Abstract
The fresh-cut produce industry has been the fastest-growing portion of the food retail market during the past 10 years, providing consumers with convenient and nutritious food. However, fresh-cut fruits and vegetables raise food safety concerns, because exposed tissue may be colonized more easily by pathogenic bacteria than intact produce. This is due to the higher availability of nutrients on cut surfaces and the greater potential for contamination because of the increased amount of handling. We found that applied Listeria monocytogenes populations survived and increased only slightly on fresh-cut Red Delicious apples stored at 10°C but increased significantly on fresh-cut honeydew melons stored at 10°C over 7 days. In addition, we examined the effect of lytic, L. monocytogenes-specific phages via two phage application methods, spraying and pipetting, on L. monocytogenes populations in artificially contaminated fresh-cut melons and apples. The phage mixture reduced L. monocytogenes populations by 2.0 to 4.6 log units over the control on honeydew melons. On apples, the reduction was below 0.4 log units. In combination with nisin (a bacteriocin), the phage mixture reduced L. monocytogenes populations by up to 5.7 log units on honeydew melon slices and by up to 2.3 log units on apple slices compared to the control. Nisin alone reduced L. monocytogenes populations by up to 3.2 log units on honeydew melon slices and by up to 2.0 log units on apple slices compared to the control. The phage titer was stable on melon slices, but declined rapidly on apple slices. The spray application of the phage and phage plus nisin reduced the bacterial numbers at least as much as the pipette application. The effectiveness of the phage treatment also depended on the initial concentration of L. monocytogenes.
Over the past decade, the frequency of reported outbreaks of illnesses due to foodborne pathogens has increased (Outbreak Alert, Center for Science in the Public Interest [www.cspinet.org]). There may be a number of possible reasons for this increase, including increased consumption of minimally processed or fresh-cut produce and better epidemiologic surveillance programs (4). Fresh-cut produce is a rapidly growing $10 to 12 billion/year industry and accounts for more than 10% of all produce sales in the United States with an annual growth rate between 10 and 20% (International Fresh-Cut Produce Association [http://www.fresh-cuts.org/fcf.html]). The growth potential of foodborne pathogens is greater on fresh-cut produce than on produce with the peel or rind intact, because there are more nutrients available on the cut surface and the only preservation used is refrigeration (10, 23).
One foodborne human pathogen, Listeria monocytogenes, has been associated with a number of serious foodborne outbreaks and recalls (6). The bacterium is a psychrotroph, since it survives and grows at temperatures as low as 8°C and can therefore become a problem in refrigerated foods (5, 10, 30). L. monocytogenes contamination has been primarily associated with the consumption of dairy products, beef, pork, poultry, and seafood. However, an increasing body of data supports and suggests that salad vegetables, such as cabbage, celery, lettuce, cucumber, onion, leeks, watercress, radish, tomatoes, and fennel, among others, can have a high incidence of L. monocytogenes, and some of these products have been implicated in outbreaks of foodborne listeriosis (4, 24, 28). Little work has been done regarding the capacity of fruit to support the survival and/or growth of this pathogen (5), but outbreaks of listeriosis usually occur at pathogen populations greater than 103 CFU per g or per ml (29). The L. monocytogenes serotypes 1/2a, 1/2b, and 4b are of particular concern, since they account for up to 96% of human listeriosis cases throughout the world (29). Because of the high case fatality rate associated with L. monocytogenes infections, the U.S. Food and Drug Administration (FDA and the U.S. Department of Agriculture Food Safety Inspection Service have established a zero-tolerance (no detectable level permitted) for L. monocytogenes in ready-to-eat foods, including processed fresh-cut fruits and vegetables. Several food recalls have been triggered by possible L. monocytogenes contamination during the last few years, including a recall of packaged fresh-cut apples (23 to 27 March 2001; see FDA website).
Previously, we reported on the control of Salmonella by phages on fresh-cut fruits (21). The use of naturally occurring, lytic phages to reduce contamination of fresh-cut produce with foodborne pathogens has several advantages over the use of chemical sanitizers and washes (21, 26). For example, methods commonly used in industry, such as aqueous washes containing chlorine formulations or plain water, are nonspecific and can achieve a less-than-10-fold reduction in Listeria populations on cut-produce surfaces (15). Alternatively, specific phages attack the targeted pathogens only, thus preserving the competitive potential of the indigenous microflora (26). They also can reduce the bioburden on produce of those bacteria that are resistant to antibiotics (18). Homologous phages and bacteriophage pools have been used against spoilage bacteria on meat (12, 13, 14). To reduce the potential for the development of phage-resistant mutants, we applied a cocktail of different phages. Lytic bacteriophages (phages) may provide an effective alternative for decontaminating fresh-cut fruits that may contain various bacterial pathogens.
Nisin, a broad-spectrum, pore-forming bacteriocin, is produced by lactic acid bacteria that are often found on produce (17, 22). It is active against many gram-positive bacteria, including L. monocytogenes (16). Nisin is especially active at the lower pH values typical of many fruits and some vegetables (27). Nisin is the only commercially available bacteriocin recognized as a safe and legal biological food preservative by the Food and Agriculture Organization and World Health Organization (technical memorandum TM 1-1e SPEC; Danisco Cultor, Brabrand, Denmark) as well as the FDA (9). Its use at different levels has been approved in more than 70 countries for application to a number of food stuffs such as dairy or fermentation products, alone and with added vegetables, fruits, or meats, baked goods, liquid egg products, canned foods, and beer.
The objectives of this model study were to determine (i) the survival and growth of L. monocytogenes applied to fresh-cut apple and honeydew melon slices at 10°C, an abusive temperature; (ii) the effectiveness of specific, lytic phages in controlling L. monocytogenes contamination on fresh-cut fruits; (iii) the effectiveness of different phage application methods and solutions; and (iv) the effectiveness of combining the phage treatment with the application of the bacteriocin nisin.
MATERIALS AND METHODS
Fruit.
Red Delicious apples and honeydew melons obtained from the market were cut into 10-mm-thick slices with a deli slicer (Model 827; Berkel Inc., La Porte, Ind.). For honeydew pieces, the 10-mm-thick melon rings were cut into equally sized squares about 30 mm2. The fruit surfaces as well as the deli meat slicer were disinfected with 70% ethanol immediately before slicing. A cork borer was used to cut tissue plugs of apple and honeydew that were 10 mm thick and 10 mm in diameter, resulting in tissue plugs of 0.785 cm3.
Phage.
The phage mixtures LM-103 and LMP-102, which contained 14 and 6 distinct lytic phages, respectively, are specific for L. monocytogenes, including the serotypes 1/2a, 1/2b, and 4b, which are predominantly associated with human listeriosis. The mixtures were provided by Intralytix, Inc. (Baltimore, Md.). The phage concentration was approximately 109 PFU/ml in 1 M phosphate-buffered saline (pH 7.4). The mixture was diluted either with 0.85% NaCl (pH 6.5 to 7.0) or with sterile water (pH 6.5 to 7.0) to approximately 5 × 107 PFU/ml immediately before application to the fruit slices. A buffered solution (phosphate-buffered saline) for phage application was tested to determine if it would improve the effectiveness in reducing L. monocytogenes populations compared to phage application in water. The reduction achieved by the two different phage mixtures, Lm-103 and Lm-102, was the same in our experiments according to the statistical analysis. The mixtures were used interchangeably.
Nisin.
The bacteriocin nisin, manufactured as Nisaplin (Aplin & Barrett Ltd., Beaminster, Dorset, United Kingdom), was obtained from Sigma (Sigma-Aldrich, St. Louis, Mo.). Solutions containing 1, 200, and 400 IU/25 μl of nisin were prepared immediately before use by dissolving Nisaplin in sterile distilled water. For experiments where only one nisin concentration was used, the concentration was 400 IU/25 μl.
Preparation of the bacterial inoculum.
The L. monocytogenes culture, strain LCDC 81-861 serotype 4b, from an outbreak from cabbage, was obtained from Robert Brackett, Department of Food Science and Technology, University of Georgia, Agricultural Experiment Station, Griffin, Georgia 30223. The strain was selected for resistance to nalidixic acid (NAL). For inoculation of the fruit slices, L. monocytogenes cultures were grown overnight on tryptic soy agar (TSA) plates (BD Diagnostic Systems, Sparks, Md.) with 100 μg of NAL/ml at 37°C and then in Luria-Bertani broth (BD Diagnostic Systems) for 6 h. The cells were harvested by centrifugation at 10,000 × g for 15 min and washed once with sterile saline solution (0.85% [wt/vol] NaCl). The pellet was resuspended in saline solution and adjusted to a concentration of 5 × 108 CFU/ml at an optical density at 420 nm of 0.15 using a SmartSpec 3000 spectrophotometer (Bio-Rad Laboratories, Richmond, Calif.). About 30 ml of the suspension was diluted to a concentration of about 5 × 105 CFU/ml before being added to the slices unless otherwise noted. The exact cell concentration of the inoculum was determined by spiral plating on TSA followed by incubation at 37°C for 1 day.
For growth of Listeria at different pH values, 50 ml of tryptic soy broth in Erlenmeyer flasks was adjusted with HCl or NaOH to a pH of 3.5, 4.0, 4.5, 5.0, 5.5, 6.0, 6.5, 7.0, 8.0, or 9.0. After inoculation with 50 μl of an L. monocytogenes overnight culture, the flasks were placed on a gyratory shaker (150 rpm) at 10°C, and samples were taken immediately after inoculation and after 90 h. In addition, flasks containing about 108 CFU of L. monocytogenes/ml grown for 3 h were inoculated with 107 PFU of the LMP-102 phage/ml and placed on a gyratory shaker (100 rpm) at 30°C. Samples were taken immediately after inoculation as well as 2 and 24 h after inoculation, and those with and without phage were compared.
Application of the treatments.
The fruit tissue plugs were randomly placed in sterile 100- by 16-mm glass test tubes. The fruit squares were placed in commercial 530-ml dome fruit plastic bowls (#518; Rock-Tenn. Co., Chicago Plastics, Franklin Park, Ill.). The fruit tissue was then inoculated with 25 μl of the L. monocytogenes suspension containing approximately 5 × 105 CFU/ml. The procedure for inoculating the pieces of fruit took approximately 10 min. Then, the phage and/or nisin treatments were applied by pipette to a 5- by 5-mm area in 25-μl aliquots or as a spray to runoff (<250 μl/square) to the entire square. The phage mixture was applied to the fruit pieces before the other treatments. There were four fruit samples per treatment at each recovery time. In the spray tests there were four samples, each including two subsamples at each recovery time. The tubes were then loosely capped for sterility. The covers on the plastic bowls allowed air exchange, which ensured that the environmental conditions (O2 and CO2 content) did not change and therefore did not create a modified atmosphere.
Efficacy of phage on various bacterial concentrations.
To determine whether the concentration of the bacterial contamination had an influence on the effectiveness of the phage treatment, we compared the decrease in the bacterial population at two initial concentrations (105 and 106 CFU/ml). Honeydew melon squares were inoculated with L. monocytogenes at 105 or 106 CFU/ml and treated with the same phage concentration at least 2 log units higher than the bacterial concentration. The bacteria were recovered after 0, 2, 5, and 7 days. The log CFU/sample values of the recovered bacterial populations were analyzed as a three-factor general linear mixed model with treatment and time as the fixed factors and experiment as a random block.
The difference between the control mean and the phage mean for concentrations of 105 and 106 was examined in one of the experiments. The hypothesis that the phage suppressed Listeria equally for both concentrations was tested at each sampling time (after 0, 2, 5, and 7 days).
Effect of magnesium.
The addition of Mg is known to aid phage packaging and adsorption to the bacteria (3, 8). The fruit squares were inoculated with L. monocytogenes as described above. To determine whether the addition of Mg increased the efficacy of the phage treatment, two concentrations of 25 μl of a magnesium-amino acid-chelate (lot# 203331; Albion Laboratories, Inc., Clearfield, Utah), 14 mM (3 mg/ml) and 42 mM (8.98 mg/ml), were added with or without the phage. Log (CFU) values of the recovered bacterial populations were analyzed as a two-factor general linear model with treatment and time as the factors. Since the time × treatment interaction was statistically significant, mean comparisons were done.
Recovery of bacteria.
Recovery and quantification of the L. monocytogenes populations from the tissue plugs were performed after 0, 2, 5, and 7 days of storage at 10°C as described previously (5). Briefly, the tissue plugs were each placed into a sterile plastic bag containing 4.5 ml of buffered peptone water and homogenized in a stomacher blender for 120 s, speed setting 8 (Bagmixer 100 Minimix; Interscience). Aliquots (50 μl) of the homogenized mixtures or dilutions thereof were plated in duplicate on TSA-agar, using a spiral plater (DW Scientific; Shipley, West Yorkshire, England). The agar contained 100 μg of NAL (Sigma)/ml. The plates were incubated overnight at 30°C. Colony counts were determined using an automated plate counter (ProtoCol; Synoptics, Cambridge, United Kingdom), and the data were plotted as CFU per sample. All experiments were repeated.
Phage titration.
Samples from phage treatments in each experiment were homogenized and then filtered through a 0.45-μm-pore-size membrane (Acrodisk; Pall Gelman, Ann Arbor, Mich.). The phage titer in the filtrates was determined using a soft agar overlay (1). The resulting plaques were counted with the ProtoCol plate counter (Synoptics), and the data were plotted as PFU per sample.
Environmental parameters.
At the beginning and the end of the experiment the internal atmosphere of the container and the pH of the fruit were determined. Six-milliliter gas samples collected from the headspace of 16- by 100-mm glass tubes containing the tissue were analyzed for O2 and CO2 levels using O2 and CO2 analyzers (S-3A and CD-3A, respectively; Ametek, Pittsburgh, Pa.). The pH values of the apple and melon slices were determined using an MI-410 combination pH microelectrode (Microelectrodes, Inc., Bedford, N.H.).
Statistical analyses.
The bacterial recovery data (CFU per sample) were transformed to log units, log 10 (x + 1). One was added to the values to allow the use of zero values in the analyses. Since most values were quite large, this had little to no effect on the analysis results. Any treatments where all the values were zero or had the same value (no variability) were omitted from the analysis.
The data were first analyzed using Proc Mixed (SAS Institute) as linear models with the most factors that would include all the treatments. The data were also analyzed as higher-order linear models, although not all treatments could be included in these models. The experiments set up at different times were treated as blocks and included in the preliminary models as both a random and a fixed effect. If the block effect accounted for very little variability, it was dropped from later models. The assumptions of the general linear model were tested. When necessary to correct for variance heterogeneity, the variance grouping technique was used. When effect(s) were statistically significant, mean comparisons were done with Sidak adjusted P values so that the experiment-wise error was 0.05.
RESULTS
Bacterial populations and pH.
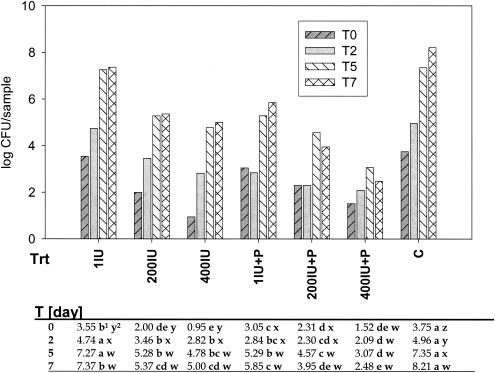
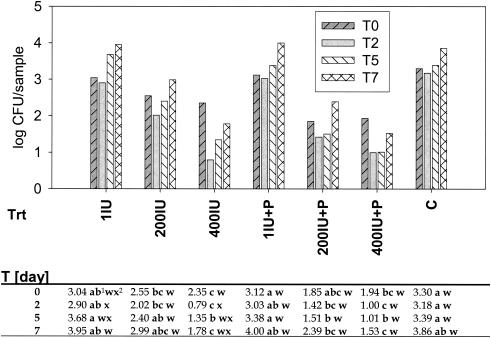
L. monocytogenes survived and populations were higher than the initial populations by 0.6 log units on Red Delicious apple tissue (pH 4.4) and by 4.5 log units on honeydew melon slices (pH 5.8) after 7 days at 10°C (Fig. 1 and 2).
FIG. 1.
Recovery of L. monocytogenes from honeydew melon slices treated with nisin or nisin plus phage and stored over 7 days (T0 to T7) at 10°C. Superscript numbers: 1, treatment means within time with different (a, b, c, or d) letters are different at the 0.05 significance level (across); 2, time means within treatment with different (w, x, y, or z) letters are different at the 0.05 significance level (down). The log CFU values were analyzed as a three-factor general linear model with block, treatment, and time as factors. Variance grouping was used to correct for variance heterogeneity. As the time × treatment interaction was statistically significant, mean comparisons were done. Nisin treatments were as follows. 1IU, 200IU, 400IU: 1, 200, or 400 IU of nisin, respectively; P, phage treatment; C, L. monocytogenes control.
FIG. 2.
Recovery of L. monocytogenes from apple slices treated with nisin or nisin plus phage and stored over 7 days (T0 to T7) at 10°C. Log (CFU) values were analyzed as a two-factor general linear model with treatment and time as the factors. Variance grouping was used to correct for variance heterogeneity. Nisin treatments were as follows. 1IU, 200IU, 400IU: 1, 200, or 400 IU of nisin, respectively; P, phage treatment; C, L. monocytogenes control. As the time × treatment interaction was statistically significant, mean comparisons were done. Superscript numbers: 1, treatment means within time with different (a, b, c, or d) letters are different at the 0.05 significance level (across); 2, time means within treatment with different (w, x, y, or z) letters are different at the 0.05 significance level (down).
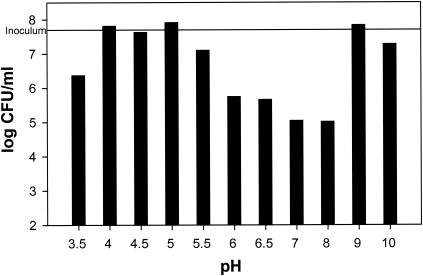
The bacterial populations on apples were lower after 2 days than at the time of inoculation, but then they recovered and were higher during the subsequent testing period (Fig. 2). Bacterial populations continuously increased on honeydew melon squares for 7 days at 10°C (Fig. 1). The growth of L. monocytogenes in broth was pH dependent, with little growth below pH 4.5, increasing growth between pH 5 and 6, and optimum growth between pH 6 and 8 (results not shown). In liquid culture, the phage also was active between pH values of 5.5 and 8. The greatest reduction of L. monocytogenes populations was achieved within a pH range of 7 to 8 (Fig. 3).
FIG. 3.
Growth (log CFU/ml) at 30°C of a L. monocytogenes broth culture (5 × 107 CFU/ml) at different pH values 2 h after inoculation with a phage mixture (107 PFU/ml).
Treatment with nisin and phages.
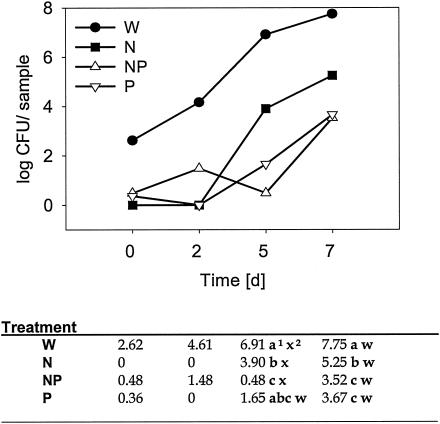
In two sets of experiments with honeydew melons and Red Delicious apples, the effect of nisin on L. monocytogenes was determined at three different concentrations alone or together with the phage treatment. Treatments with phage alone reduced the bacterial populations on honeydew melons (Fig. 4). Treatments with phage plus nisin or nisin alone reduced the bacterial populations on both apples and honeydew melons (Figs. 2 and 4). On honeydew melons, bacterial populations treated with nisin at 200 and 400 IU were 2.8 to 3.2 log units lower than the control after seven days (Fig. 1). In comparison, addition of the phage and nisin reduced the bacterial populations by 4.3 to 5.7 log units. There was a phage × nisin interaction on honeydew melons (Table 1). Combining phage and nisin concentrations of 1, 200, and 400 IU reduced the bacterial populations at different rates, with the highest reduction at 400 IU. However, without the addition of phage, the treatments with 200 and 400 IU of nisin were not different.
FIG. 4.
Populations of L. monocytogenes on honeydew melons following spray treatment with water (W), nisin (N), phage (P), or a combination of nisin and phage (NP) and stored at 10°C over 7 days. Superscript numbers: 1, treatment means within time with different (a, b, c, or d) letters are different at the 0.05 significance level; 2, time means within treatment with different w's and x's are different at the 0.05 significance level. Times zero and 2 were omitted in the statistical analysis because of zero values. The log (CFU) values were analyzed as two-factor general linear model with time and treatment as the factors. Variance grouping was used to correct for variance heterogeneity. As the time × treatment interaction was significant, mean comparisons were done. Times zero and 2 were not included in the statistical analysis because of zero values.
TABLE 1.
Recovery of L. monocytogens from honeydew melon slices treated with (P+) or without (P−) the phage in interaction with different nisin concentrationsa
| Treatment | Interaction mean (log CFU/sample) for nisin concn (IU) of:
|
||
|---|---|---|---|
| 1 | 200 | 400 | |
| P − | 5.81 ab wc | 4.07 a x | 3.62 a x |
| P + | 4.18 b w | 3.25 b x | 2.43 b y |
The log (CFU) values were analyzed as a four-factor general linear model with block, time, nisin, and phage as the factors. Variance grouping was used to correct for variance heterogeneity. The data from fruit treated only with L. monocytogenes were not included in the analysis. Since the nisin × phage interaction was statistically significant, mean comparisons were done.
Phage means within nisin with different (a or b) letters are different at the 0.05 significance level.
Nisin means within phage with different (w, x, or y) letters are different at the 0.05 significance level.
Overall, on apple slices, the bacterial populations on phage-treated slices (mean = 2.18; P = 0.05) were statistically smaller than on those without the phage (mean = 2.48; P = 0.05). The treatment with 200 and 400 IU of nisin reduced the bacterial populations by approximately 0.9 to 2.0 log units and in combination with the phage treatment by approximately 1.5 to 2.3 log units after 7 days (Fig. 2). There was no difference between the 200- and 400-IU nisin treatments on apple slices.
On both fruits, the combination of the phage with nisin at various concentrations reduced the bacterial populations more than nisin alone (Tables 1 and 2). On honeydew, phage plus 400 IU of nisin was more effective than phage plus 200 IU of nisin for the first 2 days after inoculation, but there was no difference after 5 and 7 days (Table 2). On apple slices only the reduction on fruit treated with 400 IU was greater than on those treated with 1 IU of nisin (Fig. 2).
TABLE 2.
Recovery of L. monocytogens from honeydew melon slices treated with (P+) or without (P−) the phage in interaction with different nisin concentrationsa
| Treatment | Interaction mean (log CFU/sample) for Time (days)
|
|||
|---|---|---|---|---|
| 0 | 2 | 5 | 7 | |
| Nisin | ||||
| 1 IU | 3.17 ab zc | 3.74 a y | 6.31 a x | 6.75 a w |
| 200 IU | 2.17 b y | 2.93 b x | 4.92 b w | 4.60 b w |
| 400 IU | 1.32 c y | 2.41 c x | 4.40 b w | 3.97 b w |
| Phage | ||||
| P− | 2.17 ab yc | 3.70 a x | 5.93 a w | 6.20 a w |
| P+ | 2.28 a x | 2.35 b x | 4.49 b w | 4.01 b w |
The log (CFU) values were analyzed as a four-factor general linear model with block, time, nisin, and phage as the factors. Variance grouping was used to correct for variance heterogeneity. The L. monocytogenes control was not included in the analysis. Since the time × nisin and time × phage interactions were statistically significant, mean comparisons were done.
Treatment means within time with different (a, b, or c) letters are different at the 0.05 significance level.
Time means within nisin with different (w, x, y, or z) letters are different at the 0.05 significance level.
When nisin and the phage were applied as a spray, there was a time × treatment interaction (Fig. 4). The reduction in bacterial populations was greatest after 5 and 7 days in the nisin-phage combination treatment but was not different from the phage treatment alone. The nisin treatment alone reduced pathogen populations, but not as well as the phage treatment or the phage-nisin combination treatment.
Effect of phage on different bacterial concentrations.
The reduction of the initial bacterial populations by the phage treatment was greater for 105 than for 106 CFU of bacteria/ml (Table 3). At the early sampling times the relative suppression of L. monocytogenes by the phage was the same for the two concentrations, but after 5 and 7 days of storage the phage suppressed L. monocytogenes populations of 105 CFU/ml more than at 106 CFU/ml.
TABLE 3.
Reduction of L. monocytogenes populations at two different initial concentrations by the phage treatment and comparison of the reductiona
| Time (days) | Reduction of L.m. populations (CFU/sample) with initial L.m. concn (CFU/ml) ofb:
|
||||
|---|---|---|---|---|---|
| 105
|
106
|
||||
| Ctrl − Pc | P value | Ctrl − Pc | P value | Comparison of reduction, 105 vs. 106 CFU/sample (P value) | |
| 0 | 0.6205 | 0.0058 | 0.5641 | <0.0001 | 0.7763 |
| 2 | 2.0832 | <0.0001 | 2.0669 | <0.0001 | 0.8702 |
| 5 | 2.2129 | <0.0001 | 1.6939 | <0.0001 | 0.0100 |
| 7 | 1.4356 | <0.0001 | 0.7370 | <0.0006 | 0.0100 |
The bacteria were recovered from honeydew melon slices stored for 7 days at 10°C.
L. m., L. monocytogenes.
Numbers indicate L. monocytogenes populations on the control (Ctrl) minus L. monocytogenes populations on the phage-treated samples (P).
Phage solution and application method.
Recovery of L. monocytogenes from control treatments (no phage) was greater when buffer rather than water was applied at the time of inoculation and after 7 days (Table 4). There was no statistical difference in the bacterial populations recovered from slices treated with phage in either solvent, but the trend was toward a consistently lower bacterial population in the water control and for phage in water using the spray application. However, when the phage mixture was applied with a pipette, the trend was reversed. In this case, the bacterial populations recovered from the fruit slices treated with phage in water were consistently larger than those treated with phage in buffer.
TABLE 4.
Effect of two different phage-carrying solutions and application methods on the recovery of L. monocytogenes from honeydew melon slices contaminated with 105 CFU of the bacterium/ml
| Application method | Solution | Mean recovery (log CFU/sample) for recovery time (days)d
|
|||
|---|---|---|---|---|---|
| 0 | 2 | 5 | 7 | ||
| No phage | Buffer | 3.21 aa | 4.73 a | 7.04 a | 7.73 a |
| Water | 2.51 b | 4.12 a | 6.86 a | 6.86 b | |
| Phage, pipette | Buffer | 1.70 a | 2.55 a | 4.99 a | 5.40 a |
| Water | 1.78 a | 2.98 a | 5.31 a | 5.94 a | |
| Phage, spray | Buffer | 1.52 a | 2.15 a | 4.55 a | 5.19 a |
| Water | 1.53 a | 1.85 a | 3.65 a | 4.73 a | |
| No phage | NAb | 2.86 xc | 4.43 x | 6.95 x | 7.64 x |
| Pipette | NA | 1.74 y | 2.77 y | 5.15 y | 5.67 y |
| Spray | NA | 1.52 y | 2.00 y | 4.10 yz | 4.96 y |
Solution means within application method with different (a or b) letters are different at the 0.05 significance level.
NA, data for buffer and water treatments were combined and then analyzed.
Application means within time with different (x, y, or z) letters are different at the 0.05 significance level.
The phage was applied in buffer or water as a spray or with a pipette. The log (CFU/sample) values were analyzed as a three-factor general linear model using Proc Mixed (Sas Institute) with solution, time, and application as the factors. Variance grouping was used to correct for variance heterogeneity. Means and mean comparisons are given. Time and application as well as solution × application and time × application interactions were significant.
There was no statistical difference between the application methods in respect to the efficacy of the phage (Table 4). The phage treatment applied either way reduced bacterial populations compared with the control. However, while not statistically significant, the pathogen populations were consistently smaller in the samples treated with the spray application than in those with pipette application. Addition of a magnesium chelate either had no effect or increased populations of L. monocytogenes on honeydew melons treated with the phage (data not shown). In addition, the bacterial populations increased over time.
Phage titer.
The titer of L. monocytogenes-specific phages sprayed onto honeydew melon remained between 3.9 and 5.4 logs of PFU. It increased by about 1 log unit over a period of 7 days on honeydew melon. The phage titer was similar on fruit treated with L. monocytogenes with or without nisin and was not affected at the nisin concentrations examined. On apple slices the phage titer declined rapidly to nondetectable levels within 30 min after application.
Environmental parameters.
The partial pressure of O2 and CO2 inside the tubes indicated that the atmospheric conditions did not change during 7 days of storage at 10°C. The O2 content was between 20.54 and 20.69%, and the CO2 content was between 0.08 and 0.11%. The pH measurements of the fruit tissue plugs indicated no significant change. The average pH on apples was 4.37 and on honeydew was 5.77.
DISCUSSION
We found that treatment with a Listeria-specific lytic phage cocktail alone or in combination with nisin is an effective method for reducing L. monocytogenes contamination on fresh-cut fruit. This is similar to our earlier report with respect to Salmonella and Salmonella-specific phages on honeydew melons (21).
The recovery of L. monocytogenes populations from honeydew melon pieces stored at 10°C over 7 days was several log units higher than the recovery from Red Delicious apples. The pH of honeydew melons is approximately 5.5 to 6.5, and that of Red Delicious is 3.8 to 4.2. This pH difference may be a major factor contributing to the differences in the bacterial populations on the produce and is in agreement with the growth dynamics of L. monocytogenes at various pH values with phage added, where pH values above 5 result in a greater reduction in bacterial populations. These results are similar to those of our previous experiments with Salmonella growing on lower-pH fresh-cut apples and higher-pH honeydew melons. The more rapid increase of L. monocytogenes populations compared to Salmonella on honeydew melons stored at 10°C may be due to the greater cold tolerance of L. monocytogenes (21).
Of the many bacteriocins produced by lactic acid bacteria, nisin is the only one approved as a preservative in 50 countries and the only purified bacteriocin that is commercially available (11, 27). The fact that the bactericidal activity of nisin increases at a pH below 5, due to its greater solubility at low pH (25, 31), makes it suitable for use on fruit, the pH of which is typically in the range of 3 to 6. Foods that have been implicated in Listeria outbreaks generally contain more than 3 log units of bacteria per gram or milliliter (29). Using the combination of a phage cocktail and nisin, L. monocytogenes populations decreased by 5.8 log units (to a final population of 1.4 CFU/ml) and by 3.5 log units (to a final population of 0.6 CFU/ml) on honeydew and apple slices, respectively. These reductions on fresh-cut fruit are greater than those achieved with aqueous chemical sanitizers or by washing with water, and they show that the nisin treatment complements the activity of the phage on produce with different pH values. By combining these two treatments, we further reduced the likelihood of bacteria developing resistance to both nisin and the phages. Using combined treatments is consistent with the hurdle concept by Leistner (19), who describes the effective control of foodborne pathogens by using a combination of compatible control measures to ensure the safety of food. The phage treatment is a new and effective hurdle, which can be combined with nisin and other control measures, such as pH and temperature, to maximize protection from foodborne pathogens on fresh-cut fruits (20).
On honeydew melons, generally, control of L. monocytogenes increased with increasing nisin concentration. The phage-nisin combination was more effective than the nisin treatment alone, especially after longer storage periods. The fact that the number of bacteria recovered from fruit treated with the two highest nisin concentrations was statistically different only when the phage was added to the treatment may be due to an interaction between the phage and nisin. This is conceivable, since nisin creates nonselective and temporary pores in the cytoplasmic membrane, and the phage may be better able to infect such a weakened cell (17, 22). Nisin also helps to reduce L. monocytogenes populations initially, while the phage cocktail helps to keep pathogen populations low over time. A stable phage titer on honeydew melons, independent of the addition of nisin, further confirms the usefulness of this treatment combination. The addition of a magnesium amino acid chelate as a source of Mg improved the growth of the bacterium more than it aided the phage and therefore is not recommended as an additive to increase phage efficacy.
On apple slices (pH 4.4), the initial L. monocytogenes populations recovered were smaller than those on honeydew melon, due most likely to the sensitivity of the bacteria to the lower pH of apples. The population reduction on apples was mostly due to the nisin treatment, because the phage treatment alone did not reduce the bacterial populations. We previously reported that a lower pH can reduce or inactivate Salmonella phage populations (2, 21), and the titer of the Listeria-specific phage mixture used in this study on low-pH apple slices also declined rapidly to nondetectable levels. As with honeydew melon, there was an interaction between the phage and nisin on apples. In the presence of nisin, L. monocytogenes populations were smaller on phage-treated samples than on those not treated with the phage. This indicates that there were still active phages present on the apple slices. A possible explanation is that a part of the phages' infection mechanism may have been impaired by the acidic pH of the apples, because in the presence of nisin they were again able to reduce bacterial populations. Nisin, as mentioned above, can form temporary pores in the bacterial membrane (17, 22). The successful use of phages on fresh-cut produce with a low pH, such as that of apples, may require the application of higher concentrations of phage or the development of low-pH-tolerant phage mutants. The other alternative on acidic fruit is the combination of the phage with bactericidal compounds, such as nisin, which is more effective at a low pH, resulting in a small additive effect.
The spray application was compared to the pipette application, which simulated the dipping process. Even though the amounts of liquid applied with the two application methods were different, they were comparable per contaminated area. The phage titer on honeydew squares treated with either application method was equal or higher for the pipette application than for the spray application. The method of the phage or phage and nisin application did not influence the efficacy of the treatment on honeydew melons. However, we observed a trend of consistently lower bacterial populations treated with the spray in comparison to the pipette application. Taking into consideration that a higher titer was recovered from honeydew squares treated by pipette application, in some cases, the spray application was more effective.
The phage application on honeydew melons was more effective over time than nisin. Nisin was effective for up to 2 days and could be used as an additional safety hurdle. However, nisin was not necessary for achieving the same long-term results as when the phage was applied alone on honeydew melons. While there was no difference between the sprayed phage and the phage-plus-nisin application, the trend was toward lower bacterial populations in the combination treatment. One reason may be that nisin loses activity after a short period of time, and another may be the possible development of nisin-resistant mutants. Therefore, the nisin-phage combination treatment may reduce the risk of the emergence of nisin-resistant mutants. Although nisin was initially very effective in reducing bacterial populations of Listeria, the phage application keeps the populations low over a longer time period (Fig. 4).
Increasing the initial phage/Listeria ratio enhanced the efficacy of the phage in reducing bacterial populations. Contamination of fresh-cut produce with foodborne pathogens is not likely to occur at the high bacterial levels used in our experiments. Thus, phage treatment may be a useful way to reduce and maintain low populations of foodborne pathogens before problems, which may develop at levels of 3 log units or higher (29), occur on fresh-cut produce.
In conclusion, phage and nisin applications reduce pathogenic bacterial contamination and growth on produce, and when implemented can contribute to the microbial safety of fruits and vegetables. On fruit with a lower pH, the addition of nisin at higher concentrations complements phage activity in the control of L. monocytogenes. In addition, the effect of nisin on reducing L. monocytogenes is short term compared to the phage treatment. A spray application may increase the effectiveness of the phage through better coverage of the slices. Phages may provide a novel, environmentally safe alternative for control of bacterial contamination of produce. They can be used alone or in combination with other control measures, and are compatible with at least some of the practices currently used in the food industry, to reduce the contamination of whole or fresh-cut fruits and vegetables by foodborne pathogens.
Acknowledgments
We thank A. B. Blodgett, K. Green, M. Shaw, and J. L. McEvoy for their valued assistance. Arnold Kreger and Torrey Brown are gratefully acknowledged for their helpful suggestions and editorial comments.
Funding was provided in part by Intralytix, Inc., through a CRADA agreement with the U.S. Department of Agriculture. A. Sulakvelidze holds an equity interest in Intralytix.
REFERENCES
- 1.Adams, M. H. 1959. Bacteriophages. Interscience Publishers, New York, N.Y.
- 2.Alisky, J., K. Iczkowski, A. Rapoport, and N. Troitsky. 1998. Bacteriophages show promise as antimicrobial agents. J. Infect. 36:5-15. [DOI] [PubMed] [Google Scholar]
- 3.Arber, W., L. Enquist, B. Hohn, N. Murray, and K. Murray. 1983. Experimental methods for use with Lambda. In R. Hendrix, J. Roberts, F. Stahl, and R. Weisberg (ed.), Lambda II. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 4.Beuchat, L. 2002. Ecological factors influencing survival and growth of human pathogens on raw fruits and vegetables. Microbes Infect. 4:413-423. [DOI] [PubMed] [Google Scholar]
- 5.Conway, W. S., B. Leverentz, R. A. Saftner, W. J. Janisiewicz, C. E. Sams, and E. Leblanc. 2000. Survival and growth of Listeria monocytogenes on fresh-cut apple slices and its interaction with Glomerella cingulata and Penicillium expansum. Plant Dis. 84:177-181. [DOI] [PubMed] [Google Scholar]
- 6.Farber, J. M., and P. I. Peterkin. 1991. Listeria monocytogenes, a food-borne pathogen. Microbiol. Rev. 55:476-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farber, J. M., S. L. Wang, Y. Cai, and S. Zhang. 1998. Changes in populations of Listeria monocytogenes inoculated on packaged fresh-cut vegetables. J. Food Prot. 61:192-195. [DOI] [PubMed] [Google Scholar]
- 8.Feiss, M., and A. Becker. 1983. DNA packaging and cutting. In R. Hendrix, J. Roberts, F. Stahl, and R. Weisberg (ed.), Lambda II. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 9.Food and Drug Administration/Center for Food Safety and Applied Nutrition. 2001. Direct food substances affirmed as generally recognized as safe, p. 462-464.Title 21 of the Code of Federal Regulations—Food and Drugs, chapter 1, part 184. Food and Drug Administration, Department of Health and Human Services, Washington, D.C.
- 10.Francis, G. A., C. Thomas, and D. O'Beirne. 1999. The microbial safety of minimally processed vegetables. Int. J. Food Sci. Technol. 34:1-22. [Google Scholar]
- 11.Gould, G. W. 1996. Industry perspectives on the use of natural antimicrobials and inhibitors for food applications. J. Food Prot. 1996 (Suppl.): 82-86. [DOI] [PubMed]
- 12.Greer, G. G. 1986. Homologous bacteriophage control of Pseudomonas growth and beef spoilage. J. Food Prot. 49:104-109. [DOI] [PubMed] [Google Scholar]
- 13.Greer, G. G. 1990. Inability of a bacteriophage pool to control beef spoilage. Int. J. Food Microbiol. 10:331-342. [DOI] [PubMed] [Google Scholar]
- 14.Greer, G. G., and B. D. Dilts. 2002. Control of Brochotrix thermosphacta spoilage of pork adipose tissue using bacteriophages. J. Food Prot. 65:861-863. [DOI] [PubMed] [Google Scholar]
- 15.Han, Y., R. H. Linton, S. S. Nielsen, and P. E. Nelson. 2001. Reduction of Listeria monocytogenes on green peppers (Capsicum annum L.) by gaseous and aqueous chlorine dioxide and water washing and its growth at 7C. J. Food Prot. 64:1730-1738. [DOI] [PubMed] [Google Scholar]
- 16.Holzapfel, W. H. 1995. Biological preservation of foods with reference to protective cultures, bacteriocins and food-grade enzymes. Int. J. Food Microbiol. 24:343-362. [DOI] [PubMed] [Google Scholar]
- 17.Jack, R. W., G. Bierbaum, and H.-G. Sahl. 1998. Biological activities, p. 157-190. Lantibiotics and related peptides. Springer-Verlag and Landes Bioscience, Georgetown, Tex.
- 18.Kiessling, C. R., J. H. Cutting, M. Loftis, W. M. Kiessling, A. R. Datta, and J. N. Sofos. 2002. Antimicrobial resistance of food-related Salmonella isolates, 1999-2000. J. Food Prot. 65:603-608. [DOI] [PubMed] [Google Scholar]
- 19.Leistner, L. 1992. Food preservation by combined methods. Food Res. Int. 25:151-158. [Google Scholar]
- 20.Leverentz, B., W. J. Janisiewicz, and W. S. Conway. 2002. Biological control of minimally processed fruits and vegetables, p. 319-332. In J. S. Novak, G. M. Sapers, and V. K. Juneja (ed.), Microbial safety of minimally processed foods. CRC Press, Boca Raton, Fla.
- 21.Leverentz, B., W. S. Conway, Z. Alavidze, W. J. Janisiewicz, Y. Fuchs, M. J. Camp, E. Chighladze, and A. Sulakvelidze. 2001. Examination of bacteriophage as a biocontrol method for Salmonella on fresh-cut fruit—a model study. J. Food Prot. 64:1116-1121. [DOI] [PubMed] [Google Scholar]
- 22.Li, J., M. L. Chikindas, R. D. Ludescher, and T. J. Montville. 2002. Temperature- and surfactant-induced membrane modifications that alter Listeria monocytogenes nisin sensitivity by different mechanisms. Appl. Environ. Microbiol. 68:5904-5910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nguyen-the, C., and F. Carlin. 1994. The microbiology of minimally processed fresh fruits and vegetables. Crit. Rev. Food Sci. Nutr. 34:371-401. [DOI] [PubMed] [Google Scholar]
- 24.Sizmur, J. K., and C. W. Walker. 1988. Listeria in prepacked salads. Lancet i:1167. [DOI] [PubMed] [Google Scholar]
- 25.Stiles, M. E. 1994. Potential for biological control of agents of foodborne disease. Food Res. Int. 27:245-250. [Google Scholar]
- 26.Sulakvelidze, A., Z. Alavidze, and J. G. Morris, Jr. 2001. Bacteriophage therapy (minireview). Antimicrob. Agents Chemother. 45:649-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomas, L. V., M. R. Clarkson, and J. Delves-Broughton. 2000. Nisin, p. 463-524. In A. S. Naidu (ed.), Natural food antimicrobial systems. CRC Press, Boca Raton, Fla.
- 28.Thunberg, R. L., T. T. Tran, R. W. Bennett, R. N. Matthews, and N. Belay. 2002. Microbial evaluation of selected fresh produce obtained at retail markets. J. Food Prot. 65:677-682. [DOI] [PubMed] [Google Scholar]
- 29.Tompkin, R. B. 2002. Control of Listeria monocytogenes in the food-processing environment. J. Food Prot. 65:709-725. [DOI] [PubMed] [Google Scholar]
- 30.Ukuku, D., and W. Fett. 2002. Behaviour of Listeria monocytogenes inoculated on cantaloupe surfaces and efficacy of washing treatments to reduce transfer from rind to fresh-cut pieces. J. Food Prot. 65:924-930. [DOI] [PubMed] [Google Scholar]
- 31.Vandenbergh, P. A. 1993. Lactic acid bacteria, their metabolic products and interference with microbial growth. FEMS Microbiol. Rev. 12:221-238. [Google Scholar]