Abstract
Two fungal chloroperoxidases (CPOs), the heme enzyme from Caldariomyces fumago and the vanadium enzyme from Curvularia inaequalis, chlorinated 1-(4-ethoxy-3-methoxyphenyl)-2-(2-methoxyphenoxy)-1,3-dihydroxypropane, a dimeric model compound that represents the major nonphenolic structure in lignin. Both enzymes also cleaved this dimer to give 1-chloro-4-ethoxy-3-methoxybenzene and 1,2-dichloro-4-ethoxy-5-methoxybenzene, and they depolymerized a synthetic guaiacyl lignin. Since fungal CPOs occur in soils and the fungi that produce them are common inhabitants of plant debris, CPOs may have roles in the natural production of high-molecular-weight chloroaromatics and in lignin breakdown.
Natural organochlorine compounds are widely distributed in the environment, but the processes that generate them are not completely understood. It is clear that many natural organochlorines are microbial secondary metabolites, whereas others are generated abiotically (22), but little attention has been given to the possibility that biological chlorinations of lignin, the most abundant terrestrial aromatic substance, may produce chloroaromatic compounds. If microbial mechanisms exist to oxidize Cl− in plant material, the resulting electrophilic chlorine species are likely to react with the electron-rich aromatic rings of lignin (4). This chemistry could explain, in part, why field samples of soil, litter, and decayed wood have all been shown to contain high-molecular-weight chloroaromatics (5, 14).
Certain plant-pathogenic ascomycetes that are cosmopolitan inhabitants of plant debris provide a possible route for Cl− oxidation in the vicinity of lignin (6, 8, 20). These fungi produce extracellular chloroperoxidases (CPOs), heme- or vanadium-containing enzymes that oxidize Cl− to hypochlorous acid (HOCl) or a similarly reactive chlorine electrophile (18, 21). CPOs chlorinate a variety of aromatic substrates (2, 15, 19, 23), but their reactivity with lignin is unknown. We report here that the major structures in lignin are not only chlorinated but also cleaved by the heme CPO of Caldariomyces fumago and the vanadium CPO of Curvularia inaequalis.
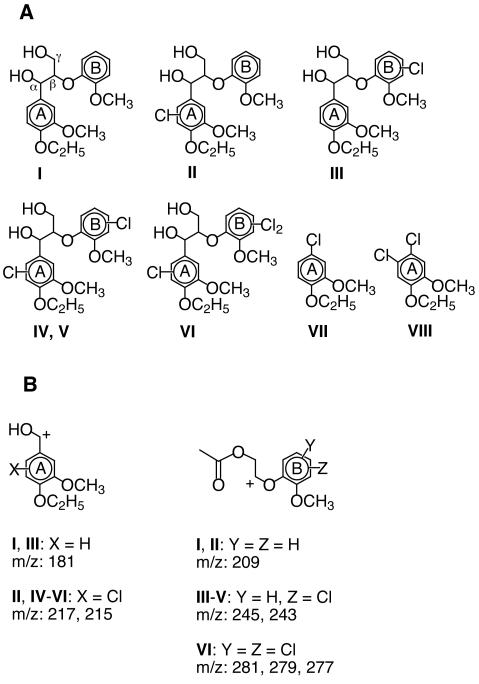
Chlorination and cleavage of a lignin model dimer.
Compound I, threo-1-(4-ethoxy-3-methoxy-ring-[14C]phenyl)-2-(2-methoxyphenoxy)-1,3-dihydroxypropane (1.0 mCi/mmol), a model of the major nonphenolic arylglycerol-β-aryl ether structure in lignin (Fig. 1A), was prepared as described previously (9, 12). The model was treated with either commercially available Caldariomyces fumago heme CPO (Sigma) or partially purified Curvularia inaequalis vanadium CPO. The latter enzyme was obtained by DEAE-Sephacel chromatography of the extracellular medium from Curvularia inaequalis cultures (ATCC 10713) as described earlier (17). The reaction mixtures (1.0 ml) contained compound I (3.4 × 105 dpm, 160 μM), KCl (20 mM), and buffer (25 mM). The buffers were potassium phosphate, pH 3.0, for heme CPO reactions and sodium acetate, pH 4.0, for vanadium CPO reactions. Enzyme was added (35 monochlorodimedone units of heme CPO or 1 monochlorodimedone unit of vanadium CPO) (6), followed by H2O2 (1.7 mM), and the reaction mixtures were stirred overnight at ambient temperature.
FIG. 1.
(A) Structures of model I and of chlorinated products produced from it by CPO. A standard of compound VII was prepared by treating 4-chloroguaiacol (Aldrich) with ethyl iodide. 1H nuclear magnetic resonance: 1.36 (t), J = 7.0 Hz; 3.83 (s); 4.02 (q), J = 7.0 Hz; 6.86 (dd), J = 8.4 Hz, 2.2 Hz; 6.91 (d), J = 8.4 Hz; 6.95 (d), J = 2.2 Hz. A standard of compound VIII was prepared by treating compound VII with one equivalent of Cl2. 1H nuclear magnetic resonance: 1.39 (t), J = 7.0 Hz; 3.86 (s); 4.08 (q), J = 7.0 Hz; 7.08 (s); 7.10 (s). (B) Diagnostic ions from the fragmentation of diacetylated compounds I to VI in the mass spectrometer. The distribution and number of chlorine substituents in each molecule were calculated from the relative frequencies of 35Cl- and 37Cl-containing ions (13). See Table 1 for the mass spectra.
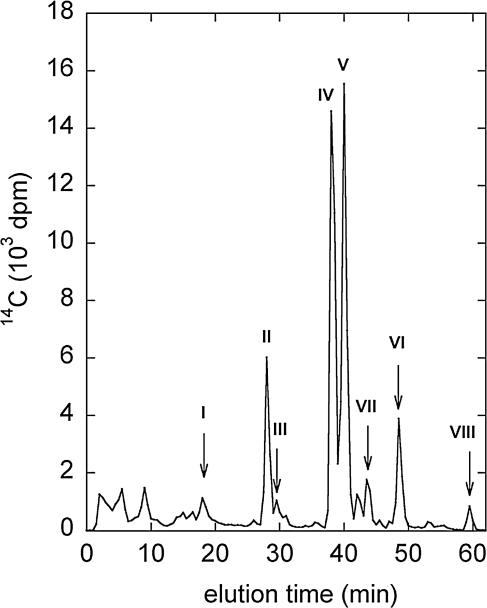
The completed reaction mixtures were stirred for 1 h with 0.25 ml of acetonitrile, and portions (0.50 ml) were analyzed by reversed-phase high-performance liquid chromatography (HPLC) on a Luna 5-μm-pore-size C18 column (Phenomenex). The column was eluted at 1.0 ml/min and ambient temperature with a linear gradient of acetonitrile ranging from 25 to 50% in water over 60 min. Fractions (0.5 ml) were collected and assayed for 14C by scintillation counting. The results showed that the CPOs generated numerous products from model I when H2O2 and Cl− were both present. No reaction occurred when either H2O2 or Cl− was omitted. Typical data for the vanadium CPO are shown in Fig. 2 and Table 1. Experiments with heme CPO or HOCl gave the same products (data not shown).
FIG. 2.
HPLC radiochromatogram of products obtained from the treatment of model I with vanadium CPO. See Fig. 1A for the structures of compounds I to VIII.
TABLE 1.
Products generated in the reaction of model I with vanadium CPO
| Compound | Yield (%)b | Mass spectrum (m/z, relative intensity)c |
|---|---|---|
| Ia | 2.6 | 432 (M+, 8), 209 (14), 181 (100) |
| IIa | 9.0 | 468 (M+ + 2, 4), 466 (M+, 11), 217, (20), 215 (65), 209 (56), 43 (100) |
| IIIa | 1.5 | 468 (M+ +2, 3), 466 (M+, 8), 245 (2), 243 (6), 181 (100) |
| IVa | 23.4 | 504 (M+ + 4, 1), 502 (M+ +2, 5), 500, (M+, 7), 245 (9), 243 (33), 217 (27), 215 (86), 43 (100) |
| Va | 25.1 | 504 (M+ + 4, 1), 502 (M+ +2, 4), 500 (M+, 6), 245 (9), 243 (37), 217, (23), 215 (72), 43 (100) |
| VIa | 6.1 | 538 (M+ + 4, 2), 536 (M+ +2, 5), 534, (M+, 5), 281 (2), 279 (9), 277 (13), 217 (28), 215 (85), 43 (100)d |
| VII | 2.9 | 188 (M+ + 2, 37), 186 (M+, 100), 160, (20), 158 (56), 145 (20), 143 (53), 117 (16), 115 (33) |
| VIII | 1.2 | 224 (M+ +4, 10), 222 (M+ +2, 56), 220 (M+, 88), 196 (9), 194 (54), 192 (84), 181 (12), 179 (60), 177 (100), 153 (5), 151 (25), 149 (40) |
Diacetylated derivatives.
Percentage of total 14C in HPLC radiochromatogram (Fig. 2).
Diagnostic ions only.
M+ + 6 ion not detected.
The fractionated products from a scaled-up reaction were extracted, acetylated, and analyzed by gas chromatography-electron impact mass spectrometry. The results showed that most of the products (II to VI) were uncleaved, chlorinated derivatives of model I (Fig. 1; Table 1). However, two of the minor products, VII and VIII, resulted from Cα-aryl cleavage of model I. HPLC and gas chromatography analyses showed that these products cochromatographed with standards of 1-chloro-4-ethoxy-3-methoxybenzene and 1,2-dichloro-4-ethoxy-5-methoxybenzene, respectively. Mass spectra of the products and standards confirmed these identifications (Table 1). Product VII probably resulted from attack by an electrophilic chlorine species at C-1 of the A ring in model I, followed by elimination of the model's aliphatic side chain (4). Product VIII presumably resulted from reactions between chlorine electrophiles and products (e.g., II or VII) that already carried one chlorine on the A ring. These results suggested that CPOs might cleave lignin when they chlorinate it.
Depolymerization of synthetic lignins.
A high-molecular-weight synthetic β-14C-labeled guaiacyl lignin (0.01 mCi/mmol of phenylpropane substructures) and a permethylated (nonphenolic) preparation of the same lignin were prepared as described previously (7, 10) and treated with CPOs. The reaction mixtures (final volume, 40.0 ml; magnetically stirred at ambient temperature) contained [14C]lignin (2.6 × 104 dpm; 30 μM in phenylpropane substructures), Tween 20 (0.25%), KCl (20 mM), and buffer (25 mM) as described above. The reactions were run by pumping in separate solutions of H2O2 (final concentration, 0.6 mM) and CPO (300 U for heme CPO and 20 U for vanadium CPO) over an 8-h period.
N,N-Dimethylformamide (DMF; 10 ml) was added to the completed reaction mixtures, which were then concentrated to less than 5 ml by rotary vacuum evaporation and clarified by centrifugation to give DMF-soluble [14C]lignin samples in a 75 to 95% yield. The samples were subjected to gel permeation chromatography (GPC) on a 1.8- by 33-cm column of Sephacryl S-100 in DMF that contained 0.1 M LiCl, and fractions (2.0 ml) were collected for quantitation of 14C by scintillation counting.
The GPC column was calibrated with polystyrene standards that spanned the molecular weight range of 500 to 35,000 and also with two lignin model compounds: a tetramer with a molecular weight of 1,062 (compound 197 in the U.S. Dairy Forage Research Center NMR Database of Lignin and Cell Wall Model Compounds [http://www.dfrc.ars.usda.gov/software.html]) and a monomer with a molecular weight of 166 (3,4-dimethoxybenzaldehyde). An iterative least-squares procedure was used to determine the relationship between the elution volume and the molecular weight for the standards. The weight-average molecular weight (Mw) and number-average molecular weight (Mn) for each lignin sample were then calculated by using the standard formulas (24).
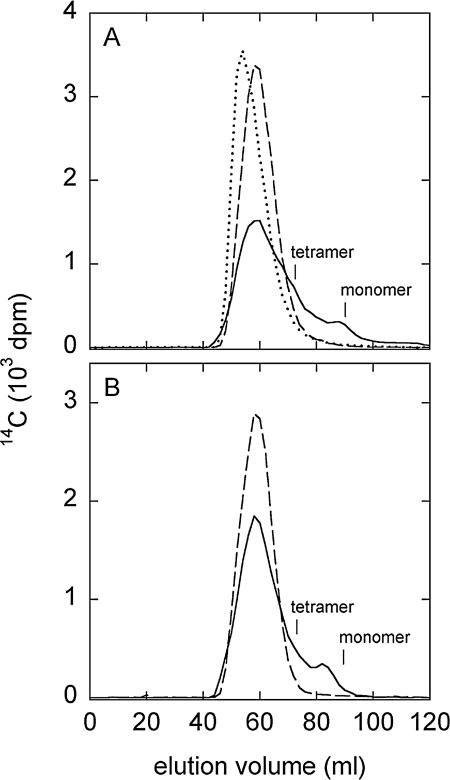
The heme CPO had no discernible effect on the lignin in control reaction mixtures that contained Cl− without H2O2, whereas it polymerized the lignin in reaction mixtures that contained H2O2 without Cl− (Fig. 3A). This result reflects the fact that heme CPO in the absence of Cl− exhibits a general peroxidase activity (3), which is expected to catalyze coupling reactions between phenolic structures in lignin. By contrast, depolymerization was the predominant fate of the lignin in heme CPO-catalyzed reaction mixtures that contained both H2O2 and Cl−. Since Cl− is the preferred reductant for CPO, its ability to reverse the enzyme's polymerizing activity suggests that an oxidized chlorine species was the ligninolytic agent in this experiment. Reactions conducted with vanadium CPO or HOCl showed that they also depolymerized lignin (data not shown).
FIG. 3.
GPC radiochromatograms showing the molecular weight distributions of synthetic lignins treated with heme CPO. (A) Results for phenolic lignin. Dashed line, reaction without H2O2 (Mw = 8,329; Mn = 3,673); dotted line, reaction without Cl− (Mw = 11,838; Mn = 4,441); solid line, complete reaction (Mw = 7,662; Mn = 1,190). (B) Results for nonphenolic lignin. Dashed line, reaction without H2O2 (Mw = 9,321; Mn = 4,316); solid line, complete reaction (Mw = 8,437; Mn = 1,700). Elution positions for the tetrameric and monomeric lignin model compound standards are indicated.
The heme CPO also cleaved synthetic lignin in which the phenolic structures had been blocked as methyl ethers (Fig. 3B). This result is significant because the chlorine electrophiles generated by CPOs are expected to be less reactive with aryl ethers, which comprise most of the linkages in lignin, than they are with phenols, which are minor structures in the polymer (4). That is, heme CPO can act as a functional, if inefficient, analogue of lignin peroxidase (7). Our supplies of vanadium CPO and permethylated lignin were too small to assess their reactivity together.
Conclusion.
Our results show that fungal CPOs are potential chlorinators of lignin in plant debris and soil and may thus account for some of the high-molecular-weight organochlorine residues that occur naturally. Because they are ligninolytic, CPOs may also contribute to the slow breakdown of lignin in soils. CPO activity (1) and H2O2 (11, 16) both occur in soils, and Cl− is of course ubiquitous in this environment. CPO-catalyzed ligninolysis provides an interesting biological parallel to the well-known industrial use of chlorine for wood pulp delignification (4).
Acknowledgments
We thank Jennie Hunter-Cevera and Ron Wever for valuable advice on CPO production. Important contributions to this work were made by Diane Dietrich, Kolby Hirth, Carl Houtman, Ken Jensen, Jr., and Sally Ralph.
This work was supported by grants from the U.S. Department of Energy (DE-FG02-94ER20140) and the U.S. Department of Agriculture (98-35103-6583) to K.E.H.
REFERENCES
- 1.Asplund, G., J. V. Christiansen, and A. Grimvall. 1993. A chloroperoxidase-like catalyst in soil: detection and characterization of some properties. Soil Biol. Biochem. 25:41-46. [Google Scholar]
- 2.Brown, F. S., and L. P. Hager. 1967. Chloroperoxidase. IV. Evidence for an ionic electrophilic substitution mechanism. J. Am. Chem. Soc. 89:719-720. [DOI] [PubMed] [Google Scholar]
- 3.Casella, L., S. Poli, M. Gullotti, C. Selvaggini, T. Beringhelli, and A. Marchesini. 1994. The chloroperoxidase-catalyzed oxidation of phenols—mechanism, selectivity, and characterization of enzyme-substrate complexes. Biochemistry 33:6377-6386. [DOI] [PubMed] [Google Scholar]
- 4.Dence, C. W. 1971. Halogenation and nitration, p. 373-432. In K. V. Sarkanen and C. H. Ludwig (ed.), Lignins: occurrence, formation, structure and reactions. Wiley-Interscience, New York, N.Y.
- 5.Flodin, C., E. Johansson, H. Boren, A. Grimvall, O. Dahlman, and R. Morck. 1997. Chlorinated structures in high molecular weight organic matter isolated from fresh and decaying plant material and soil. Environ. Sci. Technol. 31:2464-2468. [Google Scholar]
- 6.Hallenberg, P. G., and L. P. Hager. 1978. Purification of chloroperoxidase from Caldariomyces fumago. Methods Enzymol. 52:521-529. [DOI] [PubMed] [Google Scholar]
- 7.Hammel, K. E., K. A. Jensen, Jr., M. D. Mozuch, L. L. Landucci, M. Tien, and E. A. Pease. 1993. Ligninolysis by a purified lignin peroxidase. J. Biol. Chem. 268:12274-12281. [PubMed] [Google Scholar]
- 8.Hunter-Cevera, J. C., and L. Sotos. 1986. Screening for a “new”enzyme in nature: haloperoxidase production by Death Valley dematiaceous hyphomycetes. Microb. Ecol. 12:121-127. [DOI] [PubMed] [Google Scholar]
- 9.Kawai, S., K. A. Jensen, Jr., W. Bao, and K. E. Hammel. 1995. New polymeric model substrates for the study of microbial ligninolysis. Appl. Environ. Microbiol. 61:3407-3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirk, T. K., and G. Brunow. 1988. Synthetic 14C-labeled lignins. Methods Enzymol. 161:65-73. [Google Scholar]
- 11.Kok, G. L. 1980. Measurements of hydrogen peroxide in rainwater. Atmos. Environ. 14:653-656. [Google Scholar]
- 12.Landucci, L. L., S. A. Geddes, and T. K. Kirk. 1981. Synthesis of 14C labeled 3-methoxy-4-hydroxy-α-(2-methoxyphenoxy)-β-hydroxypropiophenone, a lignin model compound. Holzforschung 35:66-69. [Google Scholar]
- 13.McLafferty, F. W., and F. Turecek. 1993. Interpretation of mass spectra, 4th ed. University Science Books, Sausalito, Calif.
- 14.Myneni, S. C. B. 2002. Formation of stable chlorinated hydrocarbons in weathering plant material. Science 295:1039-1041. [DOI] [PubMed] [Google Scholar]
- 15.Niedan, V., I. Pavasars, and G. Oberg. 2000. Chloroperoxidase-mediated chlorination of aromatic groups in fulvic acid. Chemosphere 41:779-785. [DOI] [PubMed] [Google Scholar]
- 16.Petigara, B. R., N. V. Blough, and A. C. Mignerey. 2002. Mechanisms of hydrogen peroxide decomposition in soils. Environ. Sci. Technol. 36:639-645. [DOI] [PubMed] [Google Scholar]
- 17.van Schijndel, J. W. P. M., E. G. M. Vollenbroek, and R. Wever. 1993. The chloroperoxidase from the fungus Curvularia inaequalis: a novel vanadium enzyme. Biochim. Biophys. Acta 1161:249-256. [DOI] [PubMed] [Google Scholar]
- 18.van Schijndel, J. W. P. M., P. Barnett, J. Roelse, E. G. M. Vollenbroek, and R. Wever. 1994. The stability and steady-state kinetics of vanadium chloroperoxidase from the fungus Curvularia inaequalis. Eur. J. Biochem. 225:151-157. [DOI] [PubMed] [Google Scholar]
- 19.Vazquez-Duhalt, R., M. Ayala, and F. J. Marquez-Rocha. 2001. Biocatalytic chlorination of aromatic hydrocarbons by chloroperoxidase of Caldariomyces fumago. Phytochemistry 58:929-933. [DOI] [PubMed] [Google Scholar]
- 20.Vollenbroek, E. G. M., L. H. Simons, J. W. P. M. van Schijndel, P. Barnett, M. Balzar, H. Dekker, C. van der Linden, and R. Wever. 1995. Vanadium chloroperoxidases occur widely in nature. Biochem. Soc. Trans. 23:267-271. [DOI] [PubMed] [Google Scholar]
- 21.Wagenknecht, H. A., and W. D. Woggon. 1997. Identification of intermediates in the catalytic cycle of chloroperoxidase. Chem. Biol. 4:367-372. [DOI] [PubMed] [Google Scholar]
- 22.Winterton, N. 2000. Chlorine: the only green element—towards a wider acceptance of its role in natural cycles. Green Chem. 2:173-225. [Google Scholar]
- 23.Yaipakdee, P., and L. W. Robertson. 2001. Enzymatic halogenation of flavanones and flavones. Phytochemistry 57:341-347. [DOI] [PubMed] [Google Scholar]
- 24.Yau, W. W., J. J. Kirkland, and D. D. Bly. 1979. Modern size-exclusion liquid chromatography. John Wiley and Sons, New York, N.Y.