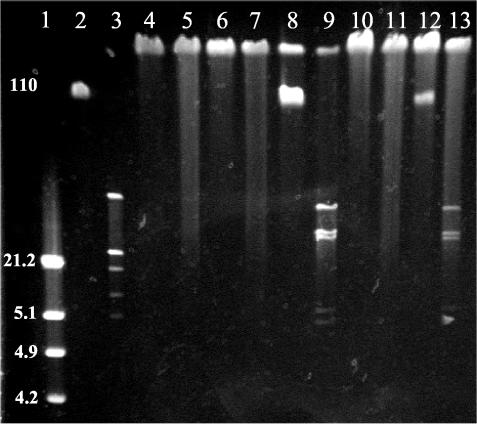
The structure of the phage genomes was compared by restriction analysis. Of the 12 restriction endonucleases tested, only HhaI yielded discriminatory patterns. On the basis of this, the phage could be placed into three restriction groups (Fig. 2). It was notable that the smaller 110-kb DNA bands in W5 and W10 could be digested by HhaI to produce similar patterns, but the DNAs corresponding to the larger bands were not cleaved by this enzyme.
FIG. 2.
Gel showing restriction fragments generated from digesting phage genomes with endonuclease HhaI. Lanes are as follows: 1, λ/HindIII marker; 2, undigested W2; 3, digested W2; 4, undigested W3; 5, digested W3; 6, undigested W4; 7, digested W4; 8, undigested W5; 9, digested W5; 10, undigested W8; 11, digested W8; 12, undigested W10; 13, digested W10.
All phages were propagated on the Campylobacter strain NCTC 12662 PT14, which does not appear to modify phage DNA to prevent digestion since at least some phage DNA could be cut with enzymes sensitive to methylation (i.e., HhaI, TaqI, and MboI). However, DNA of phages W3, W4, and W8 were completely refractory to digestion by all tested enzymes. The DNAs of these phages are probably modified by phage-encoded activities.
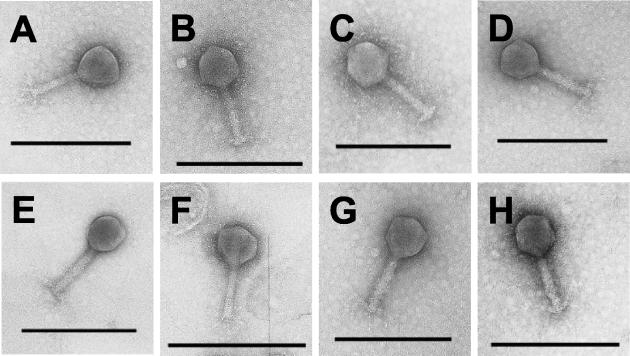
The phage selected for genome analysis were also examined by electron microscopy. Examination of the phages showed them all to have icosahedral heads and rigid contractile tails which—together with their dsDNA genomes—classify them as members of the Myoviridae family. Electron micrographs are presented in Fig. 3. Variation in the head diameters and tail lengths allowed them to be further differentiated. Class members were isolated from all types of fresh chicken products.
FIG. 3.
Electron photomicrographs of phages isolated from fresh chicken skin representing eight lytic spectra classes. The icosahedral head and rigid contractile tail are typical features of the Myoviridae family. A, W2; B, W3; C, W4; D, W5; E, W8; F, W10; G, W19; H, W20. Bar represents 250 nm. All electron photomicrographs taken at ×100,000 magnification with a JEOL 100CX transmission electron microscope.
Variation in the rates of recovery of phage isolates from chicken skin.
For those phages that had been characterized with respect to their genome size and morphology, we examined their relative recovery from the surface of inoculated chicken skin over a 10-day period. Variations in the efficiency of recovery of phage W2 (class I), W3 (class II), W4 (class III), W5 (class IV), W8 (class V), and W10 (class VI) were noted when they were reapplied to the surface of fresh chicken skin (Fig. 1B). At least 90% of the phage W5 inoculum could be recovered over the 10-day course of the experiment, whereas only 2% of the W3 inoculum could be recovered at the end of the time course. However, the recovery of all phages from the surface of frozen chicken did not differ significantly from the pattern recorded for phage φ2 shown in Fig. 1A (data not shown). In these experiments, initial high recovery rates were recorded (ranging from 84% for W3 to 98% for W5), followed by a decrease in titer 24 h postinoculation and freezing, at which time the recovery ranged from 18 to 30% and thereafter remained stable for the rest of the time course.
DISCUSSION
The successful isolation of Campylobacter phages from 34 of 300 fresh retail chicken samples has demonstrated that the methods developed in this study are practically applicable. Since the detection limit of the recovery method was determined to be 2 × 103 PFU/10 cm2 of chicken skin, isolation of phages from these products implies that at least this many bacteriophages have survived retail poultry processing and packaging. It is likely that the phages isolated in this study originate from the cecal contents of the chickens, particularly because Campylobacter phages have previously been recovered from abattoir effluent and chicken feces (8). Fecal contamination of chicken carcasses both before and after abattoir processing has been reported previously (17, 25). The Campylobacter phages were isolated from fresh chicken thighs within their prescribed shelf life; however, survival experiments demonstrated that bacteriophages can be recovered up to 10 days following inoculation—which is well beyond their stated shelf life. Phage φ2 could be efficiently recovered from the surface of frozen retail chicken that had been thawed prior to application. However, subsequent freeze and thaw resulted in a fall in the recovery by almost 80%. Birds intended for freezing are scalded at a higher temperature (14)—and this, together with the subsequent freeze-thaw cycle, is known to change the skin surface structure (2). Our results suggest that phages may not attach as well to skin surfaces following such treatment, thereby giving higher initial recovery rates. The subsequent drop occurs due to the loss of phage viability on freezing, to which members of the Myoviridae family are particularly susceptible (1).
The lytic spectra of the individual phage isolates revealed similarities in host range. Phages exhibiting the same lytic spectrum were placed in one of eight host range classes. The host ranges of all the retail poultry isolates differed markedly from the lytic spectrum of the φ2 positive control. The similarity in lytic profiles of independent phage isolates may suggest that some form of selection has taken place. This may reflect the ability of bacteriophages to survive on the chicken surface during processing and/or the ability to survive the storage conditions applied. The genome sizes and morphologies of the phages could not be correlated with any particular source of poultry meat. However, it has been reported that certain Campylobacter fla types are able to survive abattoir processing better than others (21), and this may determine the survival of any predator phages that are associated with them.
Free-range chickens are subject to environmental exposure and are therefore more likely to encounter a wider range of campylobacters and their phages. We observed a significant difference in the isolation of phages from free-range products compared with standard and economy products which may be explained in part by the frequency of Campylobacter-positive carcasses coming from the free-range sector. Greater exposure to the environment consistent with organic and free-range farming practices has been purported to be the cause of a generally higher incidence of Campylobacter in these types of flocks (11). The probability of phage isolation will increase with the presence of a susceptible host; thus, chickens with higher rates of carriage of campylobacters are more likely to be sources of phages. However, with widespread cross contamination in the abattoir, it is likely that the Campylobacter and phages found on a single carcass arise from more than one source. In addition to this, we have found that the recovery of the phage isolates inoculated onto chicken skin show marked variation. Four of the six chicken skin isolates tested yielded a higher rate of recovery over a 10-day period when applied to chicken skin and retained at 4°C (Fig. 1B) when compared with the φ2 control (Fig. 1A). Most notably, phage W5 could be recovered at >90% of the inoculum throughout the course of the experiment.
The genome sizes and morphological characteristics of the phage fall within the ranges previously reported in the literature (25). We note that phage W5 shows two distinct bands by PFGE (i.e., 110 and 150 kb) and that these bands may be distinguished by digestion with HhaI. The upper band was refractory to digestion, whereas the lower band generated fragments that correlate with the size of the lower band. This suggests that the two DNA molecules are distinct, but the reason for their presence in a purified phage preparation remains to be investigated.
Phages were only recovered from chicken samples that also harbored campylobacters—a finding that is not surprising, as phages will originate from an environment containing host cells. However, the survival data for phages inoculated onto chicken skin and incubated in retail storage conditions shows a general decline, thus suggesting that replication of the phage is not occurring on the surface of the samples. This is not surprising, as Campylobacter does not grow on food samples stored under these conditions and hence will not have an active metabolism. There are multiple variables that dictate the survival of both phage and Campylobacter throughout commercial poultry production. These factors may not necessarily allow both the phage and its host to survive at the end of processing. The multiple cross-contamination events and survival variables of Campylobacter and phages in the processing plant would make any attempt to correlate the phage with a host from a common chicken source of little value.
In conclusion, we have demonstrated that Campylobacter phages can survive on fresh and frozen retail poultry products. We have successfully implemented a method to recover Campylobacter phages from 34 independent retail chicken samples—a source not previously examined for the presence of Campylobacter phages. The finding that phages are present on retail poultry products demonstrates that the use of phages as a biocontrol agent would constitute a minimum intervention that would not ultimately introduce any entity into food products that is not already present.
Acknowledgments
This study was supported in part by the United Kingdom Department for the Environment, Food, and Rural Affairs. R.J.A. acknowledges the financial support of the University of Nottingham.
We thank Jenny Frost and colleagues at the Central Public Health Laboratory (Colindale, London, United Kingdom) for their donation of the phages and the Campylobacter strains used in their typing scheme. We also thank Stefan Hyman at the University of Leicester for his expert assistance with the phage electron microscopy.
REFERENCES
- 1.Ackermann, H., and M. S. DuBow. 1987. General properties of tailed phages, p. 28. Viruses of prokaryotes, vol. 2. CRC Press, Boca Raton, Fla.
- 2.Adams, M. R., and M. O. Moss. 1995. Food microbiology. Royal Society of Chemistry, Cambridge, United Kingdom.
- 3.Alm, R. A., P. Guerry, and T. J. Trust. 1993. Distribution and polymorphism of the flagellin genes from isolates of Campylobacter coli and Campylobacter jejuni. J. Bacteriol. 175:3051-3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beery, J. T., M. B. Hugdahl, and M. P. Doyle. 1988. Colonization of gastrointestinal tracts of chicks by Campylobacter jejuni. Appl. Environ. Microbiol. 54:2365-2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cogan, T. A., S. F. Bloomfield, and T. J. Humphrey. 1999. The effectiveness of hygiene procedures for prevention of cross-contamination from chicken carcases in the domestic kitchen. Lett. Appl. Microbiol. 29:354-358. [DOI] [PubMed] [Google Scholar]
- 6.Evans, M. R., W. Lane, J. A. Frost, and G. Nylen. 1998. A campylobacter outbreak associated with stir-fried food. Epidemiol. Infect. 121:275-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frost, J. A., J. M. Kramer, and S. A. Gillanders. 1999. Phage typing of Campylobacter jejuni and Campylobacter coli and its use as an adjunct to serotyping. Epidemiol. Infect. 123:47-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grajewski, B. A., J. W. Kusek, and H. M. Gelfand. 1985. Development of a bacteriophage typing system for Campylobacter jejuni and Campylobacter coli. J. Clin. Microbiol. 22:13-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gregory, E., H. Barnhart, D. W. Dreesen, N. J. Stern, and J. L. Corn. 1997. Epidemiological study of Campylobacter spp. in broilers: source, time of colonization, and prevalence. Avian Dis. 41:890-898. [PubMed] [Google Scholar]
- 10.Griffiths, P. L., and R. W. Park. 1990. Campylobacters associated with human diarrhoeal disease. J. Appl. Bacteriol. 69:281-301. [DOI] [PubMed] [Google Scholar]
- 11.Hald, B., E. Rattenborg, and M. Madsen. 2001. Role of batch depletion of broiler houses on the occurrence of Campylobacter spp. in chicken flocks. Lett. Appl. Microbiol. 32:253-256. [DOI] [PubMed] [Google Scholar]
- 12.Humphrey, T., and F. Jorgensen. 2000. Fit to eat? Food scares and safe food production. Microbiol. Today 27:10-12. [Google Scholar]
- 13.Humphrey, T. J., A. Henley, and D. G. Lanning. 1993. The colonization of broiler chickens with Campylobacter jejuni: some epidemiological investigations. Epidemiol. Infect. 110:601-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Humphrey, T. J., G. Mead, and B. Rowe. 1988. Poultry meat as a source of human salmonellosis in England and Wales. Epidemiol. Infect. 100:175-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Istre, G. R., M. J. Blaser, P. Shillam, and R. S. Hopkins. 1984. Campylobacter enteritis associated with undercooked barbecued chicken. Am. J. Public Health 74:1265-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobs-Reitsma, W. F. 2000. Campylobacter in the food supply, p. 497-509. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter, 2nd ed. ASM Press, Washington, D.C.
- 17.Jorgensen, F., R. Bailey, S. Williams, P. Henderson, D. R. Wareing, F. J. Bolton, J. A. Frost, L. Ward, and T. J. Humphrey. 2002. Prevalence and numbers of Salmonella and Campylobacter spp. on raw, whole chickens in relation to sampling methods. Int. J. Food Microbiol. 76:151-164. [DOI] [PubMed] [Google Scholar]
- 18.Kramer, J. M., J. A. Frost, F. J. Bolton, and D. R. Wareing. 2000. Campylobacter contamination of raw meat and poultry at retail sale: identification of multiple types and comparison with isolates from human infection. J. Food Prot. 63:1654-1659. [DOI] [PubMed] [Google Scholar]
- 19.Miles, A. A., and S. S. Misra. 1938. The estimation of the bacteriological power of the blood. J. Hyg. 38:732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy, O., J. Gray, S. Gordon, and A. J. Bint. 1995. An outbreak of campylobacter food poisoning in a health care setting. J. Hosp. Infect. 30:225-228. [DOI] [PubMed] [Google Scholar]
- 21.Newell, D. G., J. E. Shreeve, M. Toszeghy, G. Domingue, S. Bull, T. Humphrey, and G. Mead. 2001. Changes in the carriage of Campylobacter strains by poultry carcasses during processing in abattoirs. Appl. Environ. Microbiol. 67:2636-2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newell, D. G., and J. A. Wagenaar. 2000. Poultry infections and their control at the farm level, p. 497-509. In M. J. Blaser (ed.), Campylobacter, 2nd ed. ASM Press, Washington, D.C.
- 23.Phillips, C. A. 1995. Incidence, epidemiology and prevention of foodborne Campylobacter species. Trends Food Sci. Technol. 6:83-87. [Google Scholar]
- 24.Rees, J. H., N. A. Gregson, P. L. Griffiths, and R. A. Hughes. 1993. Campylobacter jejuni and Guillain-Barre syndrome. Q. J. Med. 86:623-634. [DOI] [PubMed] [Google Scholar]
- 25.Sails, A. D., D. R. Wareing, F. J. Bolton, A. J. Fox, and A. Curry. 1998. Characterisation of 16 Campylobacter jejuni and C. coli typing bacteriophages. J. Med. Microbiol. 47:123-128. [DOI] [PubMed] [Google Scholar]
- 26.Salama, S. M., F. J. Bolton, and D. N. Hutchinson. 1990. Application of a new phagetyping scheme to campylobacters isolated during outbreaks. Epidemiol. Infect. 104:405-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salama, S. M., F. J. Bolton, and D. N. Hutchinson. 1989. Improved method for the isolation of Campylobacter jejuni and Campylobacter coli bacteriophages. Lett. Appl. Microbiol. 8:5-7. [Google Scholar]
- 28.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed., vol. 2. Cold Spring Harbor Press, Cold Spring Harbor, N.Y.
- 29.Skirrow, M. B. 1977. Campylobacter enteritis: a “new” disease. BMJ 2:9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stern, N. J. 1995. Influence of season and storage on Campylobacter spp. contaminating broiler carcasses. J. Appl. Poultry Res. 4:235-238. [Google Scholar]
- 31.Stern, N. J., M. R. Clavero, J. S. Bailey, N. A. Cox, and M. C. Robach. 1995. Campylobacter spp. in broilers on the farm and after transport. Poult. Sci. 74:937-941. [DOI] [PubMed] [Google Scholar]
- 32.Tauxe, R. V. 2000. Incidence, trends and sources of campylobacteriosis in developed countries, p. 42-43. In The increasing incidence of human camylobacteriosis. Report and Proceedings of a WHO Consultation of Experts. World Health Organization, Copenhagen, Denmark.