Abstract
Results of a recent study of antibiotic resistance genes in human colonic Bacteroides strains suggested that gene transfer events between members of this genus are fairly common. The identification of Bacteroides isolates that carried an erythromycin resistance gene, ermG, whose DNA sequence was 99% identical to that of an ermG gene found previously only in gram-positive bacteria raised the further possibility that conjugal elements were moving into Bacteroides species from other genera. Six of seven ermG-containing Bacteroides strains tested were able to transfer ermG by conjugation. One of these strains was chosen for further investigation. Results of pulsed-field gel electrophoresis experiments showed that the conjugal element carrying ermG in this strain is an integrated element about 75 kb in size. Thus, the element appears to be a conjugative transposon (CTn) and was designated CTnGERM1. CTnGERM1 proved to be unrelated to the predominant type of CTn found in Bacteroides isolates—CTns of the CTnERL/CTnDOT family—which sometimes carry another type of erm gene, ermF. A 19-kbp segment of DNA from CTnGERM1 was cloned and sequenced. A 10-kbp portion of this segment hybridized not only to DNA from all the ermG-containing strains but also to DNA from strains that did not carry ermG. Thus, CTnGERM1 seems to be part of a family of CTns, some of which have acquired ermG. The percentage of G+C content of the ermG region was significantly lower than that of the chromosome of Bacteroides species—an indication that CTnGERM1 may have entered Bacteroides strains from some other bacterial genus. A survey of strains isolated before 1970 and after 1990 suggests that the CTnGERM1 type of CTn entered Bacteroides species relatively recently. One of the genes located upstream of ermG encoded a protein that had 85% amino acid sequence identity with a macrolide efflux pump, MefA, from Streptococcus pyogenes. Our having found >90% sequence identity of two upstream genes, including mefA, and the remnants of two transposon-carried genes downstream of ermG with genes found previously only in gram-positive bacteria raises the possibility that gram-positive bacteria could have been the origin of CTnGERM1.
The human intestinal tract is thought to be a site in which horizontal transfer of genes might occur fairly often. The concentration of bacteria in the colon is high, and factors such as the availability of abundant nutrients and surfaces on which biofilms could form would seem to be conducive to gene transfer (8, 11). It has been difficult to test this hypothesis, however, because of limited information about gene transfer elements in the predominant genera of colon bacteria. The numerically predominant bacteria in the colon are all obligate anaerobes, including Bacteroides species and a mixture of gram-positive genera (8). Virtually nothing is known about gene transfer elements of the gram-positive anaerobes, but some of the Bacteroides gene transfer elements have been characterized (13). Accordingly, attempts to assess the extent to which horizontal gene transfer occurs in the colon have focused on Bacteroides species.
Many Bacteroides strains carry plasmids, but a family of self-transmissible integrated elements called conjugative transposons (CTns) seems to be particularly widespread and may be the primary drivers of horizontal gene transfer in this genus (13, 18). These CTns are exemplified by CTnDOT and CTnERL, two CTns that are virtually identical except for a 13-kbp region in CTnDOT that contains an erythromycin resistance gene, ermF (23). Both CTnERL and CTnDOT carry the tetracycline resistance gene tetQ, and transfer of both of these CTns is stimulated 1,000- to 10,000-fold by exposure of donors to tetracycline (12, 13, 24). More than 80% of Bacteroides strains isolated since 1980 harbor one or more of these CTns, compared to 22 to 30% of strains isolated prior to 1970 (18). Clearly, these CTns have been in Bacteroides strains for some time and are continuing to spread actively within the genus. These data do not, however, address the question of the extent to which horizontal gene transfer might be introducing new DNA elements into Bacteroides species.
A recent survey of 289 Bacteroides isolates showed that, as expected, ermF was the predominant erythromycin (MLSB) resistance gene in Bacteroides species (18, 23). Nonetheless, nine isolates were identified that contained ermG—a class of erm gene that has been found previously only in a gram-positive bacterium, Bacillus sphaericus (7). The DNA sequences of the ermG genes found in the Bacteroides isolates were over 99% identical to the DNA sequence of the Bacillus sphaericus gene (3). Finding virtually identical antibiotic resistance genes in two such distantly related genera suggested that ermG is being transmitted horizontally, possibly from gram-positive bacteria into Bacteroides species. Moreover, since the ermG was found primarily in Bacteroides strains isolated after 1990, it was clear that ermG had come into Bacteroides species fairly recently—presumably from some other genus (3).
Broad host range transfers usually occur by conjugation, a process thought to help DNA escape restriction modification systems in the recipients, but whether ermG was on a conjugative element was not known. We report here that the ermG gene is carried on an integrated conjugative element, a CTn, which is different from CTnERL and CTnDOT and may have entered Bacteroides species much more recently than did the CTnERL- and CTnDOT-type elements. Our findings provide additional evidence which suggests that the exchange of DNA in the human colon may involve genera other than Bacteroides.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and DNA manipulations.
Strains and plasmids used in this study are described in Table 1, and the ermG-containing strains are described in Table 2. The strain designated WH was isolated from sewage by Caroline Plugge, a student in the microbial diversity summer course at the Marine Biological Laboratory (Woods Hole, Mass.). All other strains were clinical isolates obtained from various sources in the United States: DH indicates strains obtained from David Hecht, Loyola VA Hospital, Maywood, Ill.; BF6436-5 was obtained from the Yale Medical Center, and the other strains were obtained from Sydney Finegold, Wadsworth Anaerobe Laboratory, Los Angeles, Calif. (18). Species identifications were made by phenotypic testing in the laboratory of origin or by partial sequencing of the 16S rRNA gene in our laboratory or both. The species identity of one of the strains, WH713, has not been determined.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant phenotypesa | Description and source or reference |
|---|---|---|
| Bacterial strains | ||
| E. coli | ||
| DH5α MCR | RecA− McrA− McrB− Mrr− HsdM− HsdR− | Gibco BRL |
| S17-1 | RecA− Tpr TraRP4 | Contains the transfer functions of RP4 integrated in the chromosome (20) |
| B. thetaiotaomicron BT5482 strains | ||
| BT4001 | Rifr | Rifr derivative of BT5482A |
| BT4100 | Thy− Tpr | Spontaneous thymidine-requiring mutant of BT5482A |
| BT4007 | Rifr Emr Tcr | BT4001 with CTnDOT in the chromosome |
| BT4004 | Rifr Tcr | BT4001 with CTnERL in the chromosome |
| BT4009 | Rifr Emr | BT4001 with CTn7853 in the chromosome |
| BT4001ΩWH504 | Rifr Emr | BT4001 with a self-transmissible element from WH504 that confcrs low-level Emr (∼3 μg/ml) but with no known erm gene detected (this study) |
| Plasmids | ||
| pLYL001 | Tcr (Apr) | Bacteroides suicide vector that contains the oriTRK2 and tetQ cloned into pUC19; can be mobilized by IncPα plasmid RP1 or the RP4 transfer functions in E. coli S17-1 (10) |
| pYP01 | Tcr (Apr) | The 442-bp internal ermG PCR was blunted and cloned into the HincII site of pLYL001 (this study) |
| pGRW6 | Tcr (Apr) | 5-kb fragment downstream of ermG was cloned into pLYL001 by plasmid rescue of the PstI- digested chromosome DNA (this study) |
| pGRW8 | Tcr (Apr) | 10-kb fragment upstream of ermG was cloned into pLYL001 by plasmid rescue of the BamHI-digested chromosome DNA (this study) |
| pGW47 | (Knr) | A 3.0-kb AatII/NarI fragment containing the Bacteroides pBI143 rep and mob regions was purified from pFD160R (21), blunted, and then cloned into the SspI site on the p15A derivative pK184 (6) to form a low-copy-number E. coli-Bacteroides shuttle vector (G. Whittle) |
| pYP66 | (Knr) | 4.9-kb PstI-EcoRV fragment of CTnGERM1 containing mefA was cloned into the PstI-SmaI site of pGW47 to look for expression of mefA-encoded erythromycin resistance in Bacteroides hosts (this study) |
Phenotypes in parentheses are expressed only in E. coli, and phenotypes outside parentheses are expressed in Bacteroides strains. Abbreviations: Ap, ampicillin; Cm, chloramphenicol; Em, erythromycin; Gen, gentamicin; Kn, kanamycin; Rif, rifampin; Spec, spectinomycin; Tc, tetracycline; Thy−, thymidine auxotroph; Tp, trimethoprim.
TABLE 2.
Characteristics of nine Bacteroides ermG-containing strains
| Strain | Species | Genotypea | Phenotypeb | Groupc | Transfer frequenciesd
|
Retransfer frequenciesd
|
Upstream of ermGe homology | ||
|---|---|---|---|---|---|---|---|---|---|
| ermG | tetQ | ermG | tetQ | ||||||
| Bov7991 | B. ovatus | QGDNB | TE | I | 0/0 | 10−6/10−4 | NR | NT | − |
| BT7853 | B. thetaiotaomicron | QG | TE | II | 10−5/10−5 | 10−5/10−5 | 10−5/10−5 | 10−5/10−5 | + |
| DH4083 | B. fragilis | QGDN | TE | II | NT | NT | NT | NT | − |
| DH4072 | B. thetaiotaomicron | QG | TEA | II | 10−6/10−5 | 10−6/10−5 | 10−6 | 10−6 | + |
| DH3716 | B. ovatus | QGDN | TEA | III | 10−5/10−5 | 0/0 | 10−4 | NR | + |
| DH3717 | B. distasonis | QGDN | TEA | III | 10−5/10−6 | 0/0 | NT | NR | + |
| DH4140 | B. thetaiotaomicron | QGDN | TEA | III | NT | NT | NT | NT | + |
| WH713 | Bacteroides sp. | QGDN | TE | III | 10−7/10−7 | 10−8/10−7 | NT | NT | + |
| BF6436-5 | B. fragilis | QGDN | TE | IV | 10−6/10−6 | 0/0 | 10−7 | NR | + |
Genotype determined by Southern blot or dot blot hybridization to specific probes (18): Q, tetQ gene; G, ermG gene; D, CTnDOT joined ends; N, NBU1 primase and mob gene area; B, ermB gene.
Phenotype: T, tetracycline resistance (>1 μg/ml); E, erythromycin resistance (>3 μg/ml); A, ampicillin resistance (>50 μg/ml).
Group classification is based on the sequence identity of the PCR products of internal regions of the ermG genes relative to that of the ermG of Bacillus sphaericus (18).
Frequencies are given as transconjugants per recipient at the end of the mating: the transfer frequency of ermG or tetQ without Tc induction/with Tc (1 μg/ml) induction. Definitions of notations used are as follows: 0/0, <10−9; NR, not relevant (gene not present in donor); NT, not tested.
The + indicates that both the original strain and the BT4001 transconjugants hybridized to the probes made from 10-kb regions upstream of ermG of CTnGERM1 from DH3716; the − indicates the lack of same.
The methods used for cultivation of Bacteroides strains, DNA isolation, and cloning have been described previously (14, 16). The antibiotic concentrations used were as follows: ampicillin, 50 to 100 μg/ml; erythromycin (Em), 3 to 10 μg/ml; gentamicin (Gen), 200 μg/ml; tetracycline (Tc), 3 μg/ml; rifampin (Rif), 10 μg/ml; and trimethoprim (Tp), 100 μg/ml. The concentration of thymidine (Thy) was 100 μg/ml.
Mating experiments.
To test whether an ermG gene was carried on a transmissible element, mating experiments were performed as described previously for the CTnERL- and DOT-type CTns (16). Thy-requiring spontaneous mutants of the ermG-containing Bacteroides strains served as donors, and the B. thetaiotaomicron 5482A Rif-resistant strain BT4001 served as the recipient (17). The transconjugants were selected on medium containing Gen, Em, and Rif—and no Thy. Since tetQ was coresident in all of the donors, the transconjugants were tested for cotransfer of the tetracycline resistance. To confirm that ermG was on a self-transmissible element, the resulting Emr BT4001 transconjugants were used as donors in filter matings to BT4100 (Thy−,Tpr, and Rifs) recipients. The secondary transconjugants were selected on medium containing Gen, Em, Thy, and Tp and patched onto Rif plates to identify any Thy mutations of the donors. The ermG element from strain DH3716 was chosen for further study because it transferred only ermG and not tetQ—a trait which suggested that it might be a new type of Bacteroides CTn. Four of the six strains transferred ermG constitutively (i.e., they required no growth in an antibiotic for induction), and the transfer was independent of the tetQ in the donor.
PFGE.
To determine whether the DH3716-derived element was an integrated element, and if so, whether it integrated site specifically, pulsed-field gel electrophoresis (PFGE) analysis was performed. In six independent conjugation experiments, Thy− strain DH3716 was mated with a BT4001 recipient. Two transconjugants from each of the six matings (total, 12 colonies) were inoculated into 10 ml of Trypticase-yeast extract-glucose. Cells were pelleted by centrifugation and lysed in situ in an agarose plug (14). The plugs were treated with lysozyme to lyse the cells, and proteinase K was added to inactivate nucleases before loading the plug onto a 1% agarose gel.
NotI digestion (40 U per plug) produced 12 well-spaced bands. The PFGE was performed according to the manufacturer's instructions (CHEF-DR III; Bio-Rad). NotI-digested DNA from the 12 independent primary transconjugants, NotI-digested DNA from strain BT4001, and Saccharomyces cerevisiae DNA standards were loaded onto the pulsed-field gel. The gel was run at 6 V/cm for 24 h with a switching angle of 120o and a switching time of 50 to 130 s.
Cloning segments of the ermG element by using plasmid rescue.
Initial attempts to clone segments of the ermG element by constructing a cosmid library of Bacteroides chromosomal DNA and probing it with an ermG probe were unsuccessful. As an alternative strategy, we turned to a plasmid rescue approach. A 442-bp PCR product internal to ermG was cloned into the SmaI site of a Bacteroides suicide vector pLYL001 (10) in order to produce pYP01. pYP01 was then transformed into E. coli (S17-1), which contains the transfer regions of the IncPα plasmid RP4 (17). Filter mating between S17-1(pYP01) and a BT4001 transconjugant containing the CTnGERM1 element was performed. pYP01 integrates into ermG in the BT4001ΩCTnGERM1 recipient by homologous recombination. The transconjugants were selected on medium containing Gen and Tc. The chromosomal DNA of a Genr Tcr transconjugant was digested with BamHI, and a 16-kb fragment containing the vector and DNA adjacent to the ermG was isolated from low-melting-point agarose gel. DNA from this fraction was treated with T4 DNA ligase, which was used to transform Escherichia coli MCR. Transformants containing the plasmid and adjacent DNA sequences were isolated by selection for ampicillin resistance. A 16-kb plasmid, pGRW8, was isolated and was shown to contain pLYL001 (6 kb) and 10 kb of chromosomal DNA from the ermG element, which was later used as a probe. To clone more DNA from the element, the distal end of this 10-kb fragment was subcloned into pLYL001 and the process was repeated. A total of 19 kb from the regions adjacent to the ermG gene were cloned and sequenced.
DNA sequence of a 19-kb region adjacent to ermG.
By using an Applied Biosystems model 373A version 2.0.1A dye terminator automated sequencer, workers at the University of Illinois Biotechnology Genetic Engineering Facility performed DNA sequencing. The primers were synthesized by workers at the University of Illinois Biotechnology Genetic Engineering Facility or at Operon Technologies, Inc. (Alameda, Calif.).
Southern hybridization analysis.
Southern blot analysis was used to identify the band containing the ermG element in the PFGE gels and to determine the restriction fragment length polymorphisms of the various ermG transmissible elements integrated in strain BT4001 relative to CTnGERM1 probes. In both cases, the chromosomal DNAs from the agarose gels were transferred to nylon membrane filters and Southern hybridization was performed according to the previously described protocol (14). Probes were made from cloned DNA segments of CTnGERM1 and were labeled with fluorescein-dUTP by using random primers as specified in the NEN Life Sciences Renaissance Kit protocol. The Southern blots were developed by using a chemiluminescent substrate according to instructions provided by the manufacturer.
Nucleotide sequence accession number
The sequence of the 19-kb ermG region has been submitted to the EMBL nucleotide sequence database; the accession number is AJ557257.
RESULTS
Characteristics of nine ermG-containing Bacteroides strains.
Previously, the nine ermG-containing Bacteroides strains identified in our survey of Bacteroides isolates had been divided into groups (I to IV) based on small sequence differences (1 to 4 bp within a 442-bp region) between the ermG alleles (18). The ermG genes were found in strains of different Bacteroides species (Table 2), thus indicating that they might be located on transmissible elements. At least one strain from each group was chosen to test for conjugal transfer of the ermG gene in a mating experiment in which the original isolate was the donor and a Bacteroides thetaiotaomicron strain, BT4001, was the recipient. Characteristics of the ermG strains and the results of the filter mating experiments are shown in Table 2.
The group II strains, BT7853 and DH4072, cotransferred tetQ and ermG to strain BT4001, suggesting that the tetQ and the ermG genes in these strains were carried on the same mobile element. However, the group III strains, WH713, DH3716, and DH3717, and a group IV strain, BF6436-5, transferred the ermG gene independently of the coresident tetQ gene, suggesting that in these strains the mobile elements carried only the ermG gene and might thus be a novel element different from CTnERL and CTnDOT. One of these strains, DH3716, was chosen for further study. As shown in Table 2, the ermG element in DH3716 was able to retransfer in a separate mating from a BT4001 transconjugant to a BT4100 recipient and thus appears to be self-transmissible and therefore not mobilized by a CTnDOT-like element. The CTnDOT- type elements have been shown previously to mobilize a variety of plasmids and integrated elements (13). Another indication that the ermG element from DH3716 was not mobilized by a CTnDOT-type element was that the transfer of the ermG gene from DH3716 to BT4001 was not enhanced by treating the donor DH3716 with Tc. That the transfer of CTnDOT itself and of mobilization of plasmids and integrated elements such as NBU1 and NBU2 by CTnDOT is seen only after exposure of donors to Tc is typical, so this was a difference between the ermG element and CTnDOT (12). Also, the transfer frequency of the ermG element was not enhanced by exposure of donors to Em. Transfer frequencies were about 10−5 transconjugants per recipient under all conditions tested.
The ermG element from DH3716 is about 75 kb in size and integrates into the chromosome site selectively.
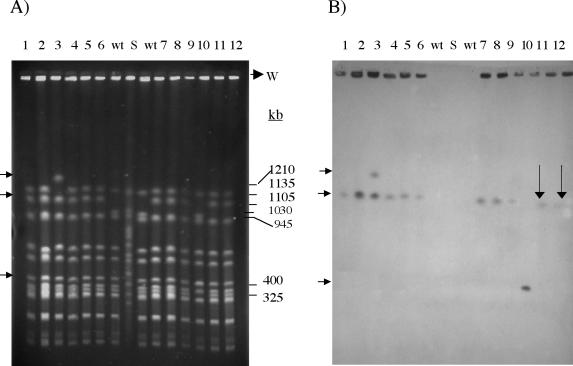
Both the donor DH3716 and the recipient BT4001 contained a 35-kb plasmid, p5482. This plasmid does not encode Em resistance, since BT4001 (which carries p5482) does not grow in the presence of 1 μg of Em per ml; therefore, the plasmid in these strains did not carry ermG. Furthermore, the ermG probe did not hybridize to the plasmid DNA preparations from either the donor or the BT4001 transconjugants, thus indicating that the ermG element was not a plasmid but instead might be integrated into the chromosome. To test the hypothesis that the ermG element was an integrated element, PFGE was performed to compare NotI digestion patterns of DNA from 12 independent transconjugants—obtained in matings between DH3716 and BT4001—to the NotI digestion pattern of the recipient BT4001 strain. Band shifts were observed in all transconjugants (Fig. 1A). In 10 of 12 transconjugants (83%), a band shifted in size from 1,030 to 1,105 kb. The increase in the size of the shifted band indicated that the integrating element was about 75 kb in size. DNA from the transconjugant in lane 3 exhibited two band shifts. Although one of the two shifts was the same as the other 10, the second shift involved a different band—suggesting that the ermG element integrated into a secondary site. Both of the shifts correspond to an increase in size of about 75 kb. Lane 10 shows the third band shift pattern. In this case, too, there was an increase of about 75 kb. Southern hybridization analysis demonstrated that all of the shifted NotI bands seen in Fig. 1A hybridized with the ermG probe and thus clearly contained the ermG element (Fig. 1B). This ermG element is an integrating, transmissible genetic element and thus appears to be a CTn. Since it carries the ermG gene, it was named CTnGERM1.
FIG. 1.
Results of PFGE and Southern blotting to determine the size and insertion sites of CTnGERM1. (A) Ethidium bromide-stained pulsed-field gel of BT4001 Emr transconjugants. The DNA from 12 ermG transconjugants, two from each of six matings between BT4001 and DH3716, was digested with NotI and run on a pulsed-field gel (lanes 1 to 12). S indicates S. cerevisiae DNA size standards. The sizes of the bands are given in kilobase pairs at the right side of the gel. Compared to the band sizes seen for BT4001, the transconjugants 1 to 9, 11, and 12 all have a band that shifted from 1,030 to 1,105 kb (arrow on left). Transconjugant 3 has an additional band that shifted from 1,135 to 1,210 kb (arrow on left). Transconjugant 10 has a band that shifted from 325 to 400 kb (arrow on left). In each case, the larger bands are 75 kb larger than the missing smaller bands, thus indicating that the integrated element is about 75 kb in size. (B) The Southern blot of the pulsed-field gel. The blot was probed with the 2.8-kb SacI-SacI ermG-containing fragment (probe F in Fig. 2). In all cases, the ermG-hybridizing bands were the ones that shifted in molecular weight. The two arrows indicate the positions of two bands that were stronger after longer exposure of the Southern blot. The three arrows on the left indicate the same band positions as the arrows in panel A.
Sequence analysis of CTnGERM1 DNA in the ermG gene region.
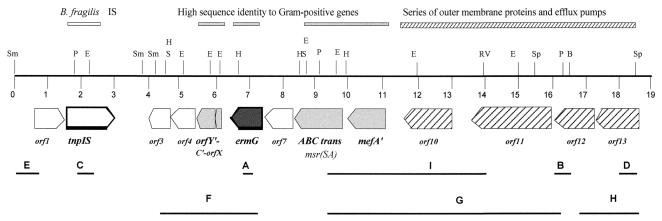
To assess the possible origin of CTnGERM1 and to ascertain whether the other ermG-containing strains carried similar CTns, we cloned 19 kb of DNA from the region containing ermG by using the plasmid rescue strategy described in Materials and Methods and obtained the complete DNA sequence of this region. The BLAST search results are summarized in Fig. 2 and Table 3. Thirteen putative ORFs were identified. According to the BLAST search results, tnpIS encodes a protein that has 90% identity with the transposase of B. fragilis IS613 (accession number BAA95632). There is 92% identity at the nucleotide level, suggesting that this is a Bacteroides insertion element transposase. Since there were 20 amino acids (aa) missing from the N-terminal end of the TnpIS protein, it was not clear if it was functional and possibly contributing to the transfer of the element. This gene was disrupted by single-crossover disruption using an internal fragment of the gene (C in Fig. 2). Southern blots were performed to confirm the result (data not shown). The transfer frequency of this mutant was the same as that of wild-type CTnGERM1 (i.e., ∼10−5 transconjugants per recipient). Thus, this putative transposase is not essential for the transfer of CTnGERM1. There are no copies of this insertion element transposase gene in the B. thetaiotaomicron 5482A genome that were detectable by Southern blot hybridization.
FIG. 2.
Schematic diagram of the 19-kb region isolated from CTnGERM1. Restriction enzymes shown include SmaI (Sm), PstI (P), EcoRI (E), SacI (S), BamHI (B), SphI (Sp), HindIII (H), and EcoRV (RV). Size in kb is indicated by numbers from 0 to 19. Fragments A, B, C, D, and E were used either as inserts cloned into pLYL001 for plasmid rescue or as probes for hybridization to the chromosome DNA. Fragment F was used as the probe for detecting the ermG-containing bands in Fig. 1B. Fragments G and H were used as probes for detecting the distribution of the upstream ermG multidrug resistance region in Bacteroides strains. Fragment I, a 4.9-kb PstI-EcoRV fragment including the mefA, was cloned into pGW47 to determine whether this fragment contained a gene that was responsible for low-level erythromycin resistance in Bacteroides hosts. Description of the functions and sequence identities are indicated by the bars above the map. Genes in light gray are homologs to known gram-positive genes, the striped arrows indicate the genes that are homologs to efflux and membrane proteins involved in antibiotic resistance mechanisms, and the putative Bacteroides IS transposase is indicated.
TABLE 3.
Sequence analysis of 19-kb region of CTnGERM1
| Location bp-bp 5′-3′ | ORF or designation | No. of aa | % aa identity (no. of aa involved/total no. of aa) | Results and descriptions of BLAST searches (accession no.) |
|---|---|---|---|---|
| 1009-1368 | 1 | 119 | 32 (16/50) | Rhizobium melioti NodM (606 aa) homology in C-terminal end (X58632) |
| 1556-2785 | tnpIS | 409 | 90 (369/409) | Bacteroides fragilis transposase (428 aa) of IS613 and closely related IS elements in imipenem-resistant strains (BAA95632.1). This protein is missing first 20 aa. There is 92% identity at the nucleotide level. Contains transposase DDE domain of the IS4 group. There are other related Bacteroides insertion elements. |
| 4006-4240 | 3 | 121 | 35 (33/93) | B. fragilis Orf9 (481 aa) of unknown function located on mobilizable transposon Tn4555 (AAD43601) |
| 5259-4764 | 4 | 164 | 58 (94/162) | Desulfitobacterium hafniense (181 aa) hypothetical protein (00098450.1); Clostridium acetobutylicum HD superfamily hydrolase (163 aa, AE007856). The translated orf4 protein has the HD and HDc motifs for metal-dependent phosphohydrolases. |
| 5260-6290 | orfY′ and C′-orfX | 230 | 85 (204/243) | Staphylococcus intermedius OrfY (244 aa) of unknown function. Associated with ermB and other antibiotic resistance genes (AF299292). The nucleotide identity is ∼92%. There is a frameshift in the sequence. The 220 nucleotides of the 3′ end of the upstream orfX (unknown function) are also present. These genes are also found on the Enterococcus faecium plasmid pUW786 (17,510 bp) that contains ermB and vancomycin resistance genes (AF516335). OrfX and OrfY are carried on Tn5404/Tn5405 originally isolated in Staphylococcus (4). |
| 6631-7365 | ermG | 244 | 99 (236/239) | Bacillus sphaericus ErmG (244 aa) on a plasmid (M15332). >99% identical at the nucleotide level. Also found on Bacteroides CTn7853 (L42817). |
| 7366-7860 | 7 | 125 | 33 (50/161) | Bacillus firmus glutamine-rich protein (AAB05373.1) |
| 9420-8137 | ABC transporter or mel | 428 | 97 (414/427) | Streptococcus pneumoniae Mel (487 aa), a homolog of the product of the macrolide-streptogramin B efflux resistance gene msr(SA) (AAK69028). Has 96% nucleotide identity. Called an ABC transporter or orf5 on Tn1207.1 (15). Located downstream of mefA. Protein missing N-terminal end due to nonsense mutations. |
| 10854-9922 | mefA | 310 | 86 (248/287) | Streptococcus pyogenes MefA (405 aa) macrolide-cfflux protein (AAC44785). 94% nucleotide identity. There are two stop codons in the end corresponding to the N terminus. Also found on Tn1207.1 in an S. pneumoniae clinical strain (15) situated upstream of ABC transporter or mel. This MefA protein is missing the N-terminal end due to nonsense mutations. |
| 12895-11537 | orf10 | 452 | 38 (179/472) | Chlorobium tepidum FusA/NodT (475 aa) family of multidrug-resistant proteins (AAM72516.1). Contains To1C and OEP and outer membrane and efflux motifs. |
| 15937-13490 | orf11 | 815 | 46 (378/821) | Chlorobium tepidum AcrB/AcrD (1,063 aa) family of multidrug-resistant proteins (AAM72517.1). Has the motif of the AcrB/AcrD/AcrF family of proteins associated with antibiotic resistance due to efflux pumps. |
| 17127-16101 | orf12 | 373 | 34 (128/367) | Chlorobium tepidum AcrA/AcrE (396 aa) family of multidrug-resistant proteins (AAM72518.1). Has a motif contained by AcrA, HlyD, and EmrA families of proteins involved with membrane fusion, secretion, and multidrug resistance efflux. |
| 18373-17171 | orf13 | 400 | N terminus, 32 (59/182); C terminus, 33 (55/164) | N-terminal end B. subtilis TagO (358 aa) tcichoic acid linkage unit synthesis (B69721). C-terminal of end acetyltransferase (GNAT) family YnaD (170 aa) B. subtilis (AAB41084.1). Contains motifs for two families of proteins: Rfe, UDP-N-acetylmuramyl pentapeptide phosphotransferase, and RimL, acetyltransferases. |
Strain BT4001 containing CTnGERM1 could grow in medium containing erythromycin concentrations as high as 200 μg/ml. A disruption of the ermG gene caused by using fragment A in Fig. 2 was made to determine whether this gene was solely responsible for the Emr phenotype. The mutant with the disrupted ermG gene still exhibited a low level of erythromycin resistance (MIC of 5 μg/ml, compared to <0.2 μg/ml for strain BT4001). This result indicates that although most of the resistance to Em is due to ermG, CTnGERM1 may carry a second Emr gene that has not yet been identified. This is presumably an erm-type gene because it confers resistance to lincosamide as well as erythromycin. If it is an erm gene, we have not yet identified it in our probe analyses as belonging to any of the known erm gene classes (18).
Six contiguous open reading frames (ORFs) of the 13 ORFs (bp 8,137 to the end) encoded putative proteins that had amino acid similarity to multidrug resistance efflux proteins (Table 3 and Fig. 2). The ABCtrans and mefA homologs had over 90% sequence identity with the genes found in Streptococcus pyogenes and S. pneumoniae. The protein translations of the ORFs indicated that there were some nonsense mutations that truncated the products or deleted the N-terminal ends in some cases (see Table 3). The fact that all of these ORFs were transcribed in the same direction raised the possibility that this collection of genes might be an integron, but the typical conserved sequence (GTTRRRY), usually found between cassettes of classical integrons (5), was not found between these ORFs. There was also no evidence of repeated sequences flanking the genes that had high nucleotide sequence identity with gram-positive genes (Table 3 and Fig. 2), although most of them are found on transposons and plasmids in Staphylococcus, Enterococcus and Streptococcus hosts (1, 4, 15).
The mefA on CTnGERM encoded a protein that had 86% amino acid sequence identity and 94% nucleotide sequence identity with a macrolide efflux protein (MefA, accession number AAC44785) of Streptococcus pyogenes. To determine whether this mefA was responsible for the low level of Emr seen in the absence of ermG, a 4.9-kb PstI-EcoRV fragment (fragment I, Fig. 2) that contained mefA and orf10 and which had homology to an efflux protein (Table 3) was cloned into a shuttle vector pGW47 to produce pYP66. S17-1(pYP66) was used as a donor to transfer pYP66 to BT4001. If the CTn-MefA was responsible for the residual Emr that was not attributable to ermG, transconjugants resistant to 3 to 5 μg of Em per ml should have been isolated. No Emr transconjugants were isolated (<10−8 per recipient). Thus, neither of the genes in this region contributes to the Emr seen in the ermG disruption strain.
More than one copy of CTnGERM1 can coexist in the same strain.
In transconjugant 3 (txg3 in lanes 3 of Fig. 1), two ermG elements had integrated in the same host. Southern blot analysis was performed to test whether these two elements were copies of CTnGERM1 or different elements. Restriction enzymes EcoRV and HindIII were used to digest txg3 and other transconjugants, which contain only one copy of CTnGERM1. Probes were made from the 19-kb fragment cloned from CTnGERM1. The digestion patterns of the two elements in txg3 were exactly the same as those seen in the transconjugants containing only one copy of CTnGERM1 (data not shown). Thus, there are two copies of CTnGERM1 integrated into the chromosome of txg3.
The ermG genes in other Bacteroides strains are associated with an element related to CTnGERM1.
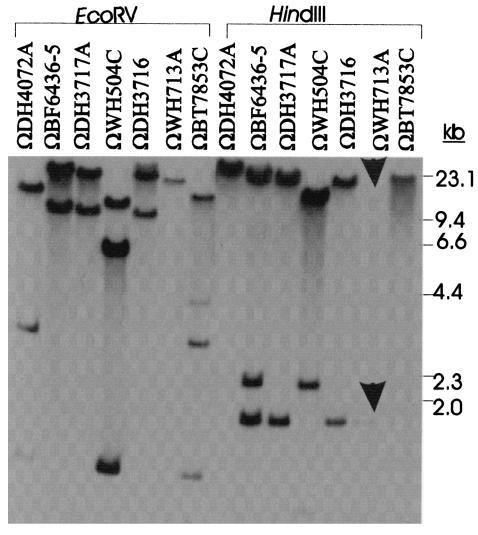
In our initial analysis of the four strains that were capable of transferring ermG without cotransferring tetQ (i.e., DH3716, DH3717, WH713, and BF6436-5), we had transferred all of the ermG elements into strain BT4001. To determine whether these four transmissible elements were all related to CTnGERM1, chromosomal DNA from the four ermG-containing transconjugants was digested with two different restriction enzymes, EcoRV and HindIII. Southern blot analysis was performed with fragment G (Fig. 3) used as the probe. Probe G (Fig. 2) was used to eliminate ermG sequences, which had already been shown to be present in all ermG strains. In all cases, the upstream region probe hybridized to DNA in the transconjugants, thus indicating that all four elements were related to CTnGERM1. This was true even of the two elements that cotransferred tetQ and ermG from BT7853 and DH4072. Although the different transconjugants contained DNA that was clearly related to DNA from CTnGERM1 from DH3716, the restriction patterns differed in the case of at least one restriction enzyme—except for the ermG element in DH3717. This indicates that even though the CTnGERM elements seem to have entered Bacteroides species only recently, either they are mutating rapidly or different variants have moved into Bacteroides species in multiple transfer events.
FIG. 3.
Southern blot analysis of BT4001 transconjugants from different donors. The Southern blot analysis was done on EcoRV- and HindIII-digested chromosomal DNA from six ermG-containing BT4001 transconjugants and an Emr transconjugant from WH504, BT4001ΩWH504. The blot was probed with fragment G, which contains sequences upstream of the ermG on CTnGERM1 (Fig. 2). The two arrows indicate the positions of two bands, which were more visible after longer exposure time of the Southern blot.
The ermG gene may be capable of moving as a cassette.
In previous studies, a CTn that cotransferred tetQ and ermG had been identified (9). This CTn was designated CTn7853 and is the ermG element that transfers out of BT7853 (ΩBT7853 in Fig. 3). A small amount of DNA around the ermG gene in CTn7853 had been cloned and sequenced (3). The sequence identity between the ermG region of CTnGERM1 and that of CTn7853 ends at 26 bp upstream of ermG (bp −26) and 96 bp downstream of ermG (bp +811; Fig. 4). Clearly, if CTn7853 is in fact a member of the CTnGERM1 family, as the results in Fig. 4 suggest, ermG is inserted in different locations in the element. A comparison between the ermG regions of CTnGERM1 and B. sphaericus revealed that the sequence identity between the ermG region on CTnGERM1 and that of B. sphaericus ended at 16 bp upstream of the gene (bp −16). In this case, however, the sequence identity downstream of the ermG gene continues an additional 100 bp (>bp +910). Thus, if ermG is on a cassette, the cassette has different endpoints from those seen in the comparison of the CTnGERM1 ermG gene and the ermG from CTn7853.
FIG. 4.
Nucleotide sequence alignment of ermG genes from CTn7853, B. sphaericus, and CTnGERM1. Each hyphen (-) represents a gap inserted because of dissimilarities in the alignment. ∗, 100% nucleotide identity of the three sequences. Lowercase letters were used when one of the sequences differed. The ermG start codon ATG and stop codon TAA are shown in boldface. The extended sequence identity between CTnGERM and the B. sphaericus ermG is continued at the bottom. The nucleotide position numbers are relative to the A of the ATG start codon: + for downstream positions and − for upstream positions. Alignment was performed with the ClustalW program. GenBank accession numbers are as follows: CTn7853, L42817; ermG region from B sphaericus, M15332; and CTnGERM1, AJ557257.
If ermG had integrated into a preexisting CTn as a cassette, we would expect to find examples of strains that carried an element related to CTnGERM1 but did not carry ermG. To determine whether such elements were found in Bacteroides species, DNA from our collection of 289 Bacteroides strains was hybridized on dot blots with probes representing the ermG upstream region, the 7.2-kb PstI-PstI fragment and the 2.5-kb BamHI-SphI fragment (fragments G and H; Fig. 2). Of the 289 strains, DNA from 36 strains (12%) hybridized to both probes. Positive strains included seven of the nine ermG-containing strains and three strains (i.e., WH504, WH505, and WH506) that were able to transfer low-level Emr but contained no known erm genes (Fig. 3). The remaining 26 cross-hybridizing strains were not Emr. The hybridization to the upstream probes G and H may be due to the presence of an ermG-like element or the presence of one or more genes acquired independently.
Location of the 19-kb ermG region in CTnGERM1.
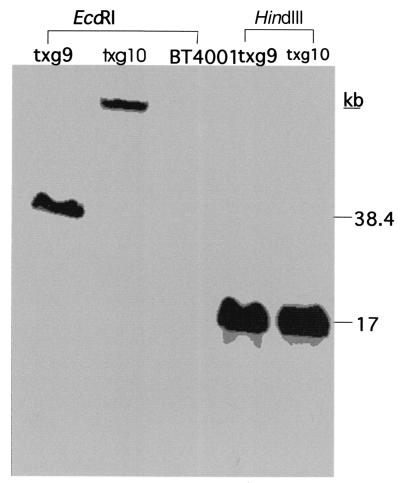
According to the PFGE results, CTnGERM1 integrated into different positions in the chromosomal DNA of txg9 and txg10. Thus, a different digestion pattern should be seen if the restriction enzyme cuts outside the element DNA. DNA from both transconjugants was digested with EcoRI and HindIII and resolved in 1% agarose field inversion electrophoresis gel and then probed with fragment B (Fig. 5). The probe hybridized to the same 17-kb HindIII in both cases, suggesting that another HindIII site is 17 kb away from the HindIII at position kb 10 in Fig. 2 and that this HindIII site is inside CTnGERM1.
FIG. 5.
Southern blot of the EcoRI- and HindIII-digested chromosomal DNA of BT4001ΩCTnGERM1 txg9 and txg10 shown in Fig. 1. The digested DNA samples were run on a field inversion electrophoresis gel, and the Southern blot of the gel was probed with labeled fragment B (Fig. 2). EcoRI-digested DNA from txg9, txg10, and BT4001 is shown in lanes 1, 2, and 3, respectively. HindIII-digested txg9 and txg10 are in lanes 4 and 5. The locations and sizes (in kb) of the relevant high-molecular-weight markers (BRL) are indicated on the right.
The EcoRI-digested chromosomal DNA of txg9 and txg10 showed different patterns. The hybridizing band of txg9 is 35 kb, but that of txg10 is >50 kb. Thus, one of the EcoRI sites is outside CTnGERM1, and the right end of CTnGERM1 is less than 35 kb away from the EcoRI site at position kb 15 in Fig. 2. Thus, the right end of CTnGERM1 is more than 10 kb away (based on HindIII digestion results) and less than 31 kb away from the end of the 19-kb cloned fragment. Since the whole element is about 75 kb in size, the left end must be 25 to 46 kb away from the left end of the cloned region (Fig. 2). These results are consistent with the hypothesis that the cloned 19-kbp ermG-containing DNA segment is near the middle of CTnGERM1.
DISCUSSION
Our results demonstrate that the transmissible element found in DH3716, CTnGERM1, is a 75-kbp element that is normally integrated into the Bacteroides chromosome. CTnGERM1 integrated into the same NotI band in most of the independently isolated transconjugants, thus indicating that integration may be relatively site specific. At this point, however, we cannot rule out the possibility that there is more than one site in the same large NotI band. By taking advantage of the fact that the CTn integrated into a different site in one of the transconjugants (i.e., tgx10), we were able to show that the cloned 19-kbp region is probably located in the middle of the 75-kbp element. Attempts to clone the ends of the CTn were unsuccessful. In our experience with CTnERL and CTnDOT, we found that regions near the ends were very difficult to clone and that deletions were common in clones that contained this DNA. Obtaining DNA sequence information from the ends of CTnGERM1 and related elements is of interest because one of the few features that seem, so far, to link the known CTns and the integrated elements they excise and mobilize is that their integrase genes are members of the phage lambda integrase family (2, 19, 22). Relatively few CTns have been characterized at this level, however, so it is not clear that this feature will prove to be a defining characteristic of integrating transmissible elements.
Even though only a 19-kbp segment of CTnGERM1 was obtained, this cloned DNA enabled us to show that all six of the elements that transferred ermG are closely related to each other and that there are CTnGERM1-type elements in Bacteroides strains that do not carry ermG. In fact, these elements that did not contain ermG were more common than the ermG-containing versions of the element. The ermG gene appears to be capable of integrating as a cassette. The fact that some of these elements also carry tetQ raises the question of whether tetQ is also integrating as a cassette into preexisting elements.
The fact that the CTnGERM1-type elements exhibited restriction length polymorphisms in the region detected by the probe used in Fig. 3 and that CTnGERM1-type elements that lacked ermG are more common than the forms that carry ermG points to the likelihood that CTnGERM1-type elements have entered members of the genus Bacteroides more than once. In this connection, it is worth noting that the strains carrying DNA that hybridizes with the CTnGERM1 probe were isolated from people in different parts of the United States. This result suggests that once these elements entered Bacteroides strains, some of them may have acquired tetQ, since tetQ appears to have been in Bacteroides strains much longer than the CTnGERM1 elements or ermG, based on our survey of Bacteroides strains. Only 3% of the 88 pre-1970 strains had DNA that hybridized to the ermG upstream probe, compared to 22 to 30% of these strains that already carried tetQ and a CTnDOT type element.
The origin of CTnGERM1 and related elements remains a mystery. The CTnGERM1 elements could have come from gram-positive bacteria. The ermG gene has previously been found only in a plasmid of B. sphaericus (7), and sequence identity of CTnGERM1 and the B. sphaericus ermG gene extends downstream of the gene. In B. sphaericus, the ermG gene is located on a plasmid, so the gene—rather than the entire CTn—seems to be what is moving between genera. Another CTnGERM1 gene had high nucleotide and amino acid sequence identity (>90%) with mefA, a macrolide efflux gene found in S. pyogenes and S. pneumonia. It is also upstream of a second gene, an ABC transporter or msr(SA) homolog, found in Streptococcus pneumoniae on transposable element Tn1207.1 (15). The nucleotide identity of the CTnGERM ORFs with these two genes is also ∼90%, but other sequences associated with the Tn-like element have not been detected. It is interesting that another homolog of MefA (MefE2) encoded by a gene found on a Bacteroides mobilizable transposon, NBU2 (22), is much less similar to the MefA on CTnGERM1: MefE2 has only 33% amino acid identity with the MefAs encoded by genes found in S. pyogenes and CTnGERM1. Thus, the high sequence identity between mefA on CTnGERM1 and the mefA in S. pyogenes is not due to conservation of the mefA sequence. The sequences of two more gram-positive genes downstream of ermG, orfX′ and orfY′, are found on Staphylococcus transposons Tn5404 and Tn5405 (1, 4), which are often located on plasmids and other transmissible elements in the gram-positive bacteria, and these sequences may be remnants of Tn insertions into the CTnGERM elements. Tn5404- and Tn5405-related elements have been found to be associated with ermB in canine staphylococcal isolates (1).
If gram-positive bacteria were the source of CTnGERM1, the source is probably not B. sphaericus itself. The percentage of G+C content of the ermG gene is 26.8%, whereas that of the Bacteroides chromosome DNA is about 42% and that of B. sphaericus is 47%. This observation indicates that ermG is a newcomer in both species. Also, in CTnGERM1, the percentage of G+C content of the ORFs adjacent to ermG are 34 and 36.2%, respectively, and many of them, as previously described, have >90% nucleotide identity with known gram-positive genes (Table 3). Low percent G+C values are typical of the gram-positive cocci, but the sequence we have now does not implicate any of the known integrated conjugal elements from these bacteria. It is interesting that despite differences in the percentage of G+C content, if CTnGERM originated in the gram-positive bacteria, enough genes on the incoming element were expressed well enough for the CTn to be fully transmissible in a foreign host. Not all the genes on CTnGERM1 are functional, however, as is indicated by the fact that the putative macrolide efflux gene did not confer resistance to erythromycin on a Bacteroides host. The lack of expression is at least partly due to mutations and deletions. Whatever the origin of CTnGERM1, it is clear that in contrast to the CTnDOT-type elements that are transferring widely within the genus Bacteroides, conjugal elements can also move into Bacteroides strains, possibly from another genus.
Acknowledgments
We thank Gabrielle Whittle for useful discussions and for constructing pGW47.
This work was supported by grant AI22383 from the U.S. National Institutes of Health.
REFERENCES
- 1.Boerlin, P., A. P. Burnens, J. Frey, P. Kuhnert, and J. Nicolet. 2001. Molecular epidemiology and genetic linkage of macrolide and aminoglycoside resistance in Staphylococcus intermedius of canine origin. Vet. Microbiol. 79:155-169. [DOI] [PubMed] [Google Scholar]
- 2.Cheng, Q., B. J. Paszkiet, N. B. Shoemaker, J. F. Gardner, and A. A. Salyers. 2000. Integration and excision of a Bacteroides conjugative transposon, CTnDOT. J. Bacteriol. 182:4035-4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper, A. J., N. B. Shoemaker, and A. A. Salyers. 1996. The erythromycin resistance gene from the Bacteroides conjugal transposon Tcr Emr 7853 is nearly identical to ermG from Bacillus sphaericus. Antimicrob. Agents Chemother. 40:506-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Derbise, A., K. G. H. Dyke, and N. El Solh. 1996. Characterization of a Staphylococcus aureus transposon, Tn5405, located within Tn5404 and carrying the aminoglycoside resistance genes aphA-3 and aadE. Plasmid 35:174-188. [DOI] [PubMed] [Google Scholar]
- 5.Hall, R. M., and C. M. Collins. 1995. Mobile gene cassettes and integrons: capture and spread of genes by site-specific recombination. Mol. Microbiol. 15:593-600. [DOI] [PubMed] [Google Scholar]
- 6.Jobling, M. G., and R. K. Holmes. 1990. Construction of vectors with the p15a replicon, kanamycin resistance, inducible lacZ α and pUC18 or pUC19 multiple cloning sites. Nucleic Acids Res. 18:5315-5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monod, M., S. Mohan, and D. Dubnau. 1987. Cloning and analysis of ermG, a new macrolide-lincosamide-streptogramin B resistance element from Bacillus sphaericus. J. Bacteriol. 169:340-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore, W. E., and L. V. Holdeman. 1974. Human fecal flora: the normal flora of 20 Japanese-Hawaiians. Appl. Microbiol. 27:961-979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nikolich, M. P., N. B. Shoemaker, G. R. Wang, and A. A. Salyers. 1994. Characterization of a new type of Bacteroides conjugative transposon, Tcr Emr 7853. J. Bacteriol. 176:6606-6612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reeves, A. R., J. N. D'Elia, J. Frias, and A. A. Salyers. 1996. A Bacteroides thetaiotaomicron outer membrane protein that is essential for utilization of maltooligosaccharides and starch. J. Bacteriol. 178:823-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roberts, A. P., P. Mullany, and M. Wilson. 2001. Gene transfer in bacterial biofilms. Methods Enzymol. 336:60-65. [DOI] [PubMed] [Google Scholar]
- 12.Salyers, A. A., N. B. Shoemaker, and A. M. Stevens. 1995. Tetracycline regulation of conjugal transfer genes, p. 393-400. In J. A. Hoch and T. J. Silhavy (ed.), Two-component signal transduction. American Society for Microbiology, Washington, D.C.
- 13.Salyers, A. A., N. B. Shoemaker, A. M. Stevens, and L. Y. Li. 1995. Conjugative transposons: an unusual and diverse set of integrated gene transfer elements. Microbiol. Rev. 59:579-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 15.Santagati, M., F. Iannelli, M. R. Oggioni, S. Stefani, and G. Pozzi. 2000. Characterization of a genetic element carrying the macrolide efflux gene mef(A) in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 44:2585-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shoemaker, N. B., C. Getty, E. P. Guthrie, and A. A. Salyers. 1986. Regions in Bacteroides plasmids pBFTM10 and pB8-51 that allow Escherichia coli-Bacteroides shuttle vectors to be mobilized by IncP plasmids and by a conjugative Bacteroides tetracycline resistance element. J. Bacteriol. 166:959-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shoemaker, N. B., and A. A. Salyers. 1987. Facilitated transfer of IncP β R751 derivatives from the chromosome of Bacteroides uniformis to Escherichia coli recipients by a conjugative Bacteroides tetracycline resistance element. J. Bacteriol. 169:3160-3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shoemaker, N. B., H. Vlamakis, K. Hayes, and A. A. Salyers. 2001. Evidence for extensive resistance gene transfer among Bacteroides spp. and among Bacteroides and other genera in the human colon. Appl. Environ. Microbiol. 67:561-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shoemaker, N. B., G. R. Wang, and A. A. Salyers. 1996. NBU1, a mobilizable site-specific integrated element from Bacteroides spp., can integrate nonspecifically in Escherichia coli. J. Bacteriol. 178:3601-3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simon, R. 1984. High-frequency mobilization of gram-negative bacterial replicons by the in vitro constructed Tn5-Mob transposon. Mol. Gen. Genet. 196:413-420. [DOI] [PubMed] [Google Scholar]
- 21.Smith, C. J., L. A. Rollins, and A. C. Parker. 1995. Nucleotide sequence determination and genetic analysis of the Bacteroides plasmid, pBI143. Plasmid 34:211-222. [DOI] [PubMed] [Google Scholar]
- 22.Wang, J., N. B. Shoemaker, G. R. Wang, and A. A. Salyers. 2000. Characterization of a Bacteroides mobilizable transposon, NBU2, which carries a functional lincomycin resistance gene. J. Bacteriol. 182:3559-3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whittle, G., B. D. Hund, N. B. Shoemaker, and A. A. Salyers. 2001. Characterization of the 13-kilobase ermF region of the Bacteroides conjugative transposon CTnDOT. Appl. Environ. Microbiol. 67:3488-3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whittle, G., N. B. Shoemaker, and A. A. Salyers. 2001. Characterization of genes involved in modulation of conjugal transfer of the Bacteroides conjugative transposon CTnDOT. J. Bacteriol. 184:3839-3847. [DOI] [PMC free article] [PubMed] [Google Scholar]