Abstract
Two hundred strains of Listeria monocytogenes collected from food and the food industry were analyzed for susceptibility to the class IIa bacteriocins sakacin P, sakacin A, and pediocin PA-1 and the class I bacteriocin nisin. The individual 50% inhibitory concentrations (IC50) were determined in a microtiter assay and expressed in nanograms per milliliter. The IC50 of sakacin P ranged from 0.01 to 0.61 ng ml−1. The corresponding values for pediocin PA-1, sakacin A, and nisin were 0.10 to 7.34, 0.16 to 44.2, and 2.2 to 781 ng ml−1, respectively. The use of a large number of strains and the accuracy of the IC50 determination revealed patterns not previously described, and for the first time it was shown that the IC50 of sakacin P divided the L. monocytogenes strains into two distinct groups. Ten strains from each group were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis of whole-cell proteins and amplified fragment length polymorphism. The results from these studies essentially confirmed the grouping based on the IC50 of sakacin P. A high correlation was found between the IC50 of sakacin P and that of pediocin PA-1 for the 200 strains. Surprisingly, the correlation between the IC50 of the two class IIa bacteriocins sakacin A and sakacin P was lower than the correlation between the IC50 of sakacin A and the class I bacteriocin nisin.
Awareness of the food-borne pathogen Listeria monocytogenes has increased in recent years. At the same time, lactic acid bacteria (LAB) have been considered and used as biopreservatives (10, 53). Many LAB produce bacteriocins—small, heat-stable, membrane-active antimicrobial peptides. The class I bacteriocin nisin, which is commercially available as a food preservative (14), and bacteriocins of class IIa (pediocin-like) are of special interest as inhibitors of Listeria spp. (19, 47). The addition of bacteriocinogenic LAB or purified bacteriocins to inhibit the growth of Listeria spp. in food products has been studied (8, 22, 35-37, 43; for a review see reference 10).
Intrinsic properties of food have a significant influence on the effect of bacteriocins on the target cells (2, 5, 27, 39). However, knowledge about the susceptibility of the target organism is also necessary when bacteriocins are added to food as biopreservatives. The susceptibilities of different strains of L. monocytogenes to both nisin and pediocin-like bacteriocins differ (22, 38, 42, 47). Identical sensitivity patterns have been reported (20, 47) for different strains of L. monocytogenes in response to different pediocin-like bacteriocins. But for comparison of the potencies of the different bacteriocins, quantitative MICs or 50% inhibitory concentrations (IC50) are needed (9, 18).
Resistance by L. monocytogenes strains to nisin and class IIa bacteriocins has been reported (29, 30, 38, 43, 47, 57, 59). The term “resistance” is often not defined clearly, so strains able to grow at the highest bacteriocin concentration available have been defined as resistant. In model systems it has been shown that high-level-resistant mutants arise spontaneously when bacteriocin-sensitive L. monocytogenes strains grow in the presence of high concentrations of a class IIa bacteriocin (29). In appropriate media the MICs for these mutants are 1,000 times higher than those for the wild-type strains (15, 29). The mode of action of class IIa bacteriocins has been elucidated recently, together with studies of mutant strains with resistance to high levels of these bacteriocins (12, 13, 15, 29, 31, 33, 34, 46, 49, 55, 61). In order to be active, class IIa bacteriocins need a target molecule at the surface of a sensitive cell, and mannose phosphotransferase system (PTS) permease is the proposed target molecule (33, 34). Gravesen et al. (29) suggest that high-level resistance to class IIa bacteriocins in L. monocytogenes is caused by prevention of synthesis of a mannose-specific PTS permease (EIItMan) and up-regulation of the synthesis of a putative β-glucoside-specific PTS enzyme II (EIIBgl) and a phospho-β-glucosidase. The up-regulations are probably a consequence of abolished expression of mptACD (mannose permease 2, encoding a putative mannose PTS permease) (29). Membrane adaptation, by increased levels of desaturated and short-acyl-chain phosphatidylglycerols, has also been suggested as part of a resistance mechanism (55).
Various methods are used to determine differences in susceptibility to bacteriocins (6, 16, 18, 38, 40, 42, 47, 52, 60). To achieve high discrimination between strains with different susceptibilities, a standardized microtiter plate assay, performed with bacteriocin solutions of known concentrations, was used to provide data necessary to determine the potencies of the bacteriocins sakacin P, pediocin PA-1, sakacin A, and nisin against L. monocytogenes. The distributions of IC50 were determined, and correlation coefficients for the IC50 were calculated, providing new information regarding the pattern of susceptibility to bacteriocins for this species.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
In this study 200 L. monocytogenes strains, originating from food and food industry environments, were investigated. The strains either originated from different sources or had been shown to differ by multilocus enzyme electrophoresis and/or restriction enzyme analysis (1; L. M. Rørvik, personal communication). Listeria ivanovii Li4 (4) was used as an indicator for the determination of bacteriocin concentrations. The Listeria strains were kept at −80°C and grown in brain heart infusion medium (Oxoid Ltd., Basingstoke, United Kingdom) at 30°C overnight prior to IC50 determination. For sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) of whole-cell proteins and for fatty acid analysis, the cells were grown for 24 h at 28°C on plates containing 30 g of Trypticase soy broth (BBL, Cockeysville, Md.) and 15 g of Bacto Agar (Difco, Detroit, Mich.) per liter of distilled water. The bacteriocin-deficient Lactobacillus sakei strain Lb790(pSAK20) (3, 4), grown in MRS medium (Oxoid Ltd.), was used as a host for the production of sakacin P, sakacin A, and pediocin PA-1 by introducing the isogenic plasmids pSPP2 (4), pSAK17B (3), and pPED2 (4), respectively. A bacteriocin-negative supernatant was obtained by introducing the isogenic plasmid pLPV111 (3) into the same Lactobacillus sakei strain.
Preparation of bacteriocin stock solutions and a bacteriocin-negative control supernatant for IC50 determinations.
Purified nisin (a gift from Danisco Innovation, Beaminster, United Kingdom) was dissolved in 0.02 N HCl with 0.1% Tween 80, the pH was adjusted to 6.2, and the solution was sterilized by filtration (pore size, 0.2 μm; Millex GS; Millipore, Molsheim, France). This nisin stock solution (12.5 μg ml−1) was stored at 4°C and used within 14 days. Cell-free supernatants (CFS) of sakacin P, sakacin A, and pediocin PA-1, as well as a bacteriocin-negative control, were produced by heterologous expression in Lactobacillus sakei Lb790 as previously described (3, 4). Cultures were grown overnight at 30°C with the appropriate antibiotics and then reinoculated in fresh MRS medium without antibiotics before further incubation at 25°C. The CFS was prepared from a dense bacterial culture (optical density at 600 nm [OD600], 3.5) by removal of cells by centrifugation at 15,000 × g for 15 min, followed by pH adjustment (pH 6.5) and heat treatment (80 to 90°C for 20 min) to eliminate protease activity. Aliquots of the CFS (stock solutions) of sakacin P, sakacin A, pediocin PA-1, and the bacteriocin-negative control were stored at −20°C. For induction of high-level resistance as described by Gravesen et al. (28), the sakacin P CFS was subjected to catalase treatment instead of heat treatment.
Production of purified bacteriocins of known concentrations.
Sakacin P, sakacin A, and pediocin PA-1 were produced as described above and purified by ammonium sulfate precipitation, cation-exchange chromatography, hydrophobic interaction chromatography, and reverse-phase chromatography as previously described (18). The concentrations of the purified bacteriocins were assessed by measuring UV absorption at 280 nm (18). Purified bacteriocins, in appropriate predilutions in Listeria enrichment broth (LEB) (Oxoid Ltd.) with 0.1% Tween 80 (Sigma), were stored at −20°C until use.
Bacteriocin assay.
L. ivanovii Li4 was used as an indicator in the microtiter plate assay for determining the concentrations of bacteriocins in the different stock solutions (CFS) essentially as previously described (4). One hundred microliters of a standardized inoculum was added to the wells (approximately 105 CFU/well) containing 100 μl of culture medium with bacteriocin at twofold dilutions. The turbidity was measured before and after incubation at 25°C for 17 h. Concentrations of bacteriocins in the various CFS were determined by correlating the activity of the CFS with the activity of the corresponding purified bacteriocin solution of known concentration applied on the same plate. Each concentration reported for the CFS is the mean of at least three different measurements of both purified bacteriocins and stock solutions. The concentrations of sakacin P, sakacin A, and pediocin PA-1 in the CFS were determined to be 2.7, 3.8, and 0.9 μg ml−l, respectively.
IC50 determinations for 200 L. monocytogenes strains.
Screening of the susceptibilities of the 200 strains to the bacteriocins sakacin P, sakacin A, pediocin PA-1, and nisin was performed as a modification of the bacteriocin assay described above. The IC50 are the calculated concentrations of a bacteriocin needed to inhibit growth by 50%, compared to uninhibited growth (i.e., average growth of the same strain in a medium without bacteriocin in eight wells on the same plate). All wells on a plate were filled with 100 μl of LEB containing 0.1% Tween 80 as in the bioassay. Duplicates of twofold dilutions of the stock solutions of sakacin P, sakacin A, pediocin-PA-1, and nisin were prepared in one microtiter plate. In addition, the probability of inhibition by bacteriocin-free CFS was tested for each strain. The inoculum for the IC50 test was prepared by diluting overnight cultures to an OD600 of 0.002 in LEB with 0.1% Tween 80. A 100-μl inoculum was added to wells containing bacteriocin as well as to the control wells containing only medium. The turbidity was measured, and the plates were incubated at 25°C until control wells for uninhibited growth reached an OD600 of 0.23 to 0.3 (approximately 17 h). The OD600 values were then used for the calculation of IC50, and the averages of parallel IC50 with a coefficient of variation less than 15% are presented (Fig. 1).
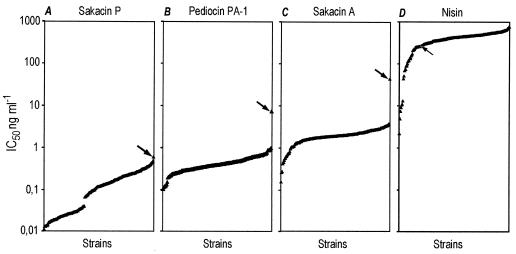
FIG. 1.
IC50 (measured as the concentration, in nanograms per milliliter, needed to achieve 50% inhibition of growth in a microtiter plate assay), on a logarithmic scale, for 200 strains of L. monocytogenes. The strains are sorted according to ascending IC50 for each of the bacteriocins: sakacin P (A), pediocin PA-1 (B), sakacin A (C), and nisin (D). Arrows indicate the IC50 of each of the bacteriocins for strain L1040; see the text for details.
Determination of correlation coefficients.
Correlation coefficients between the IC50 of the four bacteriocins for the 200 L. monocytogenes strains were calculated by using Excel (Microsoft Office XP Professional, version 2002; Microsoft Corporation).
AFLP analysis.
The primers and adapters used for amplified fragment length polymorphism (AFLP) analysis have been described by Vos et al. (58). Genomic DNAs from various strains of Listeria were prepared by using the Qiagen (Valencia, Calif.) tissue kit according to the manufacturer's instructions. Genomic DNA (approximately 0.5 μg) was incubated overnight at 37°C with 12 U of EcoRI (Promega, Madison, Wis.) and 4 U of MseI (New England Biolabs, Beverly, Mass.) in 1× NEB buffer 2 (New England Biolabs) with 1 ng of bovine serum albumin μl−1 in a total volume of 20 μl. To 5.5 μl of the digestion mix, 5.5 μl of a solution containing 2 μM EcoRI adapters and 2 μM MseI adapters in 1× T4 DNA ligase buffer (Promega), 50 mM NaCl, and 1 ng of bovine serum albumin μl−1 with 12 U of EcoRI, 4 U of MseI, and 0.5 U of T4 DNA ligase was added. Incubation was continued overnight at room temperature. After ligation, the reaction mixture was diluted 50-fold in TE buffer (10 mM Tris-HCl-0.1 mM EDTA [pH 8.0]) and stored at 4°C. The diluted ligation mix (5.0 μl) was then used as a template for PCR by adding 5.0 μl of 1× AmpliTaqGold DNA polymerase buffer, MgCl2 to 5 mM, deoxynucleoside triphosphates (Promega) to 200 μM, 30 ng of primer AFML4 (5′ GAT GAG TCC TGA GTA AC 3′) and 5 ng of primer AFEC4 (5′ GAC TGC GTA CCA ATT CC 3′), and 2.5 U of AmpliTaqGold DNA polymerase in a total volume of 50 μl. PCRs were performed on a Perkin-Elmer 9700 thermal cycler. After a 10-min activation of the polymerase at 94°C, the following cycle profile was used: cycle 1, denaturation for 2 min at 94°C, annealing for 20 s at 66°C, and elongation for 2 min at 72°C; cycles 2 to 10, 2 min at 94°C, 20 s at an annealing temperature 1°C lower than the previous cycle, starting at 65°C, and 2 min at 72°C; cycles 11 to 21, 2 min at 94°C, 20 s at 56°C, and 2 min at 72°C; cycle 22, 30 min at 60°C. Two microliters of the amplified product was added to 25 μl of loading buffer (24 μl of deionized formamide and Gene Scan 500 TAMRA standard [Applied Biosystems]). All samples were denatured for 5 min at 95°C and then rapidly cooled on ice prior to electrophoresis. Amplified fragments were separated on an ABI Prism Genetic Analyzer 310. Fragment profiles were analyzed by using GelCompare II with Pearson product moment correlation (r) and the unweighted pair group method with arithmetic mean (UPGMA; Applied Maths, Sint-Martens-Latem, Belgium).
SDS-PAGE of whole-cell proteins.
Whole-cell protein extracts were prepared, and SDS-PAGE was performed as described by Pot et al. (45). Duplicate protein extracts were prepared to check the reproducibility of the growth conditions and the preparation of the extracts. Registration of the protein patterns, normalization of the densitometric traces, and pattern storage were performed using GelCompar software (version 4.2; Applied Maths). The strains were grouped by using GelCompar software (version 4.2) with the Pearson product moment correlation coefficient (r) and UPGMA cluster analysis as previously described (45).
Fatty acid analysis.
Cells were saponified, methylated to fatty acid methyl esters, and extracted by following the Sherlock Microbial Identification System (version 3.0; MIDI, Inc., Newark, Del.). Fatty acid methyl esters were then separated on an Agilent 6890A series gas chromatograph with a 7683 autoinjector and an autosampler tray module (Agilent Technologies Inc., Wilmington, Del.). The separation was achieved with a fused-silica capillary column (25 m by 0.2 mm) with cross-linked 5% phenylmethyl silicone (film thickness, 0.33 μm; HP Ultra 2). H2 was used as the carrier. Peak integration and identification were performed by using a Chemstation (Hewlett-Packard GmbH, Waldbronn, Germany) and the Sherlock software (MIDI, Inc.).
Development of high-level resistance to class IIa bacteriocins.
Overnight cultures of L. monocytogenes were serially diluted in peptone saline before 5 μl was spotted onto tryptic soy agar (TSA) plates (Oxoid Ltd.) with or without 30% fermentate containing sakacin P as previously described (28, 29). After incubation at 30°C for 2 days, spontaneously resistant colonies on TSA plates with sakacin P and colonies from TSA plates (control without fermentate) were picked. The frequencies of mutations could be calculated, and the IC50 of sakacin P, sakacin A, pediocin PA-1, and nisin were determined for both mutants and wild-type strains.
RESULTS
IC50.
Figure 1 shows the differences in the susceptibilities of the 200 different L. monocytogenes strains to sakacin P, sakacin A, pediocin PA-1, and nisin. The IC50 of sakacin P were in the lowest range, from 0.01 to 0.61 ng ml−1. For pediocin PA-1, sakacin A, and nisin, the IC50 were 0.10 to 7.34, 0.16 to 44.2, and 2.2 to 781 ng ml−1, respectively.
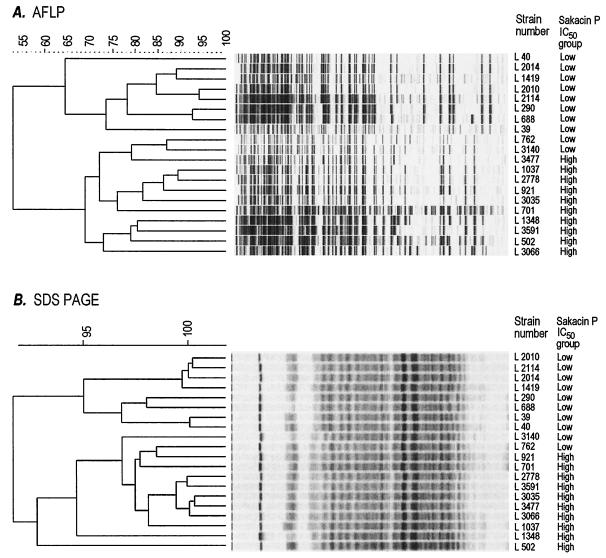
The IC50 of sakacin P divided the collection of Listeria strains into two clearly distinct groups based on susceptibility (Fig. 1). For 37% of the strains, the IC50 were equal to or less than 0.041 ng of sakacin P ml−1; for the remaining 63%, the IC50 were equal to or above 0.066 ng of sakacin P ml−1. Ten of the strains for which the IC50 were immediately above 0.066 ng ml−1 and 10 strains for which the IC50 were below 0.041 ng ml−1 were analyzed further by SDS-PAGE of whole-cell proteins, fatty acid analysis, and AFLP. Both the AFLP and SDS-PAGE studies grouped the 20 strains into two distinct groups (Fig. 2) that essentially correlated with the grouping obtained by using the IC50 of sakacin P. Such a grouping was not obtained by fatty acid analysis.
FIG. 2.
Dendrograms created with GelCompare II (see the text for details) based on AFLP analysis (A) and SDS-PAGE analysis (B) of 20 L. monocytogenes strains. Scales above the dendrograms show the percent similarity level in the cluster analysis. The strains' susceptibilities to sakacin P are measured as 50% inhibition of growth in a microtiter plate assay and are designated either Low (sakacin P IC50, ≤0.041 ng ml−1) or High (sakacin P IC50, ≥0.066 ng ml−1).
One of the 200 strains, L. monocytogenes L1040, had a special susceptibility pattern. It was the most tolerant to all three class IIa bacteriocins (IC50, 0.61, 7.34, and 44.2 ng ml−1 for sakacin P, pediocin PA-1, and sakacin A, respectively [Fig. 1A through C]). It was, however, among the 40 strains most sensitive to nisin (IC50, 257 ng ml−1).
Correlation coefficients.
There was a high correlation between the IC50 of sakacin P and that of pediocin PA-1 for the 200 strains. The correlation coefficient between the IC50 of sakacin A and that of nisin was higher than the correlation coefficient between the IC50 of sakacin A and that of sakacin P (Table 1).
TABLE 1.
Correlation coefficients between IC50 of sakacin P, sakacin A, pediocin PA-1, and nisin for 199 L. monocytogenes strainsa
| Bacteriocin | Correlation coefficient with:
|
|||
|---|---|---|---|---|
| Sakacin P | Sakacin A | Pediocin PA-1 | Nisin | |
| Sakacin P | 1.00 | |||
| Sakacin A | 0.49 | 1.00 | ||
| Pediocin PA-1 | 0.84 | 0.68 | 1.00 | |
| Nisin | 0.26 | 0.65 | 0.35 | 1.00 |
One strain displayed an extreme susceptibility pattern relative to those of the 199 others. The IC50 for this strain were removed from the data set before the correlations were calculated.
Spontaneous high-level resistance.
Four strains (two from each sakacin P IC50 group) as well as L. monocytogenes strain L1040 were spotted onto sakacin P agar plates, and mutants were obtained. With these four strains, the IC50 of sakacin P, pediocin PA-1, and sakacin A for the mutants were 1,000 times higher than those for the wild-type strains. The sakacin P IC50 for mutants of L. monocytogenes L1040 also was 1,000 times higher than that for the wild-type strain. The stock solutions of sakacin A and pediocin PA-1 were not concentrated enough to determine proper IC50, but the IC50 of sakacin A and pediocin PA-1 for the L. monocytogenes L1040 mutants were at least 64 times higher than those for the wild-type strain. Sakacin P-resistant mutants were developed with frequencies of approximately 10−5 for all five strains. The mutations did not influence the strains' susceptibility to nisin (data not shown).
DISCUSSION
Reports on the potency of bacteriocins against L. monocytogenes often suffer from the investigation of a small number of indicator strains (9, 18, 20), and it is generally difficult to compare studies on susceptibility to bacteriocins due to differences in methodology and terminology. In the present study, the susceptibility of as many as 200 different strains of L. monocytogenes to four bacteriocins, expressed as individual IC50 in nanograms per milliliter, were determined in order to obtain the detailed information needed to draw conclusions about differences in the potencies of these bacteriocins against L. monocytogenes and to reveal possible differences in susceptibility patterns between strains.
The nature of bacteriocin solutions is important in susceptibility testing. The complete purification of three class IIa bacteriocins to the amounts required for all IC50 tests was considered a laborious task. It was therefore decided to use heterologously expressed bacteriocins from the same host strain, a system already available and used (4, 5). Since the background would be constant, this was considered far better than using different (wild-type) production strains with different and unknown backgrounds. Still, there is a theoretical possibility that some compound(s) produced by the host strain with no inhibitory activity of its own (as shown by the control test) could affect the three class IIa bacteriocins differently. This would nevertheless not affect one of the main subjects of this study, namely, the differences in the susceptibilities of L. monocytogenes strains to each bacteriocin. Regarding the potency of the bacteriocins, the differences observed in this study have also been confirmed with pure bacteriocins for some of the L. monocytogenes strains (G. Fimland, personal communication). This suggests that the influence of the background supernatant on the IC50 results is very small.
All 200 strains of L. monocytogenes were sensitive to all bacteriocins tested, and the IC50 of nisin, sakacin P, sakacin A, and pediocin PA-1 demonstrate the natural differences in susceptibility to these bacteriocins. As can be seen in Fig. 1, there were large strain-to-strain differences in susceptibility for each of the bacteriocins used. This is in accordance with previous reports (6, 20, 22, 47, 54), and such strain-to-strain differences have to be considered when bacteriocins are to be used as biopreservatives.
In Fig. 1, the IC50 for the 200 strains are sorted in ascending order for each of the bacteriocins. The sakacin P response (Fig. 1A) differs from the effects of the other bacteriocins in that the bacterial strains were divided into two groups based on the sakacin P IC50. For all of the sakacin P-sensitive strains in the lower group (which contains 74 strains), the IC50 of sakacin P were below the minimum IC50 determined for any of the other bacteriocins (Fig. 1). The discriminatory power of the method used in this study is greater than that obtained by determination of the highest of twofold dilutions inhibiting growth (20) or by susceptibility determinations based on plating on a limited number of bacteriocin concentrations in agar plates (47). The discovery of such a grouping of strains as is seen in Fig. 1A shows the importance of the discrimination obtained in this study by determinations of individual IC50.
To obtain additional information about the characteristics of strains in the “low” and “high” sakacin P IC50 group, 10 strains from each group were subjected to AFLP, fatty acid analyses, and SDS-PAGE of whole-cell proteins. Statistical analysis of the fatty acid composition of the membranes from these strains did not give any distinct grouping (data not shown). Fatty acid composition has previously been correlated to induced resistance to bacteriocins (55) but may not be involved in natural differences in susceptibility. All strains were grouped equally into two major groups based on results from both AFLP and SDS-PAGE, although the grouping based on SDS-PAGE occurred at a much higher similarity level and was not clearly apparent by visual examination. As can be seen in Fig. 2, the correlation between the sakacin P grouping and the grouping based on AFLP and SDS-PAGE was high. Further investigations are needed to explain the relation between sakacin P susceptibility and other classifications, as well as the exceptions to this correlation, i.e., strains L762 and L3140 (Fig. 2). Another feature of the two sakacin P IC50 groups was connected to the distribution of serotypes, as the group for which sakacin P IC50 were low contained 31 of 33 serotype-4 L. monocytogenes strains. Since the serotype describes a surface property, we speculate that surface properties in serotype-4 strains are partly responsible for the high potency of sakacin P against these strains.
Rasch and Knøchel (47) have reported the natural differences in the susceptibilities of 350 L. monocytogenes strains to nisin and pediocin PA-1 based on growth characteristics on agar containing three different concentrations of the bacteriocins (given in arbitrary units). Susceptibility to bavaricin A (another name for sakacin P) was determined for 22 of the strains, and for these strains the resistance patterns for bavaricin A and pediocin PA-1 are reported to be in complete agreement (47). However, these investigators' use of arbitrary units and the lack of individual MIC or IC50 make a comparison to the results obtained in this study difficult. Identical susceptibility patterns for class IIa bacteriocins have also been reported for a lower number of L. monocytogenes strains (20), based on a method not as highly discriminatory as the method used in this study. Identical susceptibility patterns for all the strains would have returned very high, and equal, correlation coefficients between the IC50 of the class IIa bacteriocins. We conclude that the patterns are not identical for class IIa bacteriocins, as the correlation coefficients (Table 1) vary from 0.49 to 0.84. These findings are due to the improved discrimination in determination of IC50 combined with the high number of different strains investigated.
Figure 1 shows that the IC50 for nisin is higher than the IC50 for the class IIa bacteriocins, as reported earlier (9). This is perhaps due to the different modes of action of the lantibiotics (nisin) and class IIa bacteriocins (34). It is therefore surprising that the correlation between nisin and sakacin A is higher than the correlation between sakacin P and sakacin A. Class IIa bacteriocins are generally known to be highly effective against Listeria spp. (18, 21, 44, 50). This is the first report of individual IC50 of sakacin P, sakacin A, and pediocin PA-1, expressed in nanograms per milliliter, for this high number of L. monocytogenes strains, and sakacin P is shown to be the most potent of the class IIa bacteriocins. For approximately half of the strains, the sakacin P IC50 were lower than the lowest pediocin PA-1 IC50, and nearly all of the sakacin A IC50 were higher than the maximum sakacin P IC50 (Fig. 1). This agrees with the observations of Fimland (23), who reports that sakacin P is more potent than pediocin PA-1 against many L. monocytogenes strains. Important characteristics of class IIa bacteriocins are their cysteine content and their ability to make disulfide bridges. Pediocin PA-1/AcH, enterocin A, divercin V41, sakacin G, and plantaricin 423 are known to possess two disulfide bridges, whereas the other class IIa bacteriocins contain a single disulfide bridge (18, 21, 26, 51, 56). Structure-function analyses of pediocin-like bacteriocins (24-26) show that a less flexible mutant of sakacin P, harboring an additional disulfide bridge (more like pediocin PA-1), displays lower activity against Listeria spp. than natural sakacin P. According to Fimland (23), this clearly indicates that sakacin P, the more flexible molecule, is capable of forming more favorable interactions with some Listeria sp. strains. The significance of disulfide bridges for the potency of class IIa bacteriocins may be genus or species dependent. Based on the MICs of different class IIa bacteriocins for one single L. ivanovii strain, Guyonnet et al. (32) concluded that the peptides with two disulfide bridges are significantly more effective than those harboring a single disulfide bridge. In a study of LAB and Listeria strains, the increased activities of pediocin PA-1 and enterocin A (two disulfide bridges) compared to that of sakacin P seemed to be more pronounced for LAB strains than for Listeria spp. (18). Eijsink et al. (18) suggested that the high levels of activity in pediocin PA-1 and enterocin A are at least partly due to the extra disulfide bridge. In the present study only L. monocytogenes is analyzed, and the results indicate that for this species, factors other than the number of disulfide bridges are important for the potency of the bacteriocins. Based on susceptibility testing of 200 different strains, it is clear that sakacin P is more potent than pediocin PA-1 and sakacin A against L. monocytogenes, under the experimental conditions used here.
If bacteriocins are to be used as biopreservatives in foods, development of resistance to these antimicrobial compounds has to be considered. Recently the mechanism in L. monocytogenes for high-level resistance to class IIa bacteriocins has been reported (29). None of the 200 strains examined in this study showed high-level resistance, and none of the strains could be classified as resistant. It has been reported that high-level resistance to one class IIa bacteriocin confers cross-resistance to other class IIa bacteriocins (29, 46). This study shows that spontaneous high-level resistant mutants, cross-resistant to other class IIa bacteriocins, develop also when sakacin P is the selective agent used in the agar. However, the IC50 of nisin for the high-level-resistant mutants was the same as that for the wild type strains, in agreement with other reports (17, 46). Cross-resistance between class IIa bacteriocins has been reported frequently (7, 17, 46-48), but reports concerning cross-resistance between bacteriocins of different classes contain contradictory results (7, 11, 41, 46, 47, 52, 54).
Resistance is a relative term, and there is a need for guidelines for the classification of different species according to their susceptibilities to various bacteriocins. Such guidelines should reflect the concentrations of bacteriocins used or produced in food products when bacteriocins or bacteriocinogenic strains are added as biopreservatives. Intrinsic factors affect the activity of a bacteriocin in a food product (2, 5, 27), but studies have shown that susceptibility differences measured in microtiter plate assays are comparable to differences seen for the same strains of L. monocytogenes in food model systems (37). Based on this study, sakacin P appears to be a candidate for inhibition of L. monocytogenes in food. It has been shown previously that sakacin P inhibits L. monocytogenes without development of resistant strains in food model experiments with cold smoked salmon and chicken cold cuts (36, 37). Bacteriocins can be applied as biopreservatives in many food systems. However, they should not be the only preservative principle used; rather, they should be part of a system with multiple preservative principles, so-called “hurdles” (10, 21).
Acknowledgments
We thank Liv Marit Rørvik at the Norwegian School of Veterinary Science for the L. monocytogenes strain collection and Joss Delves-Broughton for the generous gift of purified nisin. We also thank Ingolf Nes, Trond Møretrø, Inga Marie Aasen, and Askild Holck for valuable discussions, and we are grateful to Janne Beate Utåker for critical reading of the manuscript. The expert technical assistance of Consuelo Gonzales is greatly appreciated.
This work was supported by the Norwegian Research Council (grant 120859/112). BCCM/LMG is supported by the Federal Office for Scientific, Technical and Cultural Affairs of Belgium. J.S. is indebted to the Fund for Scientific Research—Flanders (Belgium) for research grants.
REFERENCES
- 1.Aase, B., G. Sundheim, S. Langsrud, and L. M. Rørvik. 2000. Occurrence of and a possible mechanism for resistance to a quaternary ammonium compound in Listeria monocytogenes. Int. J. Food Microbiol. 62:57-63. [DOI] [PubMed] [Google Scholar]
- 2.Aasen, I. M., S. Markussen, T. Møretrø, T. Katla, L. Axelsson, and K. Naterstad. Interactions of the bacteriocins sakacin P and nisin with food constituents. Int. J. Food Microbiol., in press. [DOI] [PubMed]
- 3.Axelsson, L., and A. Holck. 1995. The genes involved in production of and immunity to sakacin A, a bacteriocin from Lactobacillus sake Lb706. J. Bacteriol. 177:2125-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Axelsson, L., T. Katla, M. Bjørnslett, V. G. H. Eijsink, and A. L. Holck. 1998. A system for heterologous expression of bacteriocins in Lactobacillus sake. FEMS Microbiol. Lett. 168:137-143. [DOI] [PubMed] [Google Scholar]
- 5.Blom, H., T. Katla, B. F. Hagen, and L. Axelsson. 1997. A model assay to demonstrate how intrinsic factors affect diffusion of bacteriocins. Int. J. Food Microbiol. 38:103-109. [DOI] [PubMed] [Google Scholar]
- 6.Bouttefroy, A., M. Linder, and J. B. Millière. 2000. Predictive models of the combined effects of curvaticin 13, NaCl and pH on the behaviour of Listeria monocytogenes ATCC 15313 in broth. J. Appl. Microbiol. 88:919-929. [DOI] [PubMed] [Google Scholar]
- 7.Bouttefroy, A., and J. B. Millière. 2000. Nisin-curvaticin 13 combinations for avoiding the regrowth of bacteriocin resistant cells of Listeria monocytogenes ATCC 15313. Int. J. Food Microbiol. 62:65-75. [DOI] [PubMed] [Google Scholar]
- 8.Buyong, N., J. Kok, and J. B. Luchansky. 1998. Use of a genetically enhanced, pediocin-producing starter culture, Lactococcus lactis subsp. lactis MM217, to control Listeria monocytogenes in cheddar cheese. Appl. Environ. Microbiol. 64:4842-4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cintas, L. M., P. Casaus, M. F. Fernández, and P. E. Hernández. 1998. Comparative antimicrobial activity of enterocin L50, pediocin PA-1, nisin A and lactocin S against spoilage and foodborne pathogenic bacteria. Food Microbiol. 15:289-298. [Google Scholar]
- 10.Cleveland, J., T. J. Montville, I. F. Nes, and M. L. Chikindas. 2001. Bacteriocins: safe, natural antimicrobials for food preservation. Int. J. Food Microbiol. 71:1-20. [DOI] [PubMed] [Google Scholar]
- 11.Crandall, A. D., and T. J. Montville. 1998. Nisin resistance in Listeria monocytogenes ATCC 700302 is a complex phenotype. Appl. Environ. Microbiol. 64:231-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dalet, K., C. Briand, Y. Cenatiempo, and Y. Héchard. 2000. The rpoN gene of Enterococcus faecalis directs sensitivity to subclass IIa bacteriocins. Curr. Microbiol. 41:441-443. [DOI] [PubMed] [Google Scholar]
- 13.Dalet, K., Y. Cenatiempo, P. Cossart, and Y. Héchard. 2001. A σ54-dependent PTS permease of the mannose family is responsible for sensitivity of Listeria monocytogenes to mesentericin Y105. Microbiology 147:3263-3269. [DOI] [PubMed] [Google Scholar]
- 14.Delves-Broughton, J., P. Blackburn, R. J. Evans, and J. Hugenholtz. 1996. Applications of the bacteriocin, nisin. Antonie Leeuwenhoek 69:193-202. [DOI] [PubMed] [Google Scholar]
- 15.Duffes, F., P. Jenoe, and P. Boyaval. 2000. Use of two-dimensional electrophoresis to study differential protein expression in divercin V41-resistant and wild-type strains of Listeria monocytogenes. Appl. Environ. Microbiol. 66:4318-4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.du Toit, E. A., and M. Rautenbach. 2000. A sensitive standardised micro-gel well diffusion assay for the determination of antimicrobial activity. J. Microbiol. Methods 42:159-165. [DOI] [PubMed] [Google Scholar]
- 17.Dykes, G. A., and J. W. Hastings. 1998. Fitness costs associated with class IIa bacteriocin resistance in Listeria monocytogenes B73. Lett. Appl. Microbiol. 26:5-8. [DOI] [PubMed] [Google Scholar]
- 18.Eijsink, V. G. H., M. Skeie, P. H. Middelhoven, M. B. Brurberg, and I. F. Nes. 1998. Comparative studies of class IIa bacteriocins of lactic acid bacteria. Appl. Environ. Microbiol. 64:3275-3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ennahar, S., and N. Deschamps. 2000. Anti-Listeria effect of enterocin A, produced by cheese-isolated Enterococcus faecium EFM01, relative to other bacteriocins from lactic acid bacteria. J. Appl. Microbiol. 88:449-457. [DOI] [PubMed] [Google Scholar]
- 20.Ennahar, S., N. Deschamps, and J. Richard. 2000. Natural variation in susceptibility of Listeria strains to class IIa bacteriocins. Curr. Microbiol. 41:1-4. [DOI] [PubMed] [Google Scholar]
- 21.Ennahar, S., T. Sashihara, K. Sonomoto, and A. Ishizaki. 2000. Class IIa bacteriocins: biosynthesis, structure and activity. FEMS Microbiol. Rev. 24:85-106. [DOI] [PubMed] [Google Scholar]
- 22.Ferreira, M. A. S. S., and B. M. Lund. 1996. The effect of nisin on Listeria monocytogenes in culture medium and long-life cottage cheese. Lett. Appl. Microbiol. 22:433-438. [DOI] [PubMed] [Google Scholar]
- 23.Fimland, G. 2002. Structure-function analysis of the pediocin-like bacteriocins and the immunity proteins conferring resistance to these bacteriocins. D.Sc. thesis. University of Oslo, Oslo, Norway.
- 24.Fimland, G., O. R. Blingsmo, K. Sletten, G. Jung, I. F. Nes, and J. Nissen-Meyer. 1996. New biologically active hybrid bacteriocins constructed by combining regions from various pediocin-like bacteriocins: the C-terminal region is important for determining specificity. Appl. Environ. Microbiol. 62:3313-3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fimland, G., V. G. H. Eijsink, and J. Nissen-Meyer. 2002. Mutational analysis of the role of tryptophan residues in an antimicrobial peptide. Biochemistry 41:9508-9515. [DOI] [PubMed] [Google Scholar]
- 26.Fimland, G., L. Johnsen, L. Axelsson, M. B. Brurberg, I. F. Nes, V. G. H. Eijsink, and J. Nissen-Meyer. 2000. A C-terminal disulfide bridge in pediocin-like bacteriocins renders bacteriocin activity less temperature dependent and is a major determinant of the antimicrobial spectrum. J. Bacteriol. 182:2643-2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gänzle, M. G., S. Weber, and W. P. Hammes. 1999. Effect of ecological factors on the inhibitory spectrum and activity of bacteriocins. Int. J. Food Microbiol. 46:207-217. [DOI] [PubMed] [Google Scholar]
- 28.Gravesen, A., A. M. J. Axelsen, J. M. da Silva, T. B. Hansen, and S. Knøchel. 2002. Frequency of bacteriocin resistance development and associated fitness costs in Listeria monocytogenes. Appl. Environ. Microbiol. 68:756-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gravesen, A., M. Ramnath, K. B. Rechinger, N. Andersen, L. Jansch, Y. Héchard, J. W. Hastings, and S. Knøchel. 2002. High-level resistance to class IIa bacteriocins is associated with one general mechanism in Listeria monocytogenes. Microbiology 148:2361-2369. [DOI] [PubMed] [Google Scholar]
- 30.Gravesen, A., K. Sørensen, F. M. Aarestrup, and S. Knøchel. 2001. Spontaneous nisin-resistant Listeria monocytogenes mutants with increased expression of a putative penicillin-binding protein and their sensitivity to various antibiotics. Microb. Drug Resist. 7:127-135. [DOI] [PubMed] [Google Scholar]
- 31.Gravesen, A., P. Warthoe, S. Knøchel, and K. Thirstrup. 2000. Restriction fragment differential display of pediocin-resistant Listeria monocytogenes 412 mutants shows consistent overexpression of a putative beta-glucoside-specific PTS system. Microbiology 146:1381-1389. [DOI] [PubMed] [Google Scholar]
- 32.Guyonnet, D., C. Fremaux, Y. Cenatiempo, and J. M. Berjeaud. 2000. Method for rapid purification of class IIa bacteriocins and comparison of their activities. Appl. Environ. Microbiol. 66:1744-1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Héchard, Y., C. Pelletier, Y. Cenatiempo, and J. Frére. 2001. Analysis of σ54-dependent genes in Enterococcus faecalis: a mannose PTS permease (EIIMan) is involved in sensitivity to a bacteriocin, mesentericin Y105. Microbiology 147:1575-1580. [DOI] [PubMed] [Google Scholar]
- 34.Héchard, Y., and H.-G. Sahl. 2002. Mode of action of modified and unmodified bacteriocins from Gram-positive bacteria. Biochimie 84:545-557. [DOI] [PubMed] [Google Scholar]
- 35.Hugas, M., F. Pagès, M. Garriga, and J. M. Monfort. 1998. Application of the bacteriocinogenic Lactobacillus sakei CTC494 to prevent growth of Listeria in fresh and cooked meat products packed with different atmospheres. Food Microbiol. 15:639-650. [Google Scholar]
- 36.Katla, T., T. Møretrø, A. Holck, L. Axelsson, and K. Naterstad. 2001. Inhibition of Listeria monocytogenes in cold smoked salmon by addition of sakacin P and/or live Lactobacillus sakei cultures. Food Microbiol. 18:431-439. [Google Scholar]
- 37.Katla, T., T. Møretrø, I. Sveen, I. M. Aasen, L. Axelsson, L. M. Rørvik, and K. Naterstad. 2002. Inhibition of Listeria monocytogenes in chicken cold cuts by addition of sakacin P and sakacin P-producing Lactobacillus sakei. J. Appl. Microbiol. 93:191-196. [DOI] [PubMed] [Google Scholar]
- 38.Larsen, A. G., and B. Norrung. 1993. Inhibition of Listeria monocytogenes by bavaricin A, a bacteriocin produced by Lactobacillus bavaricus MI401. Lett. Appl. Microbiol. 17:132-134. [Google Scholar]
- 39.Liu, W., and J. N. Hansen. 1990. Some chemical and physical properties of nisin, a small-protein antibiotic produced by Lactococcus lactis. Appl. Environ. Microbiol. 56:2551-2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McAuliffe, O., C. Hill, and R. P. Ross. 1999. Inhibition of Listeria monocytogenes in cottage cheese manufactured with a lacticin 3147-producing starter culture. J. Appl. Microbiol. 86:251-256. [DOI] [PubMed] [Google Scholar]
- 41.Messens, W., and L. De Vuyst. 2002. Inhibitory substances produced by Lactobacilli isolated from sourdoughs—a review. Int. J. Food Microbiol. 72:31-43. [DOI] [PubMed] [Google Scholar]
- 42.Motlagh, A. M., S. Holla, M. C. Johnson, B. Ray, and R. A. Field. 1992. Inhibition of Listeria spp. in sterile food systems by pediocin AcH, a bacteriocin produced by Pediococcus acidilactici H. J. Food Prot. 55:337-343. [DOI] [PubMed] [Google Scholar]
- 43.Murray, M., and J. A. Richard. 1997. Comparative study of the antilisterial activity of nisin A and pediocin AcH in fresh ground pork stored aerobically at 5°C. J. Food Prot. 60:1534-1540. [DOI] [PubMed] [Google Scholar]
- 44.Nes, I. F., and H. Holo. 2000. Class II antimicrobial peptides from lactic acid bacteria. Biopolymers 55:50-61. [DOI] [PubMed] [Google Scholar]
- 45.Pot, B., P. Vandamme, and K. Kersters. 1994. Analysis of electrophoretic whole-organism protein fingerprints, p. 493-521. In M. Goodfellow and A. G. O'Donnell (ed.), Chemical methods in prokaryotic systematics. J. Wiley and Sons, Chichester, United Kingdom.
- 46.Ramnath, M., M. Beukes, K. Tamura, and J. W. Hastings. 2000. Absence of a putative mannose-specific phosphotransferase system enzyme IIAB component in a leucocin A-resistant strain of Listeria monocytogenes, as shown by two-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Appl. Environ. Microbiol. 66:3098-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rasch, M., and S. Knøchel. 1998. Variations in tolerance of Listeria monocytogenes to nisin, pediocin PA-1 and bavaricin A. Lett. Appl. Microbiol. 27:275-278. [DOI] [PubMed] [Google Scholar]
- 48.Rekhif, N., A. Atrih, and G. Lefebvre. 1994. Selection and properties of spontaneous mutants of Listeria monocytogenes ATCC 15313 resistant to different bacteriocins produced by lactic acid bacteria strains. Curr. Microbiol. 28:237-241. [Google Scholar]
- 49.Robichon, D., E. Gouin, M. Debarbouille, P. Cossart, Y. Cenatiempo, and Y. Héchard. 1997. The rpoN (σ54) gene from Listeria monocytogenes is involved in resistance to mesentericin Y105, an antibacterial peptide from Leuconostoc mesenteroides. J. Bacteriol. 179:7591-7594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rodriguez, J. M., M. I. Martinez, and J. Kok. 2002. Pediocin PA-1, a wide-spectrum bacteriocin from lactic acid bacteria. Crit. Rev. Food Sci. Nutr. 42:91-121. [DOI] [PubMed] [Google Scholar]
- 51.Simon, L., C. Fremaux, Y. Cenatiempo, and J. M. Berjeaud. 2002. Sakacin G, a new type of antilisterial bacteriocin. Appl. Environ. Microbiol. 68:6416-6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Song, H.-J., and J. Richard. 1997. Antilisterial activity of three bacteriocins used at sub-minimal inhibitory concentrations and cross-resistance of the survivors. Int. J. Food Microbiol. 36:155-161. [DOI] [PubMed] [Google Scholar]
- 53.Stiles, M. E. 1996. Biopreservation by lactic acid bacteria. Antonie Leeuwenhoek 70:331-345. [DOI] [PubMed] [Google Scholar]
- 54.Szabo, E. A., and M. E. Cahill. 1998. The combined affects of modified atmosphere, temperature, nisin and ALTATM 2341 on the growth of Listeria monocytogenes. Int. J. Food Microbiol. 43:21-31. [DOI] [PubMed] [Google Scholar]
- 55.Vadyvaloo, V., J. W. Hastings, M. J. van der Merwe, and M. Rautenbach. 2002. Membranes of class IIa bacteriocin-resistant Listeria monocytogenes cells contain increased levels of desaturated and short-acyl-chain phosphatidylglycerols. Appl. Environ. Microbiol. 68:5223-5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van Reenen, C., M. Chikindas, W. Van Zyl, and L. Dicks. 2003. Characterization and heterologous expression of a class IIa bacteriocin, plantaricin 423 from Lactobacillus plantarum 423, in Saccharomyces cerevisiae. Int. J. Food Microbiol. 81:29-40. [DOI] [PubMed] [Google Scholar]
- 57.Verheul, A., N. J. Russell, R. Van'T Hof, F. M. Rombouts, and T. Abee. 1997. Modifications of membrane phospholipid composition in nisin-resistant Listeria monocytogenes Scott A. Appl. Environ. Microbiol. 63:3451-3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vos, P., R. Hogers, M. Bleeker, M. Reijans, T. Vandelee, M. Hornes, A. Frijters, J. Pot, J. Peleman, M. Kuiper, and M. Zabeau. 1995. AFLP—a new technique for DNA fingerprinting. Nucleic Acids Res. 23:4407-4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wan, J., K. Harmark, B. E. Davidson, A. J. Hillier, J. B. Gordon, A. Wilcock, and M. J. Coventry. 1997. Inhibition of Listeria monocytogenes by piscicolin 126 in milk and Camembert cheese manufactured with a thermophilic starter. J. Appl. Microbiol. 82:273-280. [DOI] [PubMed] [Google Scholar]
- 60.Wan, J., M. W. Hickey, and M. J. Coventry. 1995. Continuous production of bacteriocins, brevicin, nisin and pediocin, using calcium alginate-immobilized bacteria. J. Appl. Bacteriol. 79:671-676. [Google Scholar]
- 61.Yan, L., A. Gibbs, M. Stiles, D. Wishart, and J. Vederas. 2000. Analogues of bacteriocins: antimicrobial specificity and interactions of leucocin A with its enantiomer, carnobacteriocin B2, and truncated derivatives. J. Med. Chem. 43:4579-4581. [DOI] [PubMed] [Google Scholar]