Abstract
A commercial blend of essential oil (EO) compounds was added to a grass, maize silage, and concentrate diet fed to dairy cattle in order to determine their influence on protein metabolism by ruminal microorganisms. EO inhibited (P < 0.05) the rate of deamination of amino acids. Pure-culture studies indicated that the species most sensitive to EO were ammonia-hyperproducing bacteria and anaerobic fungi.
The drive toward a decrease in the use of antibiotics in animal production has intensified the search for natural products that enhance production, in the case of ruminants, by modulating ruminal fermentation. Essential oils (EO) are natural products that give plants and spices their characteristic odor and color. Oh et al. (12) found that EO from the Douglas fir pine needle have an antibacterial effect in the rumens of sheep and deer. Fernandez et al. (6) used a commercial blend of EO compounds to manipulate rumen fermentation, inhibiting the breakdown of protein, thus potentially increasing the dietary protein available to the ruminant. The aim of the present study was to investigate the influence of EO on protein metabolism by mixed ruminal microorganisms and on different ruminal bacteria, protozoa, and fungi.
In order to identify the mechanisms and microbial species affected by EO, the effects of EO were measured in ruminal microorganisms obtained from four Holstein-Friesian cows, each fitted with a ruminal cannula and fed ad libitum a diet consisting of grass, maize silage, and concentrate mix (330, 220, and 450 g kg−1 on a dry-matter basis). The concentrate contained soybean meal, rapeseed meal, sunflower meal, maize gluten feed, maize distillers grain, cottonseed meal, ground wheat, palm kernel meal, molassed sugar beet pulp, wheat feed, and minerals (106, 106, 45, 22, 89, 89, 171, 122, 111, 111, and 28 g of dry matter kg−1, respectively). Cattle received the diet with or without 1,000 mg of a blend of EO (Crina Ruminants; Akzo Nobel Surface Chemistry Ltd., Hertfordshire, United Kingdom) day−1 as part of a two-by-two factorial design with 4-week periods. Crina Ruminants is a defined, patented mixture of natural and nature-identical EO compounds that includes thymol, eugenol, vanillin, and limonene on an organic carrier (14). Measurements were made on 2 consecutive days during the last week of each period.
Samples of ruminal fluid were removed 2 h after morning feeding and strained (strained ruminal fluid [SRF]) though two layers of cheese cloth before use in experimental measurements. Ruminal degradation of proteins was investigated by using casein, lactoglobulin, hide powder azure, albumin, and elastin Congo red (Sigma Chemical Co., Poole, United Kingdom) reductively methylated with [14C]formaldehyde (18). Peptidase activities were measured with Ala2, Ala5, AspLysArgValTyr, GlyArg-4-methoxy-2-naphthylamide (GlyArg-MNA), and Ala2-p-nitroanilide (Ala2-pNA) by measuring peptide breakdown by reverse-phase high-performance liquid chromatography (20), fluorimetrically by the appearance of MNA, or spectrophotometrically by the appearance of pNA (20, 21). Deaminase activity was determined with casein acid hydrolysate as the substrate (11). Monensin, which is known to inhibit some of the major species of deaminative organisms (13), was added to samples incubated with casein acid hydrolysate at a final concentration of 5 μM.
The effect of EO on bacterial growth was measured by inoculating stock cultures grown in the liquid version of Hobson's M2 medium (8) into the same medium containing serial twofold increases in EO concentration (10). EO was added aseptically after the medium was autoclaved to give final concentrations ranging from 5 to 1,000 ppm. Bacterial growth was measured by reading the optical density at 650 nm hourly. To investigate possible adaptation of bacteria to EO, bacteria were inoculated into tubes containing the lowest concentration of EO and incubated at 39°C anaerobically for 48 h. This tube was used to inoculate (5%, vol vol−1) medium containing twice the EO concentration and so on until a concentration that inhibited growth totally was reached. The results were recorded as the EO concentration at which the optical density was half of that measured in the absence of EO (IC50). The influence of EO on rumen ciliate protozoa was determined by the bacteriolytic activity of protozoa in ruminal fluid (22). The effect of EO on the activity of Methanobrevibacter smithii PS (ATCC 35061) and Neocallimastix frontalis RE1 was determined from gas production over 10 days in the presence of increasing concentrations of EO (10). The microbial species used are all maintained in the culture collection of the Rowett Research Institute; their origins were described previously (10, 21).
Data were analyzed as a two-by-two factorial design with Genstat 5 (7).
Influence of dietary EO on protein metabolism.
The different proteins incubated with SRF in vitro were hydrolyzed at rates that spanned 2 orders of magnitude (Table 1). EO had no effect on the breakdown of any of the proteins tested (Table 1), even with their different secondary and tertiary structures. Casein and lactoglobulin are soluble proteins with little ordered structure; albumin is also soluble but contains abundant disulfide cross-links that make it more resistant to degradation (18); hide powder azure and elastin are both insoluble, and the latter is also heavily cross-linked (18).
TABLE 1.
Effect of feeding essential oils to cattle on the subsequent breakdown of proteins, peptides, and amino acids in rumen fluid in vitro
| Treatment or parameter | Amt (mg) of substrate degraded mg of protein−1 h−1
|
Amt (nmol) of peptide degraded mg of protein−1 h−1
|
Amt (nmol) of NH3 produced mg of protein−1 h−1
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Casein | Lacto- globulin | Hide powder azure | Albumin | Elastin Congo red | Ala2 | Ala5 | Arg Lys Asp Val Tyr | Ala2- pNA | GlyArg- MNA | No monensin | 5 μM monensin | |
| Control | 0.46 | 0.21 | 0.048 | 0.033 | 0.0056 | 0.60 | 1.03 | 0.33 | 1.64 | 0.36 | 410 | 280 |
| Essential oils | 0.49 | 0.24 | 0.049 | 0.035 | 0.0054 | 0.69 | 1.22 | 0.35 | 1.58 | 0.40 | 372 | 287 |
| SEDb | 0.038 | 0.032 | 0.0035 | 0.0016 | 0.00092 | 0.088 | 0.230 | 0.035 | 0.056 | 0.060 | 9.8a | 10.9 |
P < 0.05.
SED, standard error of the difference.
The peptides selected also covered a range of potential substrates, including di- and oligopeptides and substrates for dipeptidyl aminopeptidases. Once again, EO had no effect (Table 1). There was, however, a significant decrease (P < 0.05) in the rate of ammonia production from casein acid hydrolysate, which comprises free amino acids, when casein acid hydrolysate was incubated with SRF from cattle receiving EO (Table 1). A similar study with sheep indicated a 24% decrease in deamination of amino acids (our unpublished results). The decrease in ammonia production caused by EO was abolished in the presence of monensin (Table 1), and the deamination rate was reduced to similar values in both treatments. Taken together, these results suggest that EO suppresses some of the species that are inhibited by monensin but has little effect on other deaminating species.
Influence of EO on pure cultures of ruminal microorganisms.
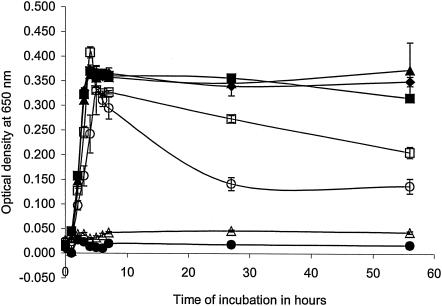
EO, when added to the culture medium, inhibited the growth of most pure cultures of ruminal bacteria at concentrations of less than 100 ppm; Streptococcus bovis was the most resistant species, and Prevotella ruminicola, Clostridium sticklandii, and Peptostreptococcus anaerobius were the most sensitive species (Table 2). Some species, including P. ruminicola and P. bryantii, adapted and were able to grow in higher concentrations of EO, while others, including C. sticklandii and P. anaerobius, remained sensitive. The last two species are among those identified by Russell and his colleagues (2, 3, 13, 15, 16) as “hyper-ammonia producing” (HAP) species detrimental to the efficiency of nitrogen retention in ruminants. Any adaptation to EO would diminish the effect in vivo. A further consideration may be the survival of bacteria in stationary phase: the loss of viability of Ruminobacter amylophilus was greatly increased by EO (Fig. 1).
TABLE 2.
Effect of essential oils on growth of pure cultures of rumen bacteria after direct inoculation or after adaptation
| Strain | IC50 of essential oils (ppm)a
|
|
|---|---|---|
| Direct inoculation | Adaptation | |
| Clostridium sticklandii 12662 | 36.0 | 35.0 |
| Peptostreptococcus anaerobius 27337 | 42.5 | 52.0 |
| Selenomonas ruminantium Z108 | 57.0 | 58.0 |
| Ruminococcus flavefaciens FD1 | 60.0 | 60.0 |
| Prevotella brevis GA33 | 57.5 | 61.0 |
| Prevotella albensis M384 | 50.0 | 65.0 |
| Eubacterium ruminantium 2377 | 70.0 | 76.0 |
| Anaerovibrio lipolytica 5S | 73.8 | 80.0 |
| Veillonella parvula L59 | 88.0 | 86.0 |
| Prevotella ruminicola 23 | 33.8 | 94.3 |
| Fibrobacter succinogenes S85 | 95.0 | 110.0 |
| Butyrivibrio fibrisolvens SH13 | 56.2 | 112.5 |
| Prevotella bryantii B14 | 54.0 | 127.5 |
| Lachnospira multipara D15d | 112.5 | 120.0 |
| Ruminococcus albus SY3 | 49.0 | 132.5 |
| Ruminobacter amylophilus WP109 | 42.6 | 150.0 |
| Mitsuokella multiacidas 46/5 | 113.5 | 160.0 |
| Megasphaera elsdenii J1 | 113.0 | 190.0 |
| Lactobacillus casei LB17 | 56.2 | 221.2 |
| Streptococcus bovis ES1 | 127.5 | 240.0 |
| Clostridium aminophilum 49906 | 94.2 | 262.5 |
IC50 is the concentration of essential oils that led to a 50% decrease in cell density at 24 h of incubation. The results shown are means of three culture tubes at each concentration.
FIG. 1.
Effect of EO on the growth of R. amylophilus WP109. Growth was determined with the following additions of EO: 0 ppm (♦), 50 ppm (▪), 100 ppm (▴), 200 ppm (□), 400 ppm (○), 800 ppm (•), and 1,600 ppm (▵). Results are mean values and standard errors of triplicates.
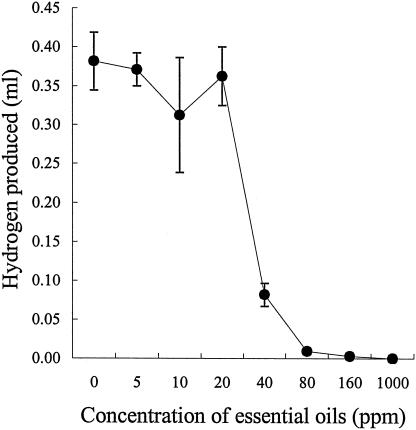
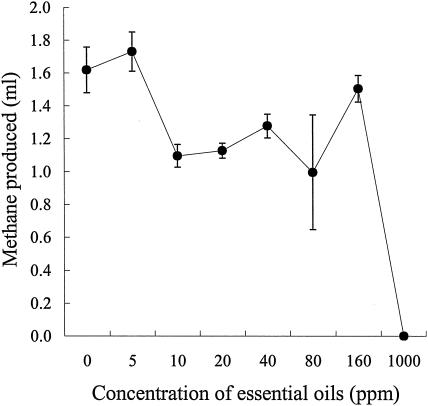
The bacteriolytic activity of ruminal protozoa was unaffected (data not shown). However, EO affected the activity of the ruminal fungus N. frontalis, with H2 production almost totally inhibited at a concentration of 40 ppm (Fig. 2). The sensitivity of N. frontalis to EO after adaptation was not determined, as the measurements of H2 production were made after 10 days of incubation, sufficient time to allow the fungi to adapt. Growth of the methanogen M. smithii was unaffected by EO concentrations of up to 160 ppm, although inhibition occurred at 1,000 ppm (Fig. 3).
FIG. 2.
Effect of EO on the growth of N. frontalis RE1. Growth was measured indirectly by the production of hydrogen in the headspace gas after 10 days of incubation. The results are mean values and standard errors of the means from three cultures.
FIG. 3.
Effect of EO on growth of M. smithii. Growth was measured indirectly by the production of methane in the headspace gas after 10 days of incubation. The results are mean values and standard errors of the means from three cultures.
Mode of action of EO.
These results suggest that the range of sensitivity to EO is rather small for most species and that none is particularly sensitive. As the rumen volume of the animals used here is about 60 liters and 1,000 mg of EO per day was fed, the likely maximum liquid concentration would be 17 ppm, assuming that no absorption or metabolism takes place. This is equivalent to half of the IC50 for the most sensitive species (Table 2). Nevertheless, local concentrations of EO, some of which are sparingly soluble, may be higher in vivo, particularly on surfaces of ingested plant materials, which may increase the potency of EO in vivo. The relatively narrow range of sensitivity contrasts with the sensitivity to ionophores such as monensin and tetronasin, for which the range of sensitivities between the most and least sensitive organisms may be 1,000-fold (4, 10). Nevertheless, the influence of EO may be greater on microorganisms associated with solids than on liquid-associated species, adaptation will undoubtedly be a major factor, and the influence of EO on survival, rather than growth, may be a potent selective characteristic. It is also possible that EO elicits an antimicrobial effect on species of ruminal bacteria that have not yet been isolated in pure culture. Cultivated species do not represent the true bacterial diversity of the rumen ecosystem. PCR-based analysis of the 16S rRNA gene of the whole population revealed that the majority of bacteria are not represented as cultivated species, and some species associated with solids are quite different from those in fluid (17).
The present results indicate that the main effect on protein catabolism is at the final stage, deamination of amino acids. Ammonia production in the rumen is predominantly performed by two distinct groups of organisms: species present in high numbers but with low specific activity, as well as those present in low numbers but with very high specific activity (16). The latter are the group of HAP species, which are characterized as being monensin sensitive and nonsaccharolytic (13). The finding that monensin-insensitive deaminating activity was similar in both treatments suggests that EO does not affect deaminating species unaffected by monensin. Furthermore, while HAP species all seem to be sensitive to monensin (1-3, 5, 15, 16), EO inhibited C. sticklandii and P. anaerobius while the other HAP species tested, Clostridium aminophilum, was not affected. This may explain why EO was less effective than monensin at inhibiting ammonia production. It would be of interest to determine the effects of EO on the wider range of HAP species now available (1, 5, 9). HAP species vary from diet to diet and perhaps geographically. It would also be informative to determine which oil in the mixture is most effective at inhibiting deamination, as well as the mechanisms of its toxicity.
EO may also affect proteolysis in vivo, however. Experiments in which protein supplements were incubated in the sheep rumen suggested that their rate of degradation was suppressed by EO (our unpublished results). The absence of an effect in vitro with any of the proteins tested here suggests that the colonization and/or hydrolysis of nonprotein components of the supplements may be affected. Furthermore, the toxicity of EO to the proteolytic and amylolytic species R. amylophilus in stationary phase may slow the breakdown of starchy and/or high-protein supplements, which may be beneficial to the animal. In the present experiments, proteolysis was measured in SRF. Similar measurements should be made in large particulate matter, where the sensitivity of ruminal fungi, which are proteolytic as well as fibrolytic (19, 23), to EO may be of greater significance.
Any beneficial effects of EO on protein metabolism may have to be balanced against possible detrimental effects on fiber breakdown. Here, both cellulolytic ruminal bacteria and fungi were affected by EO, although the bacteria showed adaptation to EO. Fernandez et al. (6) found that a 20-fold increase in the amount of EO given to sheep, from 50 to 1,000 mg day−1, caused a marked inhibition in fiber digestion in the rumen, which may be consistent with these antimicrobial effects.
Implications.
These experiments with EO demonstrate that it is possible to use natural products, particularly plant secondary metabolites, to manipulate ruminal fermentation by selective suppression of certain microbial species. EO were considered valuable as antimicrobial agents until the discovery of antibiotics overshadowed them. As microbiologists strive to retain the usefulness of antibiotics for human therapy, reexamination of the potential of EO for a variety of purposes, including growth promotion in animal production, is timely.
Acknowledgments
The Rowett Research Institute receives financial support from the Scottish Executive Environment and Rural Affairs Department.
REFERENCES
- 1.Attwood, G. T., A. V. Klieve, D. Ouwerkerk, and B. K. Patel. 1998. Ammonia-hyperproducing bacteria from New Zealand ruminants. Appl. Environ. Microbiol. 64:1796-1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen, G., and J. B. Russell. 1988. Fermentation of peptides and amino acids by a monensin-sensitive ruminal peptostreptococcus. Appl. Environ. Microbiol. 54:2742-2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen, G., and J. B. Russell. 1989. More monensin-sensitive, ammonia-producing bacteria from the rumen. Appl. Environ. Microbiol. 55:1052-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, M., and M. J. Wolin. 1979. Effect of monensin and lasalocid-sodium on the growth of methanogenic and rumen saccharolytic bacteria. Appl. Environ. Microbiol. 38:72-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eschenlauer, S. C. P., N. McKain, N. D. Walker, N. R. McEwan, C. J. Newbold, and R. J. Wallace. 2002. Ammonia production by rumen microorganisms and enumeration, isolation, and characterization of bacteria capable of growth on peptides and amino acids from the sheep rumen. Appl. Environ. Microbiol. 68:4925-4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernandez, M., E. Serrano, P. Frutos, F. J. Giraldez, A. R. Mantecon, and J. R. Llach. 1997. Effect of Crina HC supplement upon the rumen degradative activity in sheep. Inf. Tech. Econ. Agraria 18(Vol. Extra):160-162. [Google Scholar]
- 7.Genstat 5 Committee. 1987. Genstat 5 users' manual. Oxford University Press, Oxford, United Kingdom.
- 8.Hobson, P. N. 1969. Rumen bacteria. Methods Microbiol. 3B:133-149.
- 9.McSweeney, C. S., B. Palmer, R. Bunch, and D. O. Krause. 1999. Isolation and characterization of proteolytic ruminal bacteria from sheep and goats fed the tannin-containing shrub legume Calliandra calothyrsus. Appl. Environ. Microbiol. 65:3075-3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Newbold, C. J., R. J. Wallace, N. D. Watt, and A. J. Richardson. 1988. The effect of the novel ionophore tetronasin (ICI 139603) on ruminal microorganisms. Appl. Environ. Microbiol. 54:544-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newbold, C. J., R. J. Wallace, and N. McKain. 1990. Effects of the ionophore tetronasin on nitrogen metabolism by ruminal microorganisms in vitro. J. Anim. Sci. 68:1103-1109. [DOI] [PubMed] [Google Scholar]
- 12.Oh, H. K., T. Sakai, M. B. Jones, and W. M. Longhurst. 1967. Effect of various essential oils isolated from Douglas fir needles upon sheep and deer rumen microbial activity. Appl. Microbiol. 68:777-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paster, B. J., J. B. Russell, C. M. J. Yang, J. M. Chow, C. R. Woese, and R. Tanner. 1993. Phylogeny of the ammonia-producing ruminal bacteria Peptostreptococcus anaerobius, Clostridium sticklandii, and Clostridium aminophilum sp. nov. Int. J. Syst. Bacteriol. 43:107-110. [DOI] [PubMed] [Google Scholar]
- 14.Rossi, J. December 1999. Additives for animal nutrition and technique for their preparation. European patent EP 0646321 B1.
- 15.Russell, J. B., H. J. Strobel, and G. Chen. 1988. Enrichment and isolation of a ruminal bacterium with a very high specific activity of ammonia production. Appl. Environ. Microbiol. 54:872-877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Russell, J. B., R. Onodera, and T. Hino. 1991. Ruminal protein fermentation: new perspectives on previous contradictions, p. 681-697. In T. Tsuda, Y. Sasaki, and R. Kawashima (ed.), Physiological aspects of digestion, metabolism in ruminants: proceedings of the Seventh International Symposium on Ruminant Physiology. Academic Press, London, England.
- 17.Tajima, K., R. I. Aminov, T. Nagamine, K. Ogata, M. Nakamura, H. Matsui, and Y. Benno. 1999. Rumen bacterial diversity as determined by sequence analysis of 16S rDNA libraries. FEMS Microbiol. Lett. 29:159-169. [Google Scholar]
- 18.Wallace, R. J. 1983. Hydrolysis of 14C-labelled proteins by rumen micro-organisms and by proteolytic enzymes prepared from rumen bacteria. Br. J. Nutr. 50:345-355. [DOI] [PubMed] [Google Scholar]
- 19.Wallace, R. J., and K. N. Joblin. 1985. Proteolytic activity of a rumen anaerobic fungus. FEMS Microbiol. Lett. 29:19-25. [Google Scholar]
- 20.Wallace, R. J., and N. McKain. 1989. Analysis of peptide metabolism by ruminal microorganisms. Appl. Environ. Microbiol. 55:2372-2376. [DOI] [PMC free article] [PubMed]
- 21.Wallace, R. J., and N. McKain. 1991. A survey of peptidase activity in rumen bacteria. J. Gen. Microbiol. 137:2259-2264. [DOI] [PubMed] [Google Scholar]
- 22.Wallace, R. J., and C. A. McPherson. 1987. Factors affecting the rate of breakdown of bacterial protein in rumen fluid. Br. J. Nutr. 58:313-323. [DOI] [PubMed] [Google Scholar]
- 23.Yanke, L. J., Y. Dong, T. A. McAllister, and K.-J. Cheng. 1993. Comparison of amylolytic and proteolytic activities of ruminal fungi grown on cereal grains. Can. J. Microbiol. 39:817-820. [DOI] [PubMed] [Google Scholar]