Abstract
The phosphoenolpyruvate:sugar phosphotransferase system (PTS) is the major sugar uptake system in oral streptococci. The role of EIIABMan (encoded by manL) in gene regulation and sugar transport was investigated in Streptococcus mutans UA159. The manL knockout strain, JAM1, grew more slowly than the wild-type strain in glucose but grew faster in mannose and did not display diauxic growth, indicating that EIIABMan is involved in sugar uptake and in carbohydrate catabolite repression. PTS assays of JAM1, and of strains lacking the inducible (fruI) and constitutive (fruCD) EII fructose, revealed that S. mutans EIIABMan transported mannose and glucose and provided evidence that there was also a mannose-inducible or glucose-repressible mannose PTS. Additionally, there appears to be a fructose PTS that is different than FruI and FruCD. To determine whether EIIABMan controlled expression of the known virulence genes, glucosyltransferases (gtfBC) and fructosyltransferase (ftf) promoter fusions of these genes were established in the wild-type and EIIABMan-deficient strains. In the manL mutant, the level of chloramphenicol acetyltransferase activity expressed from the gtfBC promoter was up to threefold lower than that seen with the wild-type strain at pH 6 and 7, indicating that EIIABMan is required for optimal expression of gtfBC. No significant differences were observed between the mutant and the wild-type background in ftf regulation, with the exception that under glucose-limiting conditions at pH 7, the mutant exhibited a 2.1-fold increase in ftf expression. Two-dimensional gel analysis of batch-grown cells of the EIIABMan-deficient strain indicated that the expression of at least 38 proteins was altered compared to that seen with the wild-type strain, revealing that EIIABMan has a pleiotropic effect on gene expression.
Oral streptococci depend on dietary carbohydrates and carbohydrates presented in oral secretions for growth and persistence in the mouth. The ability of oral streptococci to metabolize a wide variety of carbohydrates to produce organic acids is directly related to their ability to elicit dental caries. The phosphoenolpyruvate (PEP):sugar phosphotransferase system (PTS) is the primary sugar transport system in oral streptococci, especially under carbohydrate-limiting conditions, and plays important roles in global control of gene expression (20, 29, 30, 37, 38). The PTS consists of two proteins that are common to all PTS substrates, enzyme I (EI) and the heat-stable phosphocarrier protein HPr, as well as a variety of sugar-specific permeases, known as EII complexes, which catalyze the transport and concomitant phosphorylation of the substrate. The EII complexes usually consist of three domains, A, B, and C, but sometimes a fourth domain, D, is required (20, 29). The A and B domains participate in phosphorylation of the cognate substrates, whereas the C and D domains comprise the membrane permeases. The EII domains can either be covalently linked as a single protein or can be present in various combinations of individual polypeptides.
The mannose PTS appears to play central roles in sugar transport and global regulation of gene expression in oral streptococci. Most of the research on the mannose PTS of streptococci has focused on the EIIMan complex of Streptococcus salivarius, which consists of two forms of EIIABMan (EIIABLMan and EIIABHMan) proteins and of EIICMan and EIIDMan proteins (27, 37). EIIABLMan is responsible for the phosphorylation of mannose, fructose, glucose, and the nonmetabolizable glucose analog 2-deoxyglucose (2-DG), whereas the function of EIIABHMan, which can accept a phosphate group from HPr, has not yet been determined (27). Spontaneous mutants of S. salivarius selected for resistance to 2-DG that were deficient in EIIABLMan had aberrant growth characteristics and alterations in the expression of multiple cytoplasmic and membrane components (37). Some of these mutants were still able to grow with mannose as the sole carbohydrate source, although at a slower rate than the wild-type strain, suggesting the potential for a second, lower-affinity mannose transport system (13, 37). Subsequently, the lower affinity mannose transporter was shown to be a fructose-inducible PTS that was derepressed in the absence of a functional EIIABLMan (26), indicating that EIIABLMan also has the capacity to regulate other sugar permeases (37). In addition to transporting glucose, fructose, and mannose, the EIIMan of Lactobacillus pentosus has been shown to be involved in the transport of xylose (9). Finally, the involvement of EIIMan in carbon catabolite repression (CCR) was previously suggested for S. salivarius, Streptococcus bovis, and L. pentosus (8, 12, 17). Clearly, then, EIIMan proteins are central to genetic and physiologic circuits in gram-positive bacteria with low levels of G+C.
Lortie et al. (22) demonstrated that Streptococcus mutans possesses a mannose-specific PTS that is comprised of an EIIABMan domain (with 84% identity to the EIIABLMan form of S. salivarius) as well as EIICMan and EIIDMan proteins. Previously, spontaneous mutants of S. mutans that were deficient in EIIMan grew as well as the parent strain on mannose, so the presence of an inducible mannose PTS was therefore suggested for this organism (23). A variety of studies have highlighted the importance of carbohydrate source and availability in the regulation of expression of critical virulence attributes of S. mutans. The synthesis and catabolism of exopolysaccharides, acid production, levels of resistance to environmental stress, and PTS activity are among the various factors that have been shown to be influenced by the amount and type of carbohydrate on which the organisms are grown (2, 3, 4, 5, 21, 37, 38). In light of the important roles of EIIMan proteins in related organisms, we initiated an investigation of the function of the putative EIIMan protein of S. mutans UA159 in carbohydrate transport, in regulation of known virulence determinants, and in global control of gene expression.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The S. mutans strains listed in Table 1 were grown in brain heart infusion (BHI) at 37°C in a 5% CO2 atmosphere. When required, antibiotics were added at concentrations of 10 μg ml−1 for erythromycin (Em), 10 μg ml−1 for tetracycline (Tc), and 1 mg ml−1 for kanamycin (Km). Recombinant S. mutans strains were grown in BHI or, when cells were to be assessed for diauxic growth or enzymatic assays, in tryptone-vitamin (TV) base medium (7) supplemented with the desired carbohydrates. Escherichia coli strains were cultured in Luria-Bertani broth supplemented, when needed, with 100 μg of ampicillin ml−1, 40 μg of Km ml−1, 5 μg of Em ml−1, 20 μg of chloramphenicol (Cm) ml−1, or 10 μg of Tc ml−1. To isolate spontaneous 2-DG-resistant mutants, cells were grown overnight in BHI broth and streaked onto BHI plates containing 5 mM 2-DG. Growth was observed after incubation for 24 h at 37°C in a 5% CO2 atmosphere. MICs of 2-DG were tested in BHI broth supplemented with 1, 2, 5, 7, 10, 15, 20, 30, or 40 mM 2-DG.
TABLE 1.
S. mutans strains used in this study
| Strain | Relevant genotype | Description | Source or reference |
|---|---|---|---|
| UA159 | manL+fruI+fruCD+ | Wild type | |
| JAM1 | manL− | manL::kan | This study |
| TW30 | fruI− | fruI::erm | 40 |
| TW31 | fruCD− | fruCD::tet | 40 |
| TW32 | fruI−fruCD− | fruI::erm fruCD::tet | 40 |
| JAM11 | manL−fruI− | manL::kan fruI::erm | This study |
| JAM12 | manL−fruCD | manL::kan fruCD::tet | This study |
| JAM13 | manL−fruI−fruCD− | manL::kan fruI::erm fruCD::tet | This study |
| JAM7 | manL+fruI+fruCD+Pgtfcata | UA159 harboring Pgtfcat | This study |
| JAM8 | manL−Pgtfcat | JAM1 harboring Pgtfcat | This study |
| JAM9 | manL+fruI+fruCD+Pftfcatb | UA159 harboring Pftfcat | This study |
| JAM10 | manL−Pftfcat | JAM1 harboring Pgtfcat | This study |
gtf promoter fused to chloramphenicol acetyltransferase gene (cat).
ftf promoter fused to cat.
Recombinant S. mutans strains carrying cat gene fusions were grown in a BioFloIII chemostat apparatus (New Brunswick Scientific, Edison, N.J.) with a working volume of 600 ml in TY base medium (3% tryptone, 0.5% yeast extract) supplemented with 10 μg of Tc ml−1. The pH of the culture was controlled at 5, 6, or 7 pH by automated addition of 2.0 N KOH. Glucose was present in the growth medium at a concentration of 20 mM for carbohydrate-limiting conditions or 200 mM for carbohydrate excess conditions. Cells were grown for a minimum of 10 generations at a dilution rate of D = 0.3 h−1 to achieve a steady state at each parameter studied.
DNA manipulations.
Total chromosomal DNA was isolated from S. mutans as previously described (6). Using the QIAprep Spin Miniprep kit (Qiagen, Chatsworth, Calif.), plasmid DNA was isolated from E. coli. Restriction and DNA-modifying enzymes were obtained from Life Technologies, Inc. (Gaithersburg, Md.), New England Biolabs (Beverly, Mass.), or MBI Fermentas (Amherst, N.Y.). Using VentR DNA polymerase (New England Biolabs), PCRs were carried out with 100 ng of chromosomal DNA. DNA was introduced into S. mutans by natural transformation (28) and into E. coli by electroporation (31). Southern blot analyses were carried out at high stringency (31).
Construction of a manL mutant and reporter gene fusions.
The manL gene (0.99 kbp), which encodes EIIABMan, and flanking sequences were amplified from S. mutans UA159 chromosomal DNA by recombinant PCR (15) to introduce SacI and SphI restriction sites at the 5′ and 3′ ends, respectively, and a BamHI site at the center of the manL structural gene. Briefly, the primary PCRs used the primers designated EIIManS8SacI (5′-CGGAATCGAGCTCGCCAGTCATG-3′) and EIIManAS478BamHI (5′-TAAGTTTGCCGGATCCAATGACAGTGCC-3′) to generate a product of approximately 500 bp containing engineered SacI and BamHI sites. The second set of primers, EIIManS478BamHI (5′-GGCACTGTCATTGGATCCGGCAAACTTA-3′) and EIIManAS989SphI (5′-GGACATGTGCATGCTCAATGAGTTC-3′), generated another 500-bp product containing BamHI and SphI sites. Equimolar quantities of each amplicon (50 ng) were mixed and used as templates in another PCR using the primers EIIManS8SacI and EIIManAS989SphI to generate a full-length manL gene. The final product was directly cloned into SacI- and SphI-digested pGEM5Zf(+) (Promega, Wis.) to generate pJA1. To inactivate the manL gene, a BamHI fragment containing a promoterless nonpolar Km resistance marker (18) was introduced into pJA1 in the unique internal BamHI site located 500 bp downstream of the manL start codon. The resulting construct, pJA2, was used to inactivate the manL gene of S. mutans UA159 by allelic exchange. The mutant was designated JAM1.
DNA fragments containing the promoter regions and ribosomal binding sites of the genes encoding the GTF-I and -SI enzymes (gtfBC [34, 36]) and fructosyltransferase (ftf [33]) of S. mutans UA159 were amplified by PCRs and cloned into pCW24 (10) such that transcription and translation of a promoterless E. coli Cm acetyltransferase (cat) gene were driven by the cognate S. mutans regulatory elements. DNA sequencing was performed to confirm that the amplification and cloning had yielded the desired constructs. Fragments containing the gene fusions were released by digestion with SacI and HindIII, blunt ended, and subsequently cloned into HincII-digested pSF143 (35), which carries a Tc resistance determinant and replicates in E. coli but not in S. mutans. The resulting plasmids, pJA11 (carrying the Pgtf-cat promoter fusion) and pJA12 (carrying the Pftf-cat promoter fusion), were introduced into S. mutans UA159 and JAM1 (manL−). Chromosomal DNA was extracted from the recombinant S. mutans strains carrying the gene fusions. PCR products containing the full-length promoter fusions were obtained, and nucleotide sequencing was used to confirm that the strains contained intact gene fusions in the correct orientation.
Proteomic analysis.
S. mutans strains were grown in 100 ml of BHI broth to an optical density at 600 nm of 0.5. Cells were collected by centrifugation at 4,000 × g for 10 min, washed twice with Tris-buffered saline (10 mM Tris-HCl [pH 7.4], 0.9% NaCl), and resuspended in a total volume of 1 ml of 60 mM Tris-HCl (pH 6.8)-5% sodium dodecyl sulfate-10% glycerol. Cell lysates were obtained by mechanical disruption with a Bead Beater (BioSpec, Bartlesville, Okla.) in the presence of chilled glass beads (0.1-mm diameter) for 2 cycles of 30 s, with cooling on ice for 2 min during the interval. The protein concentration of each sample was determined using bicinchoninic acid assay reagent (Sigma, St. Louis, Mo.) with bovine serum albumin as the standard. Two-dimensional (2-D) electrophoresis was performed according to the method of O'Farrell (25) at Kendrick Labs, Inc. (Madison, Wis.), and the gels were stained with either silver or Coomassie blue. Densitometric analysis was used to compare the intensities of the spots. The identity of a selected protein obtained from the 2-D gels was determined by N-terminal sequencing at the Protein Core at the University of Florida (Gainesville, Fla.) followed by BLAST searches at National Center for Biotechnology Information.
Biochemical assays.
To measure gene fusion activity in chemostat-grown cells, aliquots were rapidly aspirated from the culture vessel and cells were harvested by centrifugation, washed with 10 mM Tris-HCl (pH 7.8), and resuspended in 750 μl of the wash buffer. Cells were homogenized as described above, and the cleared lysates were used for determining Cm acetyltransferase (CAT) activity by the spectrophotometric method of Shaw et al. (32). To measure PTS activity, S. mutans strains were grown to an optical density at 600 nm of 0.6 in TV medium supplemented with 0.5% (wt/vol) mannose or glucose and PTS-specific activity with glucose, fructose, or mannose as substrate was determined as detailed by LeBlanc et al. (19). Briefly, 50 ml of late exponential phase cells was harvested, washed twice with 0.1 M sodium-potassium phosphate buffer (pH 7.2), and suspended in 5 ml of the same buffer. This cell suspension was permeabilized with 250 μl of toluene-acetone (1:9), and 10 to 50 μl of permeabilized cells was used in the assay. The assay measures the oxidation of NADH in a PEP-dependent manner, and the assay mixture contained 0.1 mM NADH, 10 U of lactic acid dehydrogenase, 5 mM PEP, 10 mM NaF, and a 10 mM concentration of the sugar of interest in a final volume of 0.1 M sodium-potassium phosphate buffer, pH 7.2. The rate of NADH oxidation was monitored for 3 min at 340 nm. No PEP was added in the reference tubes. PTS activity was expressed as nanomoles of NADH oxidized in a PEP-dependent manner per minute per milligram of protein.
RESULTS AND DISCUSSION
Arrangement of the man gene cluster and flanking regions.
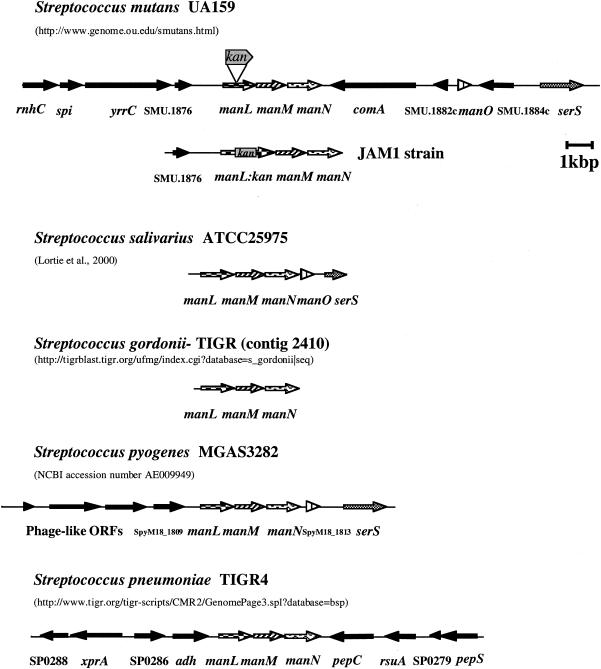
Using the S. salivarius manL sequence, computer algorithms identified a putative man gene cluster in the S. mutans UA159 genome (1), the sequence of which was completed at the Advanced Center for Genome Technology at the University of Oklahoma (http://www.genome.ou.edu/smutans.html). The organization of the man gene cluster and flanking regions in S. mutans and other streptococci is shown in Fig. 1.
FIG. 1.
The arrangement of man gene clusters and flanking regions in S. mutans UA159 and other streptococci. The location of the insertion of a nonpolar Km cassette within the manL gene to generate the strain JAM1 is indicated. Patterned arrows represent similar genes. The websites or references from which the man gene clusters and flanking regions were obtained are indicated.
The arrangement of the manL gene cluster of S. mutans is significantly different than that of S. salivarius, which is composed of four open reading frames (ORFs) (22), manLMNO, but lacks comA and SMU.1882c-like genes between manN and manO. In both species, however, the serS gene is located downstream of manO. Lortie et al. (22) suggested that this region of the genome of S. mutans was probably subjected to chromosomal rearrangement, as suggested by the presence of additional genes and relatively long noncoding regions that are not present in the S. salivarius and Streptococcus pyogenes man operons and flanking regions. Of potential significance, the presence of phage-like ORFs directly upstream of the manL gene cluster was observed in the S. pyogenes MGAS8232 genome. In Streptococcus pneumoniae TIGR4, this gene cluster is also composed of manLMN but manO was not observed in the flanking regions. Also, the flanking regions of the man cluster of S. pneumoniae differed significantly from those of S. mutans, S. salivarius, and S. pyogenes. Lortie et al. (22) suggested that the manO gene has a regulatory function in S. salivarius because this ORF can be also cotranscribed with the man operon. The presence and location of this gene differ among the streptococci; however, the importance of manO in the functionality of EIIMan is unclear.
Characteristics of EIIABMan-deficient S. mutans.
An otherwise isogenic manL mutant of S. mutans UA159 was constructed by allelic exchange with a nonpolar Km resistance determinant inserted 0.5 kbp downstream of the manL start codon, as detailed in Materials and Methods. Insertion of the marker into manL was confirmed by Southern blot analysis using probes specific for manL and for the Km resistance element. To determine the role of S. mutans EIIABMan in glucose and mannose uptake, growth rates in TV medium containing glucose or mannose as the sole carbohydrate source were determined and sugar-specific PTS activities were measured for the parental strain and JAM1, the manL mutant. S. mutans JAM1 grew more slowly than UA159 in TV medium with 0.2% glucose, with a doubling time of 92 min for JAM1 compared to 82 min for UA159 (Table 2). Growth in 0.2% mannose was faster for JAM1 (doubling time ≅180 min) than for the wild-type strain (doubling time ≅255 min). This finding is in contrast with that reported for S. mutans GS-5, in which a spontaneously arising EIIMan-deficient strain grew more slowly than the parent in glucose but growth in mannose was unaffected (23, 24). Of note, no significant differences in growth rates were observed between the wild-type strain and JAM1 when 0.2% fructose was used as the sole carbohydrate source (Table 2). Thus, the behavior of the JAM1 strain also differs from that of an EIIBMan mutant of L. pentosus, which was shown to grow faster on fructose but which displayed slower growth on glucose and mannose than the wild-type strain (8). Therefore, it seems that EIIABMan of S. mutans UA159 may constitute an ortholog of the EIIABLMan of S. salivarius and EIIMan of L. pentosus.
TABLE 2.
Doubling time of S. mutans strains in TV broth supplemented with 0.2% (wt/vol) of glucose, mannose and fructose
| Strain | Doubling time (min) ± SD in broth with:
|
||
|---|---|---|---|
| Glucose | Mannose | Fructose | |
| UA159 | 82 ± 1 | 255 ± 12 | 73 ± 6 |
| JAM1 | 92 ± 4 | 180 ± 17 | 74 ± 4 |
| TW30 | 88 ± 2 | 243 ± 30 | 85 ± 4 |
| TW31 | 89 ± 3 | 234 ± 15 | 72 ± 6 |
| TW32 | 79 ± 8 | 244 ± 15 | 80 ± 6 |
| JAM11 | 102 ± 0 | 190 ± 23 | 77 ± 5 |
| JAM12 | 108 ± 5 | 201 ± 26 | 77 ± 6 |
| JAM13 | 110 ± 14 | 187 ± 8 | 73 ± 2 |
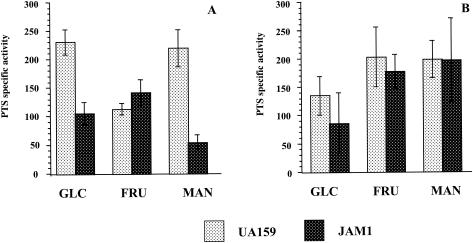
To assess PTS activity, UA159 and JAM1 were grown in TV medium with various carbohydrate sources. Glucose-specific PTS activity of JAM1 grown in glucose was markedly lower (P ≤ 0.001) than that of the wild-type strain grown under the same conditions (Fig. 2A), indicating that EIIMan of S. mutans is probably involved in glucose transport and confirming the observation of Néron and Vadeboncoeur with strain GS-5 (23). Fructose PTS activity of JAM1 was not affected by the lack of a functional EIIABMan (Fig. 2A), a finding consistent with the similar growth rates of UA159 and JAM1 in 0.2% fructose. These results indicate that unlike the case seen with S. salivarius, the S. mutans EIIABMan does not appear to take up fructose. However, we cannot exclude the possibility that EIIMan is able to repress either fructose PTS gene expression or permease activity.
FIG. 2.
Sugar-specific PTS activity of S. mutans UA159 and JAM1 grown in glucose (A) and mannose (B). The glucose-, fructose-, and mannose-specific PTS activities are indicated as GLC, FRU, and MAN, respectively. The values are the means ± standard deviations (SD) from at least three individual experiments.
Lower levels of glucose-specific PTS activity were observed in the wild-type strain grown in mannose than were seen for the strain grown in glucose (Fig. 2B), suggesting the presence of both a constitutive EIIGlu and a glucose-inducible, or mannose-repressible, EIIGlu in S. mutans, which is consistent with the findings of Néron and Vadeboncoeur (23). When JAM1 was grown in mannose, the mannose PTS activity was restored to the levels seen in the wild-type strain grown under the same conditions (Fig. 2B), providing compelling evidence for the presence of a mannose-inducible mannose PTS. Alternatively, EIIABMan may down-regulate this second mannose PTS, which may explain why the absolute level of mannose PTS activity does not differ markedly between UA159 grown in glucose or mannose and JAM1 grown in mannose. The fructose PTS activity of the wild-type strain was enhanced when cells were grown in mannose as substrate (P ≤ 0.02) (Fig. 2B), suggesting that the fructose porters may also be glucose repressible or mannose inducible. If the latter is the case, a role for EIIFru in mannose uptake would be logical.
Role of fructose-specific II enzymes.
In S. salivarius, an inducible EIIFru was shown to be able to transport mannose in spontaneously arising, EIIMan-deficient strains (13). To explore whether EIIFru enzymes of S. mutans could be responsible for the increased growth rate of JAM1 in mannose and for the restored mannose PTS activity of JAM1 grown in mannose (Fig. 2B), S. mutans strains TW30 (deficient in the inducible fructose PTS [fruI−]), TW31 (deficient in the constitutive fructose PTS [fruCD−]), and TW32 (lacking both EIIFru enzymes) (40) were examined in this study. Growth in 0.2% glucose was only slightly slower for TW30 and TW31 than for the wild-type strain, whereas strain TW32 showed no change in its growth rate on glucose. The doubling times of the fructose PTS mutants TW30, TW31, and TW32 growing on mannose were indistinguishable from that of UA159, the parent strain (Table 2).
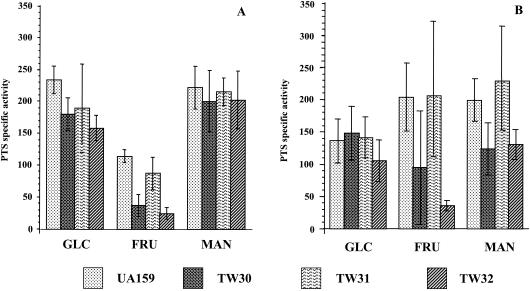
Strains that were deficient in the fructose-inducible EIIFru (TW30 and TW32) expressed modestly lower levels of glucose PTS activity than the wild-type strain when grown in glucose (Fig. 3A). For strain TW30, this was shown not to be statistically significant, although the differences between the wild-type strain and TW32 were (P ≤ 0.05). The strain that was deficient in the constitutive EIIFru only (TW31) did not show any alteration in glucose-specific PTS activity compared to the wild-type strain growing under the same conditions (Fig. 3A). At this stage, therefore, it cannot be ruled out that that the IIFru enzymes, and in particular FruI, might be capable of transporting glucose. No differences were observed for glucose PTS activity when all strains were grown in mannose as the sole carbohydrate source, albeit glucose PTS activity was consistently lower than in glucose-grown cells (Fig. 3). As expected, all strains that carried mutations in the inducible fructose PTS genes (fruI) expressed lower levels of fructose-specific PTS activity, with the lowest level observed for strains that carried mutations in both of the fructose-specific EII genes (TW32) (P ≤ 0.001). Interestingly, fructose-specific PTS activity was up-regulated in all strains growing in mannose (Fig. 3B) compared to that of glucose-grown cells (Fig. 3A), with the exception of TW32 (fruI−/fruCD−). This result indicates that growth in mannose can induce, or more likely allow for derepression of, the fructose PTS. In L. pentosus, cells grown on substrates for EIIMan had repressed fructose PTS activity, suggesting that EIIMan does have the capacity to negatively regulate EIIFru (8), at least in some lactobacilli.
FIG. 3.
Sugar-specific PTS activity of S. mutans UA159 and EIIFru− strains (TW30, TW31, and TW32) grown in glucose (A) and mannose (B). The glucose-, fructose-, and mannose-specific PTS activities are indicated as GLC, FRU, and MAN, respectively. The values are the means ± SD from at least three individual experiments.
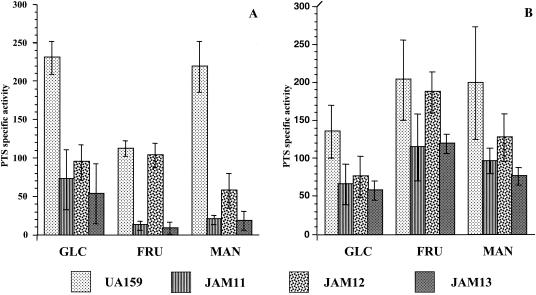
The manL gene was insertionally inactivated in strains TW30, TW31, and TW32 to generate JAM11 (manL−/fruI−), JAM12 (manL−/fruCD−), and JAM13 (manL−/fruI−/fruCD−). The strains JAM11, JAM12, and JAM13 displayed the same growth pattern as the strains carrying only the EIIFru mutations when fructose was provided as the sole carbohydrate (Table 2). Growth of JAM11-13 in glucose and mannose was essentially identical to that of the parental manL− strain, JAM1 (Table 2). The levels of fructose PTS activity in JAM11, -12, and -13 (Fig. 4) generally paralleled those observed in the parental strains TW30, -31, and -32 (Fig. 3). However, strain TW32 did not show enhanced fructose PTS activity when grown in mannose (Fig. 3B), whereas JAM13 (manL−/fruI−/fruCD−) did (P ≤ 0.0007) (Fig. 4B). These results suggested that S. mutans possesses yet another EII (different from FruI, FruCD, and EIIMan) that is capable of transporting fructose and that the expression of this gene is probably negatively regulated by EIIABMan.
FIG. 4.
Sugar-specific PTS activity of S. mutans UA159 and EIIABMan−/EIIFru− strains (JAM11, JAM12, and JAM13) grown in glucose (A) and mannose (B). The glucose-, fructose-, and mannose-specific PTS activities are indicated as GLC, FRU, and MAN, respectively. The values are the means ± SD from at least three individual experiments.
Another interesting finding arising from this series of experiments was that JAM13, which is deficient in EIIABMan, FruI, and FruCD, showed enhanced mannose PTS activity when grown in mannose. This result indicated that there might be an inducible PTS permease that is capable of transporting mannose (one that is distinct from IIMan and the known IIFru enzymes). Also of interest, when the strain that was deficient in EIIABMan and FruI (JAM11) was grown in glucose (Fig. 4A), mannose PTS activity was lower than in the EIIABMan-deficient strain (JAM1) growing under the same conditions (Fig. 2A). The strains TW30 and TW32 (fruI− and fruI−/fruCD−), when grown in glucose (Fig. 3A), had the same mannose PTS activity levels as the wild-type strain, although these same strains exhibited lower mannose PTS activity when grown in mannose (Fig. 3B). In light of these findings, and because there was no enhancement of mannose transport activity in strains that were grown in mannose and carried the fruI mutation, it is possible that FruI is capable of transporting mannose.
Resistance to 2-DG.
EIIABMan of S. salivarius participates in the transport of 2-DG, the nonmetabolizable glucose analog. In S. salivarius, spontaneous mutants can generally be selected using 5 mM 2-DG on rich solid medium with a variety of PTS substrates. In contrast, both UA159 and JAM1 grew well on BHI agar supplemented with 5 mM 2-DG. The inherent high level of resistance of S. mutans UA159 to 2-DG suggests either that the affinity of EIIMan of S. mutans for 2-DG is markedly lower than that of ManL of S. salivarius or that EIIABMan of S. mutans does not transport 2-DG. We then tested the ability of S. mutans UA159 and JAM1 to grow in BHI broth containing 2-DG in concentrations ranging from 1 to 40 mM. Under these conditions, there was some inhibition of growth of S. mutans UA159 at 1 mM 2-DG and growth was completely inhibited at 15 mM 2-DG (data not shown). In contrast, there was no evidence of inhibition of growth of JAM1 at concentrations of 2-DG as high as 5 mM, although complete inhibition was also observed in BHI with 15 mM 2-DG. These findings would seem to indicate that EIIMan of S. mutans is capable of transporting 2-DG, perhaps with lower affinity than EIIMan of S. salivarius. Also, since complete inhibition of growth of JAM1 could only be achieved in the presence of relatively high concentrations of 2DG, it is likely that there is another low-affinity 2-DG transporter in S. mutans. Whether a similar secondary pathway for 2-DG uptake at elevated concentrations of the analog exists in S. salivarius has not been tested to our knowledge.
Involvement of EIIABMan in catabolite repression.
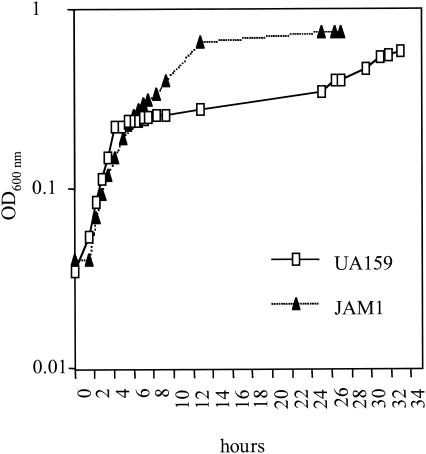
Strains of S. mutans that lack ManL (JAM1 and JAM11-13) did not exhibit diauxic growth in the presence of glucose and a nonpreferred carbohydrate source (Fig. 5). Thus, as has been observed with S. salivarius and L. pentosus, EIIABMan of S. mutans appears to be an effector of CCR. Whether EIIABMan is able to effect CCR through phosphorylation of regulatory proteins, as has been observed in a variety of saccharolytic operons of B. subtilis, or through direct interaction with sugar permeases, similar to EIIGlu of E. coli, remains to be determined. Interestingly, a putative catabolite response element (CRE), which is a conserved DNA sequence to which the CcpA-HPr complex binds to effect catabolite repression in gram-positive bacteria (4, 11, 14, 16), was found 103 bp upstream of the S. mutans manL start codon. This putative CRE differs from the consensus sequence in only the last two bases (TGTAAACGTTTTAC); thus, residues that have been shown to be necessary for efficient CRE function are conserved (14, 39). If this sequence represents a functional CRE, the EIIMan operon may be under the control of CCR via the CcpA pathway. Such an arrangement could allow for CcpA to act as a master regulator of CCR and for proteins such as EIIMan to act to fine tune gene expression in response to more subtle fluctuations in carbohydrate availability or at lower overall carbohydrate concentrations. Alternatively, the cells could utilize EIIMan to respond specifically to cognate sugars of this transporter, whereas the CcpA pathway, which senses carbon flow through fructose-1,6-bisphosphate, may govern gene expression in a less specific fashion in response to total available carbohydrate, thus allowing for monitoring of levels of sugars other than mannose and glucose.
FIG. 5.
Growth of UA159 and JAM1 in TV medium supplemented with 0.05% glucose and 0.5% inulin. The empty squares represent the wild-type strain UA159, whereas the filled triangles represent JAM1, the EIIABMan− strain. The results shown are representative of three independent experiments.
Protein expression patterns differ in the manL mutant.
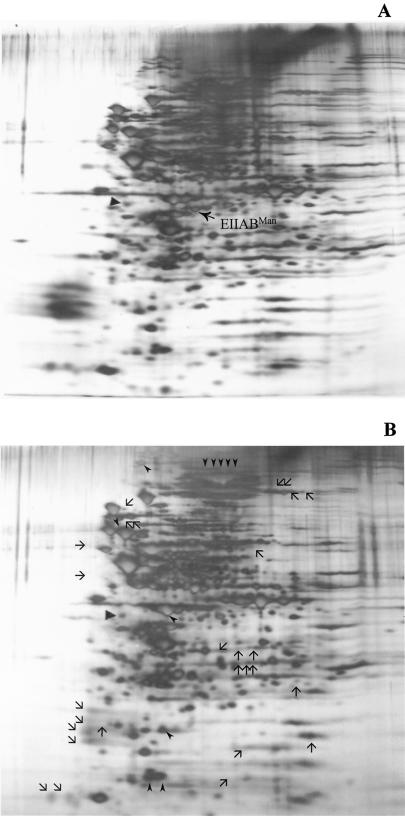
The effect on expression of the products of a variety of genes in cells lacking a functional EIIABMan was evident in 2-D gels that were silver stained, revealing that at least 11 proteins were up-regulated and 27 were down-regulated in the manL− strain (Fig. 6). Thus, manL inactivation has a pleiotropic effect on gene expression. This effect could be exerted through the pathways for catabolite repression, through indirect effects caused by alterations in carbon catabolism, or through direct involvement of ManL in the regulation of expression (or allosteric regulation of activity) of various genes or their products. In any case, it is clear that ManL is of major importance in global regulation of gene expression in S. mutans. This finding is consistent with those reported for S. salivarius that showed alterations in the 2-D gel patterns of cytoplasmic and membrane preparations from wild-type cells and a spontaneously arising EIILMan mutant (37). A spot (which was present in the wild-type strain and absent in the manL mutant in Coomassie blue-stained gels) that we suspected on the basis of molecular weight and pI measurements to be EIIABMan was extracted and analyzed by N-terminal sequencing. The sequence data (XIGIVIAXHGEF) demonstrated that this protein was EIIABMan.
FIG. 6.
Silver stained 2-D gels of total cell lysates of UA159 (A) and JAM1 (B) grown in BHI. (A) EIIABMan (arrow) is indicated in a 2-D gel of proteins from the wild-type strain. (B) Thin arrowheads represent up-regulated proteins, whereas down-regulated proteins are represented by thin arrows. Tropomyosin, the internal control, is indicated by a wide arrowhead in each of the panels.
Expression of the exopolysaccharide machinery of S. mutans is influenced by EIIABMan.
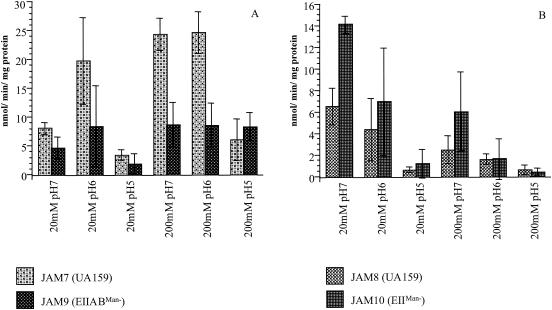
The ability to synthesize and degrade extracellular glucans and fructans has been shown to be a primary virulence attribute of S. mutans (5, 6, 7, 21). The use of sucrose to produce α1,3- and α1,6-linked glucans via glucosyltransferases is an integral part of formation of tenacious oral biofilms and is an essential component of the elicitation of smooth surface caries. Similarly, conversion of sucrose to fructan homopolymers by fructosyltransferase allows the organisms to create substantial stores of extracellular polysaccharides, which augment the acid challenge to the tooth surface. Previous studies by Li and Burne have revealed a strong relationship between carbohydrate source and availability and the expression of genes involved in exopolysaccharide synthesis (21). To determine whether EIIABMan was involved in regulation of gtfBC or ftf gene expression, S. mutans strains carrying cat gene fusions to the promoters of these genes (created as detailed in the Materials and Methods section) were grown in continuous chemostat culture to a steady state under glucose-limiting or glucose excess conditions (Fig. 7). In agreement with previous studies (21, 41), in the wild-type background (JAM7), gtfBC expression was markedly enhanced under conditions of excess carbohydrate, with optimal expression at pH 6. At pH 5, a considerably lower level of expression from the gtfBC promoter was observed in the wild-type background under both glucose excess and glucose-limiting conditions (Fig. 7A). JAM9, which is the manL− strain carrying the gtfBC promoter-cat fusion, appears to display a 2- or 2.8-fold reduction in CAT activity expressed from the gtfBC promoter in cells growing under glucose-limiting conditions (20 mM) at pH 7 or 6, respectively, compared with the expression levels in the wild-type background (Fig. 7A). In cells grown under glucose excess conditions, CAT activity was 2.9- and 3.1-fold lower in the mutant at pH 7 and 6 than in the wild-type strain grown under the same conditions. The decrease in expression from PgtfBC observed in the EIIABMan mutant strain grown under carbohydrate-limiting or carbohydrate excess conditions at pH 7 and 6 suggests that EIIABMan is required for optimal expression of these known virulence genes of S. mutans.
FIG. 7.
CAT-specific activity driven by gtfBC and ftf promoters in the wild-type and manL− backgrounds under carbohydrate excess (200 mM glucose) and carbohydrate-limiting conditions (20 mM glucose) at pH 7, 6, and 5. Values shown are means ± SD from three independent chemostat runs. The results are expressed as nanomoles of Cm acetylated per minute per milligram of protein. (A) CAT activity driven by gtfBC promoter; (B) CAT activity driven by ftf promoter.
There are a number of observations that could account for these findings. First, ManL could effect changes in expression of gtfBC through phosphorylation of (or through direct interaction with) a gtf regulatory protein or through the modulation of expression of factors needed for optimal gtf transcription. Alternatively, alterations in the transport of carbohydrates induced by ManL deficiency could be relayed to the gtf regulator(s) via phosphorelay circuits or through other proteins that participate in global regulation of genes in response to carbohydrate availability. Since the factors required for differential expression of gtfBC have not yet been identified, information from the 2-D gels on the manL mutant cannot yet be used to disclose a likely pathway for altered regulation of gtf in the JAM8 mutant.
Expression of ftf as measured by monitoring CAT activity in both strains (JAM9 and JAM10) under glucose-limiting conditions was highest at pH 7, and expression diminished as the pH was lowered, with almost no detectable CAT activity at pH 5 (Fig. 7B). Expression of ftf was not significantly influenced by the lack of a functional EIIABMan under the conditions tested, with one exception. At pH 7 under glucose-limiting conditions, JAM10 had a 2.2-fold increase in CAT activity expressed from the ftf promoter (Fig. 7B). The possible reasons for these observations could be the same as those detailed for altered gtfBC expression. However, since it seems that loss of ManL does not consistently alter ftf expression, it is likely that indirect consequences of manL inactivation result in changes in ftf expression at neutral pH. It is noteworthy that PTS activity in oral streptococci is optimal around pH 7 and at low carbohydrate concentrations and that activity declines at lower pH values (38), so it is not completely surprising that the effects of manL inactivation are manifested when PTS activity is optimal.
Summary.
It has been demonstrated that EIIABMan of S. mutans is involved in the transport of glucose and mannose as well as in the uptake of 2-DG. Also, it appears that an additional, lower-affinity 2-DG transporter is operable in S. mutans. The use of the ManL-deficient strain in growth and PTS assays revealed a possible role in mannose transport for the inducible fructose PTS permease (FruI) as well as an as-yet-unidentified, mannose-inducible PTS permease. Further, our results suggest the presence of a glucose-inducible glucose PTS and a mannose-inducible fructose PTS. Major changes in protein expression were revealed by proteomic analysis, and the participation of EIIABMan in CCR in S. mutans was unequivocally demonstrated. Finally, the participation of ManL in the expression of two essential virulence genes, gtfBC and ftf, revealed the importance of ManL in regulation of genes that are affected by carbohydrate source and availability.
Acknowledgments
We thank J. A. C. Lemos and Z. T. Wen for their valuable comments. We also thank Mark Yang for his assistance with statistical analysis.
This work was supported by NIDCR grants DE12236 and DE13239.
REFERENCES
- 1.Ajdic, D., W. M. McShan, R. E. McLaughlin, G. Savic, J. Chang, M. B. Carson, C. Primeaux, R. Tian, S. Kenton, H. Jia, S. Lin, Y. Qian, S. Li, H. Zhu, F. Najar, H. Lai, J. White, B. A. Roe, and J. J. Ferretti. 2002. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc. Natl. Acad. Sci. USA 99:14434-14439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belli, W. A., and R. E. Marquis. 1994. Catabolite modification of acid tolerance of Streptococcus mutans GS-5. Oral Microbiol. Immunol. 9:29-34. [DOI] [PubMed] [Google Scholar]
- 3.Belli, W. A., and R. E. Marquis. 1991. Adaptation of Streptococcus mutans and Enterococcus hirae to acid stress in continuous culture. Appl. Environ. Microbiol. 57:1134-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruckner, R., and F. Titgemeyer. 2002. Carbon catabolite repression in bacteria: choice of the carbon source and autoregulatory limitation of sugar utilization. FEMS Microbiol. Lett. 209:141-148. [DOI] [PubMed] [Google Scholar]
- 5.Burne, R. A. 1998. Oral streptococci: products of their environment. J. Dent. Res. 77:445-452. [DOI] [PubMed] [Google Scholar]
- 6.Burne, R. A., K. Schilling, W. H. Bowen, and R. E. Yasbin. 1987. Expression, purification, and characterization of an exo-beta-d-fructosidase of Streptococcus mutans. J. Bacteriol. 169:4507-4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burne, R. A., Z. T. Wen, Y.-Y. M. Chen, and J. E. C. Penders. 1999. Regulation of expression of the fructan hydrolase gene of Streptococcus mutans GS-5 by induction and carbon catabolite repression. J. Bacteriol. 181:2863-2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaillou, S., P. W. Postma, and P. H. Pouwels. 2001. Contribution of the phosphoenolpyruvate:mannose phosphotransferase system to carbon catabolite repression in Lactobacillus pentosus. Microbiology 147:671-679. [DOI] [PubMed] [Google Scholar]
- 9.Chaillou, S., P. H. Pouwels, and P. W. Postma. 1999. Transport of d-xylose in Lactobacillus pentosus, Lactobacillus casei, and Lactobacillus plantarum: evidence for a mechanism of facilitated diffusion via the phosphoenolpyruvate:mannose phosphotransferase system. J. Bacteriol. 181:4768-4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, Y.-Y. M., C. A. Weaver, D. R. Mendelsohn, and R. A. Burne. 1998. Transcriptional regulation of the Streptococcus salivarius 57.I urease operon. J. Bacteriol. 180:5769-5775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujita, Y., Y. Miwa, A. Galinier, and J. Deutscher. 1995. Specific recognition of the Bacillus subtilis gnt cis-acting catabolite-responsive element by a protein complex formed between CcpA and seryl-phosphorylated HPr. Mol. Microbiol. 17:953-960. [DOI] [PubMed] [Google Scholar]
- 12.Gauthier, L., S. Bourassa, D. Brochu, and C. Vadeboncoeur. 1990. Control of sugar utilization in oral streptococci. Properties of phenotypically distinct 2-deoxyglucose-resistant mutants of Streptococcus salivarius. Oral Microbiol. Immunol. 5:352-359. [DOI] [PubMed] [Google Scholar]
- 13.Gauthier, L., S. Thomas, G. Gagnon, M. Frenette, L. Trahan, and C. Vadeboncoeur. 1994. Positive selection for resistance to 2-deoxyglucose gives rise, in Streptococcus salivarius, to seven classes of pleiotropic mutants, including ptsH and ptsI missense mutants. Mol. Microbiol. 13:1101-1109. [DOI] [PubMed] [Google Scholar]
- 14.Hueck, C. J., and W. Hillen. 1995. Catabolite repression in Bacillus subtilis: a global regulatory mechanism for the gram-positive bacteria? Mol. Microbiol. 15:395-401. [DOI] [PubMed] [Google Scholar]
- 15.Innis, M. A., D. H. Gelfand, J. J. Sninsky, and T. J. White. 1990. PCR protocols: a guide to methods and applications, 1st ed. Academic Press, San Diego, Calif.
- 16.Jones, B. E., V. Dossonnet, E. Kuster, W. Hillen, J. Deutscher, and R. E. Klevit. 1997. Binding of the catabolite repressor protein CcpA to its DNA target is regulated by phosphorylation of its corepressor HPr. J. Biol. Chem. 272:26530-26535. [DOI] [PubMed] [Google Scholar]
- 17.Kearns, D. B., and J. B. Russell. 1996. Catabolite regulation in a diauxic strain and a nondiauxic strain of Streptococcus bovis. Curr. Microbiol. 33:216-219. [DOI] [PubMed] [Google Scholar]
- 18.Kremer, B. H., M. van der Kraan, P. J. Crowley, I. R. Hamilton, L. J. Brady, and A. S. Bleiweis. 2001. Characterization of the sat operon in Streptococcus mutans: evidence for a role of Ffh in acid tolerance. J. Bacteriol. 183:2543-2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LeBlanc, D. J., V. L. Crow, L. N. Lee, and C. F. Garon. 1979. Influence of the lactose plasmid on the metabolism of galactose by Streptococcus lactis. J. Bacteriol. 137:878-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lengeler, J. W., K. Jahreis, and U. F. Wehmeier. 1994. Enzymes II of the phosphoenol pyruvate-dependent phosphotransferase systems: their structure and function in carbohydrate transport. Biochim. Biophys. Acta 1188:1-28. [DOI] [PubMed] [Google Scholar]
- 21.Li, Y., and R. A. Burne. 2001. Regulation of the gtfBC and ftf genes of Streptococcus mutans in biofilms in response to pH and carbohydrate. Microbiology 147:2841-2848. [DOI] [PubMed] [Google Scholar]
- 22.Lortie, L. A., M. Pelletier, C. Vadeboncoeur, and M. Frenette. 2000. The gene encoding IIAB(Man)L in Streptococcus salivarius is part of a tetracistronic operon encoding a phosphoenolpyruvate:mannose/glucose phosphotransferase system. Microbiology 146:677-685. [DOI] [PubMed] [Google Scholar]
- 23.Néron, S., and C. Vadeboncoeur. 1987. Two functionally different glucose phosphotransferase transport systems in Streptococcus mutans and Streptococcus sobrinus. Oral Microbiol. Immunol. 2:171-177. [DOI] [PubMed] [Google Scholar]
- 24.Néron, S., and C. Vadeboncoeur. 1987. Evidence for the presence of two distinct phosphoenolpyruvate:mannose phosphotransferase systems in Streptococcus mutans GS5-2. FEMS Microbiol. Lett. 42:7-11. [Google Scholar]
- 25.O'Farrell, P. H. 1975. High resolution two-dimensional electrophoresis of proteins. J. Biol. Chem. 250:4007-4021. [PMC free article] [PubMed] [Google Scholar]
- 26.Pelletier, G., M. Frenette, and C. Vadeboncoeur. 1994. Transport of mannose by an inducible phosphoenolpyruvate:fructose phosphotransferase system in Streptococcus salivarius. Microbiology 140:2433-2438. [DOI] [PubMed] [Google Scholar]
- 27.Pelletier, M., L. A. Lortie, M. Frenette, and C. Vadeboncoeur. 1998. The phosphoenolpyruvate:mannose phosphotransferase system of Streptococcus salivarius. Functional and biochemical characterization of IIABL(Man) and IIABH(Man). Biochemistry 37:1604-1612. [DOI] [PubMed] [Google Scholar]
- 28.Perry, D., and H. K. Kuramitsu. 1981. Genetic transformation of Streptococcus mutans. Infect. Immun. 32:1295-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Postma, P. W., J. W. Lengeler, and G. R. Jacobson. 1993. Phosphoenolpyruvate:carbohydrate phosphotransferase systems of bacteria. Microbiol. Rev. 57:543-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saier, M. H., Jr., S. Chauvaux, G. M. Cook, J. Deutscher, I. T. Paulsen, J. Reizer, and J. J. Ye. 1996. Catabolite repression and inducer control in Gram-positive bacteria. Microbiology 142:217-230. [DOI] [PubMed] [Google Scholar]
- 31.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 32.Shaw, W. V., L. C. Packman, B. D. Burleigh, A. Dell, H. R. Morris, and B. S. Hartley. 1979. Primary structure of a chloramphenicol acetyltransferase specified by R plasmids. Nature 282:870-872. [DOI] [PubMed] [Google Scholar]
- 33.Shiroza, T., and H. K. Kuramitsu. 1988. Sequence analysis of the Streptococcus mutans fructosyltransferase gene and flanking regions. J. Bacteriol. 170:810-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shiroza, T., S. Ueda, and H. K. Kuramitsu. 1987. Sequence analysis of the gtfB gene from Streptococcus mutans. J. Bacteriol. 169:4263-4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tao, L., and J. J. Ferretti. 1991. Cloning vectors with streptococcal resistance genes and an Escherichia coli origin of replication which can be used for cloning of streptococcal origins of replication or for gene inactivation in streptococci. American Society for Microbiology, Washington, D.C.
- 36.Ueda, S., T. Shiroza, and H. K. Kuramitsu. 1988. Sequence analysis of the gtfC gene from Streptococcus mutans GS-5. Gene 69:101-109. [DOI] [PubMed] [Google Scholar]
- 37.Vadeboncoeur, C., and M. Pelletier. 1997. The phosphoenolpyruvate:sugar phosphotransferase system of oral streptococci and its role in the control of sugar metabolism. FEMS Microbiol. Rev. 19:187-207. [DOI] [PubMed] [Google Scholar]
- 38.Vadeboncoeur, C., L. Thibault, S. Neron, H. Halvorson, and I. R. Hamilton. 1987. Effect of growth conditions on levels of components of the phosphoenolpyruvate:sugar phosphotransferase system in Streptococcus mutans and Streptococcus sobrinus grown in continuous culture. J. Bacteriol. 169:5686-5691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weickert, M. J., and G. H. Chambliss. 1990. Site-directed mutagenesis of a catabolite repression operator sequence in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 87:6238-6242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wen, Z. T., C. Browngardt, and R. A. Burne. 2001. Characterization of two operons that encode components of fructose-specific enzyme II of the sugar:phosphotransferase system of Streptococcus mutans. FEMS Microbiol. Lett. 205:337-342. [DOI] [PubMed] [Google Scholar]
- 41.Wexler, D. L., M. C. Hudson, and R. A. Burne. 1993. Streptococcus mutans fructosyltransferase (ftf) and glucosyltransferase (gtfBC) operon fusion strains in continuous culture. Infect. Immun. 61:1259-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]