Abstract
The nematode Heterorhabditis bacteriophora transmits a monoculture of Photorhabdus luminescens bacteria to insect hosts, where it requires the bacteria for efficient insect pathogenicity and as a substrate for growth and reproduction. Siderophore production was implicated as being involved in the symbiosis because an ngrA mutant inadequate for supporting nematode growth and reproduction was also deficient in producing siderophore activity and ngrA is homologous to a siderophore biosynthetic gene, entD. The role of the siderophore in the symbiosis with the nematode was determined by isolating and characterizing a mini-Tn5-induced mutant, NS414, producing no detectable siderophore activity. This mutant, being defective for growth in iron-depleted medium, was normal in supporting nematode growth and reproduction, in transmission by the dauer juvenile nematode, and in insect pathogenicity. The mini-Tn5 transposon was inserted into phbH; whose protein product is a putative peptidyl carrier protein homologous to the nonribosomal peptide synthetase VibF of Vibrio cholerae. Other putative siderophore biosynthetic and transport genes flanking phbH were characterized. The catecholate siderophore was purified, its structure was determined to be 2-(2,3-dihydroxyphenyl)-5-methyl-4,5-dihydro-oxazole-4-carboxylic acid [4-(2,3-dihydroxybenzoylamino)-butyl]-amide, and it was given the generic name photobactin. Antibiotic activity was detected with purified photobactin, indicating that the siderophore may contribute to antibiosis of the insect cadaver. These results eliminate the lack of siderophore activity as the cause for the inadequacy of the ngrA mutant in supporting nematode growth and reproduction.
Photorhabdus luminescens (Enterobacteriaceae) is an insect pathogen mutually associated with and transmitted by the entomopathogenic nematode Heterorhabditis bacteriophora (29, 42; for reviews, see references 21 and 22). The specialized dauer juvenile (DJ) stage of the nematode contains a pure culture of P. luminescens cells in the anterior region of the gut mucosa (14, 19). The DJ nematodes seek insect larvae and enter the hemocoel through natural openings or by penetrating the cuticle and then regurgitate their charge of P. luminescens (14, 35, 42). The bacteria alone are highly lethal to insect larvae when injected into the hemocoel, with less than 30 P. luminescens cells causing 50% mortality to insect larvae (LD50), but are not pathogenic when ingested by insect larvae (29, 35). The nematode requires P. luminescens for growth and reproduction in insect larvae and on artificial medium (2, 18). The bacteria produce antibiotics and bacteriocins that appear to inhibit other saprophytic microorganisms in the infected insect cadaver (1, 40, 44). Before nutrients in the insect are depleted, the nematodes again differentiate to the DJ stage, each colonized by P. luminescens in the intestine and disperse from the cadaver in search of another insect victim.
Iron is essential to most bacteria and is often found at limiting concentrations in soil and water habitats and in eukaryotic hosts. Larval stages of the tobacco hornworm, Manduca sexta, contain a ferritin-type iron binding protein in the hemolymph (26, 38). One mechanism that bacteria use to acquire iron from eukaryotic hosts is to produce siderophore molecules that have high affinity for iron and form soluble iron complexes to sequester and transfer ferric iron into the bacterial cells (for reviews, see references 16, 37, and 43). Members of the family Enterobacteriaceae typically produce catechol and hydroxamate siderophores (16, 17, 43), some of which are considered to be virulence factors that capture iron from its bound form, usually as ferritin, in eukaryotic hosts (43). Siderophores can also function in antibiosis; i.e., siderophores produced by rhizobacteria can inhibit the growth of pathogenic organisms in the rhizosphere and enhance plant growth (30, 54). Siderophore activity was detected in cultures of P. luminescens (4) but its role in nematode symbiosis or insect virulence was not studied.
We recently reported a mini-Tn5 transposon mutant of P. luminescens that was inadequate for nematode growth and reproduction and unable to express siderophore and antibiotic activities (13). The transposon-disrupted gene, ngrA, encodes a protein that is homologous to entD, a 4′-phosphopantetheinyl transferase (PPTase) that is required for the biosynthesis of the catechol siderophore enterobactin (15, 31). Members of the PPTase superfamily are required for the acyl or peptidyl carrier protein (ACP or PCP) activity (9, 31) involved in the biosynthesis of a great diversity of fatty acid, polyketide, and nonribosomally synthesized peptide molecules. It is plausible that the defect of the ngrA mutant in supporting nematode growth and reproduction is directly linked to siderophore biosynthesis since P. luminescens may produce a siderophore that requires a holo-PCP that is covalently modified by a PPTase enzyme for siderophore biosynthesis.
To determine if siderophore activity is necessary for P. luminescens to support the growth and reproduction of its nematode host, we used mini-Tn5 mutagenesis to produce mutants producing no detectable activity. One such mutant was tested for its effect on nematode symbiosis and for virulence to insect larvae. Purification and structure determination of the siderophore molecule and analysis of the genes involved in its synthesis and transport are also described.
MATERIALS AND METHODS
Microbial methods.
The sources of the strains and plasmids used in this study are shown in Table 1. Dyes, antibiotics, and other chemicals were purchased from Sigma Chemical Co. (St. Louis, Mo.), and microbiological media were obtained from Difco (Detroit, Mich.). Cells of P. luminescens were grown in 2% Proteose Peptone no. 3, with 1.5% agar added when required, at 29°C in the dark. Kanamycin (10 μg/ml), streptomycin (20 μg/ml), spectinomycin (20 μg/ml), and sucrose (7.5%, wt/vol) were added when required. For siderophore production, cells were grown in acid-washed glassware with iron-depleted A-2 minimal medium [20 mM glucose, 10 mM K2HPO4-KH2PO4 buffer (pH 7.0), 5 mM (NH4)2SO4, 0.5 mM MgSO4, 0.5 mM NaCl] or morpholinepropanesulfonic acid (MOPS) defined medium (36) supplemented with 3% (vol/vol) glycerol and 1× minimal essential medium-vitamin solution (Gibco Invitrogen Corp., Carlsbad, Calif.) that was prepared with Milli-Q purified water (Millipore Corp., Bedford, Mass.) and batch treated with Chelex resin in accordance with the manufacturer's (Bio-Rad Laboratories, Hercules, Calif.) instructions. Escherichia coli was propagated at 37°C in Luria-Bertani medium with 1.5% agar; ampicillin (100 μg/ml), chloramphenicol (20 μg/ml), kanamycin (10 μg/ml), streptomycin (20 μg/ml), spectinomycin (20 μg/ml), and sucrose (7.5%, wt/vol) were added when required.
TABLE 1.
Strains and plasmids used in this study
| Organism or plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| Nematodes | ||
| Heterorhabditis bacterio- phora | Strain NC1 | 29 |
| Heterorhabditis megidis | Meg | 41; H. Kaya |
| Bacteria | ||
| Photorhabdus lum- inescens | ||
| NP1/1 | Primary phase, isolated from H. bacteriophora (identical to ATCC 29304, strain NC-19) | 29 |
| NC1/2 | Secondary phase, isolated from NP100 | 13 |
| Meg/1 | Primary phase, isolated from H. megidis | 41 |
| NP394 | NP1/1 with pUB394 | 13 |
| NGR209 | NP1/1 ngrA::mini-Tn5 | 13 |
| NS414 | NP1/1 phbH::mini-Tn5 | This study |
| E. coli DH5α | Cloning strain | Gibco BRL |
| Plasmid vectors | ||
| pGEM11Z(+) | Cloning vector | Promega |
| pBC SK(+) | Cloning vector | Stratagene |
| pUB394 | Mini-Tn5 delivery vector; Kmr, Smr Spr sucrose | 13 |
| p414 | Retrieved plasmid with mini- Tn5 and flanking P. lumi- nescens DNA from NS414 | This study |
Transposon mutagenesis and screen for siderophore mutants.
Transposon-induced mutants were generated from NP394, which contains a transposon delivery vector, as described previously (13). Siderophore activity was assayed on chrome azurol S (CAS) medium (45). Bacteria producing siderophore activity formed a clear halo around the colony on the blue CAS medium because of the removal of Fe3+. One mutant, named NS414, not producing a halo on CAS medium was isolated and characterized.
Phenotypic characterization.
Expression of phase-dependent characteristics by P. luminescens cells was determined as described previously (4, 13) and repeated three times. The ability of P. luminescens mutants to support nematode growth and reproduction was determined by adding 30 DJ nematodes to lipid agar containing a lawn of P. luminescens cells as described previously (13). Nematode growth and reproduction were characterized by the recovery of DJ nematodes, development to hermaphrodites 7 days after the addition of DJ nematodes, and total DJ nematode yields 20 days after DJ nematode addition. The ability of P. luminescens cells to be retained in the DJ nematode gut mucosa was also determined as described previously by counting the CFU from homogenized, surface-sterilized, freshly harvested (<3 days old) DJ nematodes and DJ nematodes that had been incubated in saline for 30 days (14).
Insect pathogenicity was tested by injecting third-instar larvae of Manduca sexta (Carolina Biological Supply, Burlington, NC) or Galleria mellonella larvae (Ja-Da Bait Co., Antigo, Wis.) with serial dilutions of P. luminescens cells as described previously (13). Oral insecticidal activity (5) was assayed with 15×-concentrated cell-free supernatants obtained from 72-h Proteose Peptone no. 3 cultures. A 0.05-ml portion of the concentrate was applied to the surface of a 1-cm3 disk of insect diet, and a single first- or second-instar larva of M. sexta was added.
Molecular biological techniques.
Plasmid preparations were performed with Wizard minipreps in accordance with the manufacture's (Promega Corp., Madison, Wis.) instructions. Restriction enzymes were used in accordance with the manufacturer's (Promega Corp.) instructions, as were NsiI and T4 ligase (New England Biolabs Inc., Beverly, Mass.). The bacterial DNA was purified with a modified cetyltrimethylammonium bromide method (10). Transformation of E. coli and P. luminescens was done by electroporation with a Bio-Rad Gene Pulser under the conditions suggested for E. coli by the supplier (Bio-Rad).
Retrieval of DNA adjacent to the transposon insertion.
The DNA from NS414 was purified, restriction enzyme digested with NsiI (the mini-Tn5 transposon contains no NsiI sites), intramolecularly ligated (ligation reaction carried out in a 0.25-ml volume), ethanol precipitated, and transformed by electroporation into E. coli DH5α cells. Transformed cells containing the transposon were selected by their resistance to kanamycin. The plasmid was purified and restriction enzyme digested with NsiI and SfiI to verify that it contained a single restriction fragment and the mini-Tn5 transposon (determined by the presence of a 2.9-kb SfiI restriction fragment). A 26-kb plasmid containing the mini-Tn5 transposon and DNA flanking the insertion was retrieved from mutant NS414 and named p414.
Sequence analysis of p414.
The sequence of DNA flanking the transposon insertion of p414 was obtained by using M13 forward and reverse primers located 60 or 40 bp from the I-end or O-end inverted repeats of the transposon and by primer walking. The oligonucleotide primer 5′TAAGCGCCTTCCTGCATGGCTT3′ was used to sequence DNA flanking the alternative O end (13). An EcoRI restriction fragment from p414 was cloned into pGEM11Zf(+) (Promega Corp.) and sequenced with M13 forward and reverse sequencing primers and by primer walking. Dye terminator cycle sequencing with an ABI terminator mixture was performed under the conditions suggested by the supplier (Perkin-Elmer Corp., Foster City, Calif.), and the products were analyzed on an ABI 377 automated sequencer. The similarity of the DNA sequences to known sequences was determined by BLAST analysis (3).
Siderophore purification.
The siderophore was initially purified from 1.5 liters of culture broth obtained from P. luminescens cells grown while shaken at 29°C to stationary phase (72 h) in iron-depleted A-2 minimal medium (250 ml in each of eight 2-liter flasks). The cells were sedimented from the culture by centrifugation at 6,000 × g for 15 min. The supernatant fluid was extracted twice, for 15 min each time, with equal volumes of ethyl acetate, and the combined extracts were evaporated to dryness under vacuum at 50°C. The residue was dissolved in 1.0 ml of ethyl acetate to which 4.0 ml of methanol-water-acetic acid (90:9:1) was added. The resulting solution was diluted to 100 ml with water, and an insoluble residue was removed by centrifugation. The supernatant was applied to a C18 cartridge (Burdick & Jackson, Inc., Muskegon, Mich.) equilibrated with water. The cartridge was washed with 10 ml of water and then eluted sequentially with 10 ml each of 20, 40, 60, 80, and 100% acetonitrile. Siderophore activity was confined to the 40% acetonitrile fraction. Siderophore purification was performed on a Hewlett Packard 1100 high-performance liquid chromatograph equipped with a diode array detector. Aliquots from the 40% acetonitrile fractions were diluted fourfold with 0.1% trifluoroacetic acid (TFA), applied to a C4 column (4.66 by 250 mm; Vydac; Hesperia, Calif.), and eluted with a 1% min−1 gradient of acetonitrile in 0.1% TFA at 0.5 ml min−1.
Siderophore purification was improved by modifying the above-described procedure as follows. Siderophore was produced in 4.75 liters of MOPS defined medium and extracted twice overnight with 0.5 volume of ethyl acetate. The combined extract was evaporated to dryness under vacuum at 50°C and resuspended in 4.8 ml of 100% methanol, and then 115.2 ml of Milli-Q water was added. Ten-milliliter aliquots were applied to C18 SepPak cartridges (Alltech Associates, Deerfield, Ill.) equilibrated with water, and 20, 40, 60, 80, or 100% methanol was used instead of acetonitrile where the siderophore eluted in the 60% methanol fraction. Active fractions were pooled; methanol evaporated under a gentle stream of nitrogen gas; extracted with ethyl acetate, which was then evaporated under nitrogen gas; dissolved with 0.4 ml of 100% methanol, where 3.6 ml of Milli-Q water was added; applied to a Nucleosil C18 5-μm column (4.6 by 250 mm Supelco, Bellefonte, Pa.); and eluted with a 0.5% min−1 30 to 80% methanol gradient at 0.5 ml min−1 on an Isco 2350 high-performance liquid chromatograph equipped with a 2360 gradient programmer and a V4 absorbance detector set at 320 nm. A single peak containing siderophore activity was collected (92- to 94-min eluates); evaporated; dissolved in 0.05 ml of 100% methanol, where 0.45 ml of Milli-Q water was added; and applied to and eluted from the C18 column as described above. This procedure yielded 10.5 mg of pure siderophore that was used for subsequent structure determination.
Photobactin characterization.
Accurate mass measurements were performed on an Applied Biosystems Mariner time-of-flight spectrometer configured with a turbo-ion spray source operated in positive-ion mode (spray tip potential, 4,500 V; spray chamber temperature, 400°C; nozzle potential, 110 V). 1H (400 MHz) and 13C (100 MHz) nuclear magnetic resonance (NMR) spectra were recorded as a CD3OD solution at 300 K with a Bruker DRX 400 spectrometer equipped with a Nalorac 3-mm MdG-400B probe head. Chemical shifts were referenced to δ 3.30 and 49.0 for 1H and 13C spectra, respectively. A series of two-dimensional (2D) NMR experiments were used to secure the structure of photobactin. One-bond proton-carbon couplings were determined with a multiplicity-edited heteronuclear single quantum coherence experiment. Constant-time heteronuclear proton-carbon multiple-bond coherence data were used to establish long-range proton-carbon couplings. Homonuclear proton couplings were measured with a pure-absorption total correlated spectroscopy experiment.
Nucleotide sequence accession number.
The GenBank accession number for the DNA sequence flanking the mini-Tn5 transposon of p414 is AY042783.
RESULTS
Isolation and characterization of a siderophore-deficient mutant.
A mutant producing no detectable siderophore activity with the CAS plate assay (45), NS414, was obtained by screening 650 mini-Tn5-induced mutants.
NS414 resembles the wild type in most characteristics except in siderophore production and growth in iron-deficient medium. The NS414 cells grew poorly in iron-depleted A-2 minimal medium, reaching a maximal A600 of 0.05. Cells of NC1/1 reached an A600 of 0.3 in this medium. The addition of 10 μM (final concentration) ferric chloride or ferric sulfate to the A-2 medium allowed the NS414 cells to grow to an A600 of 0.3. These data indicate that siderophore production is involved in sequestering Fe3+ and is required for P. luminescens to grow efficiently under iron-limiting conditions.
NS414 and NC1/1 (wild type) were indistinguishable with respect to the symbiotic interactions with the nematode under the same experimental conditions in which an ngrA mutant (also unable to produce detectable siderophore activity) was unable to support nematode growth and reproduction (Table 2). Nematodes grew equally well on LA medium (13) in the presence of NS414 or NC1/1; inoculated DJ nematodes developed to hermaphrodites to the same extent, and final DJ nematode yields were similar. The DJ nematodes retained the NS414 and NC1/1 cells equally well, and these bacteria persisted equally well in DJ nematodes incubated in saline for 30 days (Table 2). Furthermore, DJ nematodes retaining NS414 and NC1/1 cells were equally pathogenic to insects (LD50 at 72 h, <30 DJ nematodes). NS414 and NC1/1 were similar in insect virulence when injected into G. mellonella or M. sexta larvae (LD50 at 72 h, <30 cells). The levels of insecticidal activity of NS414 and NC1/1 given orally were also similar. In summary, NS414 supported nematode growth and reproduction, colonized and persisted in DJ nematodes, and had insect virulence indistinguishable from that wild-type NC1/1 P. luminescens.
TABLE 2.
Comparison of the phenotypes of NS414 with those of NC1/1, NC1/2, and NGR209
| Phenotype assayed | Reaction of strain:
|
|||
|---|---|---|---|---|
| NC1/1 (primary) | NC1/2 (secondary) | NGR209 ngrA | NS414 | |
| Dye absorption | ||||
| Eosin Y-methylene blue | + | − | + | + |
| Neutral red | + | − | + | + |
| Bromthymol blue | + | − | + | + |
| Bioluminescence | + | − | + | + |
| Extracellular products | ||||
| Lipase activity | + | − | + | + |
| Hemolytic activity | + | − | + | + |
| Protease activity | + | − | + | + |
| Antibiotic activity | + | − | − | + |
| Siderophore activity | + | − | − | − |
| Colony morphology | Convex mucoid | Flat nonmucoid | Convex mucoid | Convex mucoid |
| Pigmentation | + | − | + | + |
| CipA and CipB production | + | − | + | + |
| Support of nematode growth and reproductionc | ||||
| Development of DJ nematodesa | 19 (6) | 0 | 0 | 18 (3) |
| Final yields of DJ nematodesb | >10,000 | 0 | 0 | >10,000 |
| Colonization of DJ nematodes | ||||
| 3 days postcollection | 88 (42) | NDd | 72 (27)e | 94 (37) |
| 30 days postcollection | 122 (60) | ND | 102 (39) | 110 (25) |
| G. melonella infectivity | + | ND | + | + |
| Insect pathogenicity | ||||
| Injected cellsf | + | + | + | + |
| Oral cell-free activityg | + | ND | + | + |
Mean numbers of adult hermaphrodites present from 30 DJ nematodes added; standard deviations are in parentheses.
Nematode growth and reproduction on LA and LCM (13) and in G. mellonella hosts from an inoculum of 30 DJ nematodes.
Numbers of CFU per DJ 3 or 30 days after collection of DJ nematodes. G. melonella infectivity is positive if the LD50 is <30 DJ nematodes 72 h after addition.
ND, Not determined.
When cocultured with Meg/1 to support nematode growth and reproduction.
LD50 of <10 CFU 72 h postinjection of third-instar M. sexta larvae.
No weight gain of third-instar M. sexta larvae when 0.05 ml of concentrated 20× cell-free supernatant from a 72-h culture was added to a 1-cm piece of diet.
NS414 cells were identical to NC1/1 cells in all of the characteristics observed, including the production of antibiotics, pigments, exopolysaccharides, and crystalline inclusion proteins; bioluminescence; and dye uptake (Table 2). The only detectable difference between NS414 and NC1/1 cells was the loss of siderophore production by NS414. Furthermore, no phase instability greater than that of NC1/1 was noticed when NS414 was subcultured for more than 6 months. It is evident that siderophore activity does not affect the expression of many primary-phase phenotypes or alter the stability of the primary phase.
The mini-Tn5 transposon of NS414 was inserted into an ORF homologous to nonribosomal peptide synthetases.
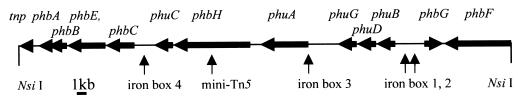
A 26-kb plasmid, p414, containing 22 kb of DNA flanking the mini-Tn5 transposon insertion was retrieved from NS414 cells. The physical map of this DNA region is shown in Fig. 1. The mini-Tn5 transposon was inserted into an ORF that is homologous to PCP modules of nonribosomal peptide synthetases (34, 46). The protein product of this gene, named phbH (for photobactin synthetase protein), is 34% identical and 50% similar to amino acid residues 1423 to 2155 of VibF (accession no. AAF02102), which are required for production of the siderophore vibriobactin by Vibrio cholerae (6, 27) (Table 3). This region corresponds to the thiolation and condensation domains of PCP modules (6, 34, 46). A serine residue present in the thiolation domain is indicative for covalent binding of phosphopantetheinate by a PPTase (31).
FIG. 1.
Structure of the DNA flanking the mini-Tn5 transposon insertion of NS414. Shown are the locations of the site of the mini-Tn5 transposon insertion and the locations of putative iron boxes.
TABLE 3.
Characteristics of genes flanking the mini-Tn5 transposon in mutant NS414a
| Gene | % Similarity/ % identity | Significance (E value) | Accession no. | Protein | Function(s) | Organism |
|---|---|---|---|---|---|---|
| phbF | 49/65 | 0 | AAF02102 | VibF | Nonribosomal peptide synthetase | V. cholerae |
| phbG | 32/49 | 4e − 54 | AAD48879 | VibH | Nonribosomal peptide synthetase | V. cholerae |
| phuB | 56/73 | 2e − 44 | AAD48880 | ViuP | Periplasmic binding protein | V. cholerae |
| phuG | 46/61 | 5e − 78 | AAD48882 | ViuG | Cytoplasmic membrane permease | V. cholerae |
| phuD | 52/65 | 7e − 75 | AAD48881 | ViuD | Cytoplasmic membrane permease | V. cholerae |
| phuA | 46/64 | 1e − 163 | AAF28471 | VuuA | Vulnibactin outer membrane receptor | V. vulnificus |
| phbH | 34/50 | 1e − 106 | AAF02102 | VibF | Nonribosomal peptide synthetase | V. cholerae |
| phuC | 64/77 | 1e − 92 | AAD29087 | FepC | Siderophore transport ATP binding | Y. enterocolitica |
| phbC | 56/68 | 8e − 89 | AAA16100 | EntC | Isochorismate synthase | E. coli |
| phbE | 66/79 | 0 | AAC73695 | EntE | 2,3-Dihydroxybenzoate-AMP ligase | E. coli |
| phbB | 55/70 | 1e − 85 | AAB40795 | EntB | 2,3-Dihydro-2,3-dihydroxy, benzoate synthase | E. coli |
| phbA | 63/79 | 2e − 88 | AAA76836 | EntA | 2,3-Dihydro-2,3-dihydroxy, benzoate dehydrogenase | E. coli |
| tnpA | 56/76 | 2e − 89 | AAA58209 | YhgA | Hypothetical transposase | E. coli |
Similarity values are for the most similar proteins, determined by BLASTp analysis (3), whose accession numbers, names, functions, and source organisms are also shown.
Two other genes, phbF and phbG, which are also homologous to nonribosomal peptide synthetases, were found in the vicinity of phbH. The protein product of phbF, like PhbH, is also similar to VibF, but to amino acid residues 187 to 391 and 490 to 1392, which correspond to aminoadenylation domains A1 to A10 (34). The adenylation domain determines the substrate specificity of the module, and “codons” have been suggested in 10 important amino acid residues that are indicative of the molecule to be adenylated (47). The codon of PhbF is 80% identical to the VibF codon (6, 28), which adenylates threonine, forming each of the two oxazoline rings found in the vibriobactin molecule (25, 27). The third putative nonribosomal peptide synthetase gene is phbG, whose protein product is similar to VibH of V. cholerae and EntF of E. coli (Table 2). Only the condensation-elongation C3 (His) domain characteristic of nonribosomal peptide synthetases is present in PhbG (34).
The phbA, phbB, phbC, and phbE genes are homologous to entA, entB, entC, and entE, which encode 2,3-dihydro-2,3-dihydroxybenzoate dehydrogenase, 2,3-dihydro-2,3-dihydroxybenzoate synthase, isochorismate synthase, and 2,3-dihydroxybenzoate-AMP ligase, respectively (Table 3) (32, 33, 55). These proteins are involved in the synthesis of the 2,3-dihydroxybenzoic acid moiety that is characteristic of the catechol class of siderophores. Homologous genes are also required for the biosynthesis of vibriobactin, which contains three 2,3-dihydroxybenzoic acid moieties (25, 52; see Fig. 4B). EntB is a multifunctional protein that has a C-terminal aryl carrier protein containing a thiolation domain that requires covalent modification by a PPTase (31). The predicted protein encoded by phbB also contains the thiolation domain (34). The protein encoded by phbE contains an adenylation domain with a codon that is similar to domains that adenylate 2,3-dihydroxybenzoate like EntE and VibE (28).
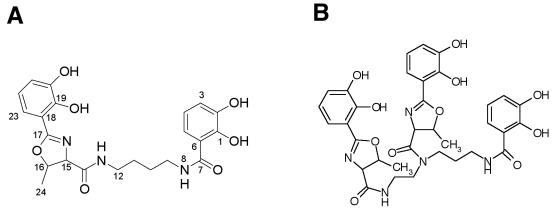
FIG. 4.
Structures of photobactin (A) and vibriobactin (B). The atom numbering scheme used to describe data from the NMR experiments is shown for photobactin.
The phuA, phuG, phuB, and phuC (photobactin uptake) genes are homologous to genes whose products are involved in TonB-dependent high-affinity transport systems (Fep) (17, 50). (Table 3). The phuA gene encodes a protein that is homologous to the outer membrane receptor for vulnibactin, VuuA, in V. vulnificus (51) and ViuA for vibriobactin in V. cholerae (7). The phuG gene encodes a protein that is homologous to ViuG, FepG, and other cytoplasmic membrane permease proteins (11, 12, 53). The phuB gene likely encodes a periplasmic ferric-siderophore binding protein analogous to the lipoprotein ViuP (53). The phuC gene encodes a protein that is homologous to ViuC and FepC, which are homologous to ATPase proteins of ABC transporters (11, 12, 53).
The tnpA gene is located at one 5′ end of the photobactin region, and its protein product is homologous to a group of hypothetical transposases and is most similar to YhgA from E. coli.
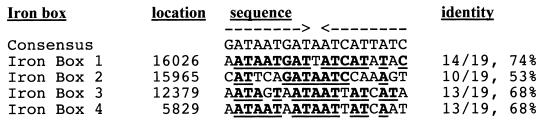
The photobactin gene region also contains four potential iron boxes (Fig. 1). Iron boxes 1 and 2, upstream of phuB, are 74 and 53% identical to the consensus iron box (8, 20) (Fig. 2). Iron box 3, located 5′ of phuA, is 68% identical to the consensus iron box. Iron box 4, located 5′ of phbC, is also 68% identical to the consensus iron box.
FIG. 2.
Alignment of putative iron boxes in p414. Bold and underlined residues are identical to the consensus sequence. Arrows above the sequence represent the inverted repeat of the iron box. The locations and percent identities of the four putative iron boxes are also shown.
Photobactin is a novel catechol-type siderophore.
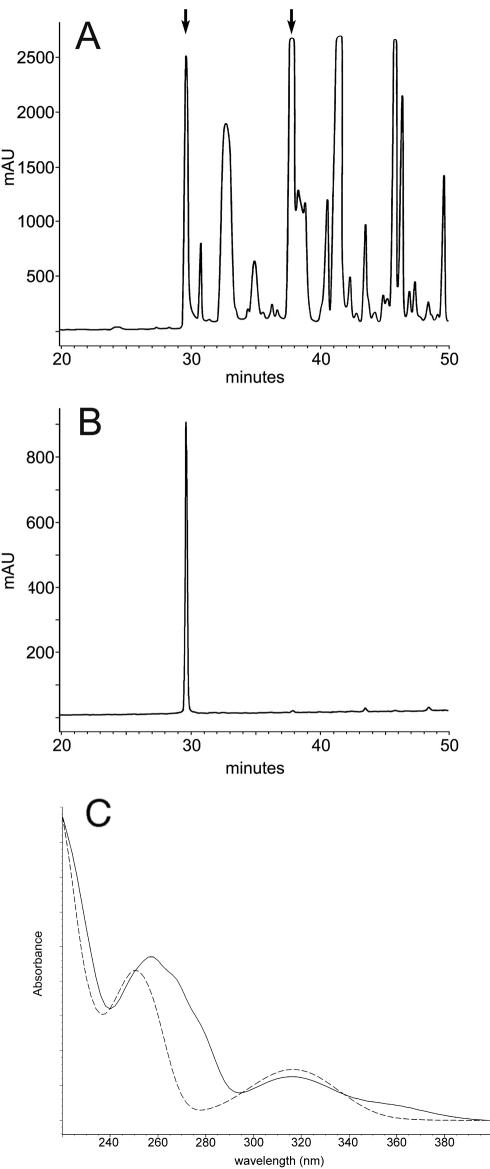
Two peaks with siderophore activity were detected from the C4 column eluted with a gradient of acetonitrile in 0.1% TFA. Most of the activity was observed in a peak eluting at 37 min, with lesser activity eluting at 29 min (Fig. 3A). However, on standing in the presence of 0.1% TFA, material from the 37-min peak also eluted at 29 min when reanalyzed under identical conditions, indicating alteration of the siderophore under acidic conditions (Fig. 3B). The UV spectrum of the 37-min peak obtained with the diode array detector was characteristic of catechol siderophores such as vibriobactin (25) and agrobactin (39), with absorbance maxima at 257 and 316 nm (Fig. 3C). The UV spectrum of the 29-min peak exhibited UV maxima at 250 and 316 nm. The shift of the absorbance maximum from 257 to 250 nm and the loss of absorbance in the 280-nm range were similar to those observed for the siderophore agrobactin under acidic conditions and indicate the presence of an oxazoline ring (39).
FIG. 3.
(A) C4 reverse-phase chromatograph of the siderophore-containing fraction obtained from the C18 cartridge. The arrows denote the peaks containing siderophore activity (29 to 30 min and 38 min). (B) When stored in 0.1% TFA, the 38-min peak shifted to 29 to 30 min. (C) UV spectra of the 38-min (solid line) and 29- to 30-min (dashed line) siderophore peaks. The shift of the absorbance maximum from 257 to 250 nm and the loss of absorbance in the 280-nm range indicate the opening of an oxazoline ring.
No alteration of the siderophore was observed when a methanol rather than an acetonitrile-0.1% TFA gradient was used to elute the siderophore from the C18 columns. This preparation of photobactin was used for structure determination. A molecular formula of C22H25N3O7 for photobactin was determined from electrospray ionization time-of-flight mass spectrometry (m/z 444.1765 [M+ + H calculated 444.1765]) and 13C NMR data. The structure of photobactin (Fig. 4A) was readily determined from a series of 2D NMR experiments. The full chemical shift assignments and key 2D NMR correlations appear in Table 4. The International Union of Pure and Applied Chemistry name for this molecule is 2-(2,3-dihydroxyphenyl)-5-methyl-4,5-dihydro-oxazole-4-carboxylic acid [4-(2,3-dihydroxybenzoylamino)-butyl]-amide. The structure of photobactin (Fig. 4A) is related to that of vibriobactin (Fig. 4B).
TABLE 4.
NMR data for photobactin
| Positiona | δC | δH, multiplicity, J (Hz) | HMBCb |
|---|---|---|---|
| 1 | 150.3 | ||
| 2 | 147.3 | ||
| 3 | 119.56 or 119.52 | 6.91, dd, 8.0, 1.5 | C-1, C-5 |
| 4 | 119.56 or 119.52 | 6.69, t, 8.0 | C-2, C-6 |
| 5 | 118.6 | 7.18, dd, 8.0, 1.5 | C-1, C-3, C-7 |
| 6 | 116.7 | ||
| 7 | 171.5 | ||
| 9 | 40.13 or 40.06 | 3.38, br t, 6.5 | C-7, C-10 or C-11 |
| 10 | 27.79 or 27.75 | 1.61, m | C-11 |
| 11 | 27.79 or 27.75 | 1.61, m | C-10 |
| 12 | 40.13 or 40.06 | 3.28, br t, 6.5 | C-10 or C-110, C-14 |
| 14 | 172.9 | ||
| 15 | 75.8 | 4.41, d, 7.5 | C-14, C-16, C-17, C-24 |
| 16 | 80.7 | 4.86, dd, 7.5, 6.5 | C-14, C-17 |
| 17 | 168.4 | ||
| 18 | 111.8 | ||
| 19 | 149.5 | ||
| 20 | 146.7 | ||
| 21 | 120.3 | 6.94, dd, 8.0, 1.5 | C-19, C-24 |
| 22 | 119.92 or 119.87 | 6.72, t, 8.0 | C-18, C-20 |
| 23 | 119.92 or 119.87 | 7.15, dd, 8.0, 1.5 | C-17, C-19, C-21 |
| 24 | 21.3 | 1.51, t, 6.5 | C-15, C-16 |
Position as indicated in Fig. 4A.
HMBC, heteronuclear multibond conductivity.
A limited amount of pure photobactin was assayed for antibiotic activity. Approximately 100 μg was dissolved in 0.1 ml of 100% methanol, and 10 μg was applied to a 6-mm-diameter filter disk, which was placed on an overnight culture of indicator bacteria. These experiments were repeated twice. Antibiotic activity was detected with Micrococcus luteus (3-mm zones of inhibition), Bacillus cereus (4-mm zones of inhibition), and Staphylococcus aureus (1.5-mm zones of inhibition) but not with E. coli or Bacillus subtilis.
DISCUSSION
Here we report the purification, the proposed structure, and most of the genes involved in the biosynthesis and transport of a novel catecholate siderophore, photobactin, produced by P. luminescens. This study was initiated to determine if siderophore production is required for P. luminescens to support the growth and reproduction of its nematode host. The rationale for this hypothesis was that ngrA, encoding a putative PPTase often involved in siderophore biosynthesis, is necessary for the bacteria to support the growth and reproduction of its nematode host and to produce siderophore activity (13). Indeed, phbH, encoding a putative PCP likely requiring a PPTase for its activity, is required for siderophore activity but not for symbiosis with the nematode. Thus, the siderophore is not the nematode growth- and reproduction-promoting factor that was absent in the ngrA mutant.
The proposed structure of photobactin (Fig. 4A) is related to the siderophore molecules vibriobactin (25; Fig. 4B) and agrobactin (39) produced by V. cholerae and Agrobacterium tumefaciens, respectively. Photobactin contains a putrescine backbone versus the spermidine and norspermidine backbones of agrobactin and vibriobactin, respectively, and as a consequence of one less amide group of putrescine, photobactin contains one less oxazoline-2,3-dihydroxybenzoicacid or 2,3-dihydroxybenzoic acid moiety.
Since photobactin and vibriobactin are structurally related, it is not surprising that the corresponding biosynthetic and transport genes are also similar, with some important differences. Genes involved in photobactin biosynthesis and transport are clustered in a single gene region, in contrast to the vibriobactin biosynthesis uptake and transport genes that are present in two regions of the V. cholerae chromosome, and many of these genes are arranged differently. One terminus of the photobactin gene cluster contains a transposase-like gene that may indicate that this region is present in or originated from a mobile genetic element like a virulence plasmid or pathogenicity island. The thiolation and adenylation domains of the unusual nonribosomal peptide synthetase VibF (27) are present as two subunits in P. luminescens, with PhbH containing the thiolation domain and PhbF containing the adenylation and cyclization domains. Because putrescine, versus norspermidine, is the amine backbone of photobactin, biochemical characterization of PhbF may reveal different specificities for acylation of primary amines than that of VibF (27). These unique characteristics of PhbH and PhbF may be useful in engineering polyketide or nonribosomal peptide synthetases for the biosynthesis of novel natural products. Finally, it is interesting that a PPTase seems to be absent from the photobactin gene cluster while it is present in the vibriobactin gene cluster, suggesting that NgrA (13) may perform this function.
NgrA is most similar to E. coli EntD, which is a PPTase required for the biosynthesis of the catechol siderophore enterobactin (15, 23, 31) and to the putative V. cholerae VibD PPTase found in the vicinity of vibriobactin biosynthesis and uptake genes (51). The disrupted phbH gene of NS414 encodes a PCP-containing thiolation domain. Covalent modification of PCPs with a 4′-phosphopantetheinyl moiety by a PPTase is required for PCP activity (24, 48, 49). Another gene, phbB, located in the vicinity of phbH, likely encodes an aryl carrier protein that also requires a PPTase for its activity (23). NgrA may covalently modify the thiolation domains of PhbH or PhbB, since a PPTase was not found in this siderophore region. However, the ability of the phbH mutant NS414 to support nematode growth and reproduction suggests that the phosphopantethenylation of another PCP or ACP, involved in the biosynthesis of an unknown metabolite, is the cause for the inadequacy of the ngrA mutant of P. luminescens in supporting nematode growth and reproduction. It is possible that NgrA regulates the activity of multiple PCPs or ACPs through posttranslational phosphopantethenylation.
Mutant NS414 resembled the wild-type bacteria in all characteristics other than siderophore production. It is significant that the characteristics of nematode symbiosis, insect virulence, and phase variation were unchanged. Therefore, the mini-Tn5 transposon-disrupted siderophore gene phbH is not essential for nematode symbiosis or insect virulence. However, mutant NS414 grows poorly in iron-deficient medium, indicating that phbH is required for growth in low-iron environments. Insects, like humans, produce high-affinity iron binding proteins, possibly to maintain a low concentration of iron in the hemolymph (26, 38). Since NS414 grows poorly in iron-deficient medium, it appears that the insect cadaver is not iron limited or that P. luminescens may have other mechanisms by which to obtain iron from insects, such as the action of secreted hemolysins, lipases, or proteases. Although NS414 does produce antibiotic activity, probably through the production of other broad-spectrum antibiotics (1, 40, 44), siderophore production may contribute to antibiosis in the insect host since purified siderophore has antibiotic activity. When growing in insect larvae, P. luminescens produces antibiotics that are thought to inhibit competing saprophytic bacteria (1, 40, 44). Photobactin may perform a similar role by sequestering iron in the insect cadaver.
The data presented herein clearly show that the inability of the ngrA mutant to support nematode growth and reproduction is not due to loss of siderophore activity. The symbiotic defect of the ngrA mutant must therefore be due to the inability to produce another nonribosomally synthesized peptide, polyketide, or lipopeptide molecules that require PPTase for its biosynthesis. The search for the NgrA target required for nematode symbiosis is thus narrowed.
Acknowledgments
This research was supported by a S. C. Johnson Wax Distinguished Scientist Fellowship awarded to T.A.C. and by the College of Agriculture and Life Sciences, University of Wisconsin—Madison.
REFERENCES
- 1.Akhurst, R. J. 1982. Antibiotic activity of Xenorhabditis spp., bacteria associated with insect pathogenic nematodes of the families Heterorhabditidae and Steinernematidae. J. Gen. Microbiol. 128:3061-3065. [DOI] [PubMed] [Google Scholar]
- 2.Akhurst, R. J., R. G. Mourant, L. Baud, and N. E. Boemare. 1996. Phenotypic and DNA relatedness study between nematode-symbiotic and clinical strains of the genus Photorhabdus (Enterobacteriaceae). Int. J. Syst. Bacteriol. 43:249-255. [DOI] [PubMed] [Google Scholar]
- 3.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 4.Bintrim, S. B., and J. C. Ensign. 1998. Insertional inactivation of genes encoding the crystalline inclusion proteins of Photorhabdus luminescens results in mutants with pleiotropic phenotypes. J. Bacteriol. 180:1261-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bowen, D. J., and J. C. Ensign. 1998. Purification and characterization of a high-molecular-weight insecticidal protein complex produced by the entomopathogenic bacterium Photorhabdus luminescens. Appl. Environ. Microbiol. 64:3029-3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butterton, J. R., M. H. Choi, P. I. Watnick, P. A. Carroll, and S. B. Calderwood. 2000. Vibrio cholerae VibF is required for vibriobactin synthesis and is a member of the family of nonribosomal peptide synthetases. J. Bacteriol. 182:1731-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butterton, J. R., J. A. Stoebner, S. M. Payne, and S. B. Calderwood. 1992. Cloning, sequencing, and transcriptional regulation of viuA, the gene encoding the ferric vibriobactin receptor of Vibrio cholerae. J. Bacteriol. 174:3729-3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calderwood, S. B., and J. J. Mekalanos. 1988. Confirmation of the Fur operator site by insertion of a synthetic oligonucleotide into an operator fusion plasmid. J. Bacteriol. 170:1015-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cane, D. E., C. T. Walsh, and C. Khosla. 1998. Harnessing the biosynthetic code: combinations, permutations and mutations. Science 282:63-68. [DOI] [PubMed] [Google Scholar]
- 10.Chan, J. W. Y. F., and P. H. Goodwin. 1994. Extraction of genomic DNA from extracellular polysaccharide-synthesizing Gram-negative bacteria. BioTechniques 18:419-422. [PubMed] [Google Scholar]
- 11.Chenault, S. S., and C. F. Earhart. 1991. Organization of genes encoding membrane proteins of the Escherichia coli ferrienterobactin permease. Mol. Microbiol. 5:1405-1413. [DOI] [PubMed] [Google Scholar]
- 12.Chenault, S. S., and C. F. Earhart. 1992. Identification of hydrophobic proteins FepD and FepG of the Escherichia coli ferrienterobactin permease. J. Gen. Microbiol. 138:2167-2171. [DOI] [PubMed] [Google Scholar]
- 13.Ciche, T. A., S. B. Bintrim, A. H. Horswill, and J. C. Ensign. 2001. A phosphopantetheinyl transferase homolog is essential for Photorhabdus luminescens to support the growth and reproduction of the entomopathogenic nematode Heterorhabditis bacteriophora. J. Bacteriol. 183:3117-3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ciche, T. A., and J. C. Ensign. 2003. For the insect pathogen Photorhabdus luminescens, which end of a nematode is out? Appl. Environ. Microbiol. 69:1890-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coderre, P. E., and C. F. Earhart. 1989. The entD gene of the Escherichia coli K12 enterobactin gene cluster. J. Gen. Microbiol. 135:3043-3055. [DOI] [PubMed] [Google Scholar]
- 16.Crosa, J. H., and C. T. Walsh. 2002. Genetics and assembly line enzymology of siderophore biosynthesis in bacteria. Microbiol. Mol. Biol. Rev. 66:223-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Earhart, C. F. 1987. Ferrienterobactin transport in Escherichia coli, p. 67-84. In G. Winkelmann, D. Van der Helm, and J. B. Neilands (ed.), Iron transport in microbes, plants and animals. VCH Publishers, Weinheim, Federal Republic of Germany.
- 18.Ehlers, R. D., S. Stoessel, and U. Whyss. 1990. The influence of phase variants of Xenorhabdus spp. and Escherichia coli (Enterobacteriaceae) on the propagation of entomopathogenic nematodes of the genera Steinernema and Heterorhabditis. Rev. Nematol. 13:417-424. [Google Scholar]
- 19.Endo, B. Y., and W. R. Nickle. 1991. Ultrastructure of the intestinal epithelium, lumen, and associated bacteria in Heterorhabditis bacteriophora. J. Helminthol. Soc. Wash. 58:202-212. [Google Scholar]
- 20.Escolar, L., J. Pèrez-Marìn, and V. de Lorenzo. 1998. Binding of the Fur (ferric uptake regulator) repressor of Escherichia coli to arrays of the GATAAT sequence. J. Mol. Biol. 283:537-547. [DOI] [PubMed] [Google Scholar]
- 21.Forst, S., and K. H. Nealson. 1996. Molecular biology of the symbiotic-pathogenic bacteria Xenorhabdus spp. and Photorhabdus spp. Microbiol. Rev. 60:21-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forst, S., B. Dowds, N. Boemare, and E. Stackebrandt. 1997. Xenorhabdus spp. and Photorhabdus spp.: bugs that kill bugs. Annu. Rev. Microbiol. 51:47-72. [DOI] [PubMed] [Google Scholar]
- 23.Gehring, A. M., K. A. Bradley, and C. T. Walsh. 1997. Enterobactin biosynthesis in Escherichia coli: isochorismate lyase (EntB) is a bifunctional enzyme that is phophopantetheinylated by EntD and then acylated by EntE using ATP and 2,3-dihydroxybenzoate. Biochemistry 36:8495-8503. [DOI] [PubMed] [Google Scholar]
- 24.Gocht, M., and M. A. Marahiel. 1994. Analysis of core sequences in the D-phe activating domain of the multifunctional peptide synthetase TycA by site-directed mutagenesis. J. Bacteriol. 176:2654-2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griffiths, G. L., S. P. Sigel, S. M. Payne, and J. B. Neilands. 1984. Vibriobactin, a siderophore from Vibrio cholerae. J. Biol. Chem. 259:383-385. [PubMed] [Google Scholar]
- 26.Huebers, H. A., E. Huebers, C. A. Finch, B. A. Webb, J. W. Truman, L. M. Riddiford, A. W. Marin, and W. H. Massover. 1988. Iron binding proteins and their roles in the tobacco hornworm, Manduca sexta (L.). J. Comp. Physiol. B 158:291-300. [DOI] [PubMed] [Google Scholar]
- 27.Keating, T. A., C. G. Marshall, and C. T. Walsh. 2000. Reconstitution and characterization of the Vibrio cholerae vibriobactin synthetase from VibB, VibE, VibF and VibH. Biochemistry 39:15522-15530. [DOI] [PubMed] [Google Scholar]
- 28.Keating, T. A., and C. T. Walsh. 1999. Initiation, elongation, and termination strategies in polyketide and polypeptide antibiotics. Curr. Opin. Chem. Biol. 3:598-606. [DOI] [PubMed] [Google Scholar]
- 29.Khan, A., and W. M. Brooks. 1976. A chromogenic bioluminescent bacterium associated with the entomophilic nematode Chromonema heliothidis. J. Invertebr. Pathol. 29:253-261. [Google Scholar]
- 30.Kloepper, J. W., J. Leong, M. Teintze, and M. N. Schroth. 1980. Enhanced plant growth by siderophores produced by plant growth-promoting rhizobacteria. Nature 286:885-886. [Google Scholar]
- 31.Lambalot, R. H., A. M. Gehring, R. S. Flugel, P. Zuber, M. LaCelle, M. A. Marahiel, R. Reid, C. Khosla, and C. T. Walsh. 1996. A new enzyme superfamily—the phosphopantetheinyl transferases. Chem. Biol. 3:923-936. [DOI] [PubMed] [Google Scholar]
- 32.Liu, J., K. Duncan, and C. T. Walsh. 1989. Nucleotide sequence of a cluster of Escherichia coli enterobactin biosynthesis genes: identification of entA and purification of its product 2,3-dihydro-2,3-dihydroxybenzoate dehydrogenase. J. Bacteriol. 171:791-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luke, R. K. J., and F. Gibson. 1971. Location of three genes concerned with the conversion of 2,3-dihydroxybenzoate into enterochelin in Escherichia coli K-12. J. Bacteriol. 107:557-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marahiel, M. A., T. Stachelhaus, and H. D. Mootz. 1997. Modular peptide synthetases involved in nonribosomal peptide synthesis. Chem. Rev. 97:2651-2674. [DOI] [PubMed] [Google Scholar]
- 35.Milstead, J. E. 1979. Heterorhabditis bacteriophora as a vector for introducing its associated bacterium into the haemocoel of Galleria mellonella larvae. J. Invertebr. Pathol. 33:324-327. [Google Scholar]
- 36.Neidhardt, F. C., P. L. Bloch, and D. F. Smith. 1974. Culture medium for enterobacteria. J. Bacteriol. 119:736-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neilands, J. B. 1981. Microbial iron compounds. Annu. Rev. Biochem. 50:715-731. [DOI] [PubMed] [Google Scholar]
- 38.Nichol, H., J. H. Law, and J. J. Winzerling. 2002. Iron metabolism in insects. Annu. Rev. Entomol. 47:535-539. [DOI] [PubMed] [Google Scholar]
- 39.Ong, S. A., T. Peterson, and J. B. Neilands. 1979. Agrobactin, a siderophore from Agrobacterium tumefaciens. J. Biol. Chem. 254:1860-1865. [PubMed] [Google Scholar]
- 40.Paul, V. J., S. Frautschy, W. Fenical, and K. H. Nealson. 1981. Antibiotics in microbial ecology: isolation and structure assignment of several new antibacterial compounds from the insect-symbiotic bacteria Xenorhabdus spp. J. Chem. Ecol. 7:589-597. [DOI] [PubMed] [Google Scholar]
- 41.Poinar, G. O., Jr., T. Jackson, and M. Klein. 1987. Heterorhabditis megidis sp. n. (Heterorhabditae: Rhabditida), parasitic in the Japanese beetle, Popillia japonica (Scarabaeidae: Coleoptera), Ohio. Proc. Helminthol. Soc. Wash. 54:53-59. [Google Scholar]
- 42.Poinar, G. O., Jr., G. M. Thomas, and R. Hess. 1977. Characteristics of the specific bacterium associated with Heterorhabditis bacteriophora (Heterorhabditidae: Rhabditida). Nematologica 23:97-102. [Google Scholar]
- 43.Ratledge, C., and L. G. Dover. 2000. Iron metabolism in pathogenic bacteria. Annu. Rev. Microbiol. 54:881-941. [DOI] [PubMed] [Google Scholar]
- 44.Richardson, W. H., T. M. Schmidt, and K. H. Nealson. 1988. Identification of an anthraquinone pigment and a hydroxystilbene antibiotic from Xenorhabdus luminescens. Appl. Environ. Microbiol. 54:1602-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwyn, B., and J. B. Neilands. 1987. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 160:47-56. [DOI] [PubMed] [Google Scholar]
- 46.Stachelhaus, T., A. Hüser, and M. A. Marahiel. 1996. Biochemical characterization of peptidyl carrier protein (PCP), the thiolation domain of multifunctional peptide synthetases. Chem. Biol. 3:913-921. [DOI] [PubMed] [Google Scholar]
- 47.Stachelhaus, T., H. D. Mootz, and M. A. Marahiel. 1999. The specificity-conferring code of adenylation domains in nonribosomal peptide synthetases. Chem. Biol. 6:493-505. [DOI] [PubMed] [Google Scholar]
- 48.Stein, T., B. Kluge, J. Vater, P. Franke, A. Otto, and B. Wittmann-Liebold. 1995. Gramicidin S synthetase 1 (phenylalanine racemase), a prototype of amino acid racemases containing the cofactor 4′-phosphopantetheine. Biochemistry 34:4633-4642. [DOI] [PubMed] [Google Scholar]
- 49.Walsh, C. T., A. M. Gehring, P. H. Weinreb, L. E. N. Quadri, and R. S. Flugel. 1997. Post-translational modification of polyketide and nonribosomal peptide synthetases. Curr. Opin. Chem. Biol. 1:309-315. [DOI] [PubMed] [Google Scholar]
- 50.Wang, C. C., and A. Newton. 1971. An additional step in the transport of iron defined by the tonB locus of Escherichia coli. J. Biol. Chem. 121:497-503. [PubMed] [Google Scholar]
- 51.Webster, A. C. D., and C. M. Litwin. 2000. Cloning and characterization of vuuA, a gene encoding the Vibrio vulnificus ferric vulnibactin receptor. Infect. Immun. 68:526-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wyckoff, E. E., J. A. Stoebner, K. E. Reed, and S. M. Payne. 1997. Cloning of a Vibrio cholerae gene cluster: identification of genes required for early steps in siderophore biosynthesis. J. Bacteriol. 179:7055-7062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wyckoff, E. E., A. M. Valle, S. L. Smith, and S. M. Payne. 1999. A multifunctional ATP-binding cassette transporter system from Vibrio cholerae transports vibriobactin and enterobactin. J. Bacteriol. 181:7588-7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu, G.-W., and D. C. Gross. 1986. Selection of fluorescent pseudomonads antagonistic to Erwinia carotovora and suppressive of potato seed piece decay. Phytopathology 76:414-422. [Google Scholar]
- 55.Young, I. G., L. Langman, R. K. J. Luke, and F. Gibson. 1971. Biosynthesis of the iron-transport compound enterochelin: mutants of Escherichia coli unable to synthesize 2,3-dihydroxybenzoate. J. Bacteriol. 106:51-57. [DOI] [PMC free article] [PubMed] [Google Scholar]