Abstract
Each summer, the nuisance green alga Cladophora (mostly Cladophora glomerata) amasses along Lake Michigan beaches, creating nearshore anoxia and unsightly, malodorous mats that can attract problem animals and detract from visitor enjoyment. Traditionally, elevated counts of Escherichia coli are presumed to indicate the presence of sewage, mostly derived from nearby point sources. The relationship between fecal indicator bacteria and Cladophora remains essentially unstudied. This investigation describes the local and regional density of Escherichia coli and enterococci in Cladophora mats along beaches in the four states (Wisconsin, Illinois, Indiana, and Michigan) bordering Lake Michigan. Samples of Cladophora strands collected from 10 beaches (n = 41) were assayed for concentrations of E. coli and enterococci during the summer of 2002. Both E. coli and enterococci were ubiquitous (up to 97% occurrence), with overall log mean densities (± standard errors) of 5.3 (± 4.8) and 4.8 (± 4.5) per g (dry weight). E. coli and enterococci were strongly correlated in southern Lake Michigan beaches (P < 0.001, R2 = 0.73, n = 17) but not in northern beaches (P = 0.892, n = 16). Both E. coli and enterococci survived for over 6 months in sun-dried Cladophora mats stored at 4°C; the residual bacteria in the dried alga readily grew upon rehydration. These findings suggest that Cladophora amassing along the beaches of Lake Michigan may be an important environmental source of indicator bacteria and call into question the reliability of E. coli and enterococci as indicators of water quality for freshwater recreational beaches.
Escherichia coli is a widely used indicator of contamination originating from domestic sewage. High levels of this bacterium have been a chronic problem throughout southern Lake Michigan during summer months, resulting in numerous beach closures (19, 24, 34, 35). There is evidence that a significant portion of E. coli may arise from nonpoint sources originating within the beach area (e.g., birds, sand, and sediment storage) or from nearby inputs (riparian and wetland runoff) (16, 19, 26, 29, 32-34). Many beach closures challenge traditional paradigms since they are not associated with waste releases, recent rainfall, wind, or known pollution events. Bacterial multiplication both within the water column and along the shoreline has been suggested (2, 11, 29), but this idea has been questioned due to the limited availability of organic nutrients and adverse environmental conditions for survival of indicator bacteria, such as sunlight, temperature, and open beach desiccation (4, 10, 25; G. J. Medema, M. Bahar, and F. M. Schets, presented at the International Symposium on Health-Related Water Microbiology, Mallorca, Spain, 1997).
This study investigated the association and persistence of E. coli and enterococci in mats of the green alga Cladophora [almost exclusively Cladophora glomerata (L.) Kütz]. Cladophora is found in both fresh and marine waters worldwide (12). In the Great Lakes, Cladophora growing on rocks and other substrates in nearshore water can become detached and accumulate along the shoreline as large mats. The accumulation is common in bays with rocky substrates, particularly from June through September. These algal masses can result in offensive, malodorous conditions that may pose a public health risk. Although Cladophora is perennial, it tends to grow as an annual due to wintry conditions (5), and it is found primarily on shelving rocks and boulders (31). This alga provides shelter and nourishment to a wide variety of organisms, such as epiphytes (cyanobacteria and diatoms) and grazers (protozoa, mollusks, rotifers, and young crayfish) (8, 20, 30, 31).
The general assumption that traditional fecal indicators (e.g., E. coli and enterococci) do not occur in natural environments (soil or water) has recently been challenged. These bacteria occur in soils (6, 15, 16, 29) and riparian sediments (32) and perhaps as epiphytic microflora on terrestrial plants (22, 23, 28). Observations of these indicators living on aquatic plants, including algae, are lacking, but such associations would be significant, since aquatic macrophytes have the potential to harbor, shed, and possibly support the growth of these indicator bacteria. The presence of indicators associated with aquatic macrophytes may lead to the misinterpretation of water quality tests or misidentification of the source of indicator bacteria.
The specific objectives of this study were to (i) describe the relative association of E. coli and enterococci with floating, attached, and stranded Cladophora, (ii) characterize the regional distribution and density of these indicator bacteria in selected areas of Michigan, Indiana, Illinois, and Wisconsin, and (iii) determine whether Cladophora may act as a nonpoint source of E. coli and enterococci.
MATERIALS AND METHODS
In this paper, we refer to Cladophora algae that have become stranded on the beach, whether in mats or filaments, as “strands.” Detached algae submerged in the beach water are referred to as “floating” algae, whether they are within the water column or on the bottom. Finally, Cladophora algae growing on pilings, rocks, outcroppings, or piers are referred to as “attached” algae.
Sampling locations: regional surveys.
A survey was conducted along 10 beaches on Lake Michigan located in Wisconsin, Illinois, Indiana, and Michigan to determine the relative abundance of E. coli and enterococci within Cladophora (Fig. 1). The sites in Wisconsin included Bradford Beach, Milwaukee, and North Beach, Racine. The sites in Illinois were Illinois State Park Beach, Zion; Waukegan Municipal Beach, Waukegan; and 63rd Street Beach, Chicago. The site in Indiana was Washington Park Beach, Michigan City. Sites in Michigan included Good Harbor Bay, Sleeping Bear Bay, Platte Bay, and South Manitou Island along the Sleeping Bear Dunes National Lakeshore, near Traverse City.
FIG. 1.
Lake Michigan beaches surveyed between June and November 2002.
Site description.
All sites were sandy beaches with medium- to fine-grain sands, gradual slopes, and moderate wave exposure. Although there were domestic sewage sources near Bradford Beach, Waukegan Municipal Beach, and Washington Park Beach, most locations were not suspected to be directly or chronically impacted by sewage. Algal conditions varied substantially. At Bradford Beach, stranded algae occurred in large mats mixed with zebra mussels (Dreissena polymorpha) and were in an advanced stage of decomposition. At Illinois State Park Beach, thick mats of attached fresh algae were collected off riprap boulders within a cove. Most algal samples were 0.5 to 5 cm thick, except at Sleeping Bear Dunes National Lakeshore beaches, where algal mats were about 50 cm thick and several meters offshore. All of the beaches had the potential to accumulate large masses of Cladophora, given the appropriate season, wind, and currents.
Sample collection.
Cladophora samples were collected between 24 June and 7 November 2002; more intensive sampling occurred during July 29 to 31. Samples from all locations were gathered from water, rock pilings, or beach sand. Algal samples were aseptically collected by hand and put into Whirl-Pak bags or glass jars. The samples were placed on ice and immediately taken to the laboratory. Air, water, and sand temperature were recorded at most of the locations. Samples from Indiana and Illinois were analyzed within 4 to 6 h of collection, but samples from the most distant locations were held for as long as 24 h at 4°C.
Sand-lake water relationships.
Three randomly chosen transects were established at 63rd Street Beach and Washington Park Beach. Along each transect, two 0.5-m2 quadrants were set: in the water, 1 m from shore (nearshore); on the sand, 1 m inland from the shore (beach). All Cladophora within the quadrants was retrieved. A water sample was collected from each of the nearshore quadrants. Beach sand immediately underlying the strands in each beach quadrant was collected to a depth of 2 cm and placed in separate plastic bags.
Microbiological analyses.
E. coli and/or enterococci were analyzed by membrane filtration (9). Generally, undiluted lake water samples were filtered in volumes ranging from 10 to 100 ml. For analyzing algae and sand samples, an initial elutriation step was necessary to release the bound bacterial cells. One-gram portions of homogenized algal samples were weighed and placed in sterile 15-ml centrifuge tubes, to which 9 ml of sterile phosphate-buffered diluent water (PBW) (pH 6.8) was added. The alga-PBW mixture was vigorously shaken for 2 min and centrifuged briefly (45 s) at 2,000 rpm (653 × g) to allow the large particles to settle. If necessary, the supernatant was further diluted in PBW, and appropriate volumes ranging from 1 to 30 ml were filtered. Filters were placed on thermotolerant-E. coli medium (mTEC) or enterococcal medium (mE) and incubated at 44.5°C (E. coli) or 41°C (enterococci) (9). Sequential rinsing of algae showed that an average of 55% of bacteria were recovered with this technique (data not shown).
Fifty grams of representative sand was added to 100 ml of PBW. The mixture was shaken for 2 min and allowed to settle for 30 s. The supernatant was serially diluted, and appropriate volumes were analyzed for E. coli and enterococci as previously described. Fresh (wet) samples of sand or algae were dried at 100°C for 24 h to determine dry weight. All bacterial concentrations in algae or sand are expressed in grams (dry weight) unless otherwise noted. Sequential rinsing showed that a mean range of 86 to 100% of bacteria was recovered from the sand-algae mixture using this technique.
E. coli and enterococci determinations included suitable blanks and reference cultures of E. coli (ATCC 25922) and Enterococcus faecalis (ATCC 29212) for quality control purposes. At least 10% of the presumptive colonies for both E. coli and enterococci were confirmed by standard tests (9).
Effects of sunlight exposure and mat thickness.
Fresh algae were arranged on circular no. 30 mesh nylon screens cut to fit over standard petri dishes (150 by 15 mm) that had been filled with sterile sand. Algae were loosely spread onto the screens at 6-, 4-, 2-, and 1-mm thicknesses. Sterile sand without algae acted as a control.
The experimental array was placed outside in full sunlight daily for four consecutive days (15 to 18 July 2002): for 8 h (7 a.m. to 3 p.m.) on the first three days and for 5 hours (7 a.m. to 10 a.m.) on the fourth day, for a total of 27 h. During this period, skies were sunny with mean winds of 4.5 km/h. Mean (range) high temperatures for air, sand, and surface of algae were 30°C (28 to 31°C), 48°C (47 to 49°C) and 48°C (42 to 49°C). The experimental array was covered and held indoors between exposures at 22°C. At 9, 18, and 27 h of cumulative outdoor exposure time, approximately one-fourth of the sample from each of the four algal mats was removed and analyzed for moisture content and culturable E. coli and enterococci. At the end of the experiment, the underlying sands were also analyzed for E. coli.
Persistence of indicator bacteria and their growth potential in rehydrated Cladophora mats.
Cladophora algae were laid flat on vinyl-coated, 2.5-cm-mesh racks (0.33 by 0.37 m) to a depth of about 2.5 cm and placed outdoors in sunlight for four consecutive days (24 to 27 June 2002) from 7 a.m. to 3 p.m. the first three days and from 7 a.m. to 11 a.m. the fourth day, for a total of 28 h. The air temperature at the surface of the algae varied between 28.5 and 31°C and averaged 30°C. Skies were generally sunny, except on day 4. Algae were analyzed for E. coli and enterococci both before and after sun exposure. The dried, sun-bleached algae were then stored at 4°C in airtight plastic bags for 6 months. Samples of algal mats were then rehydrated, and growth of E. coli and enterococci was assessed. To ascertain survival and growth potential, 0.1 g of dried Cladophora was added to centrifuge tubes containing 9.9 ml of sterile PBW. The tube contents were gently mixed and incubated at 35°C, which is not unlike temperatures of exposed beach or shallow water. Triplicate tubes were randomly drawn and analyzed for E. coli and enterococci at 0, 24, 48, 72, and 96 h of incubation.
Statistical analyses.
Statistical analyses and graphics preparation were performed with SPSS version 10.01. Statistical procedures were performed on log10-transformed data to meet parametric assumptions; nonparametric testing (Kruskal-Wallis) was used where normality could not be achieved, and correlation analysis was used to compare means. The statistical significance level was set at a P of 0.05 unless otherwise stated.
RESULTS
Regional surveys.
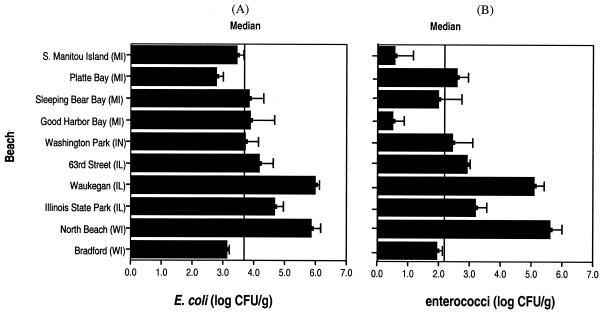
E. coli densities in Cladophora for the 10 beaches surveyed were generally high but highly variable; the overall log mean E. coli density was 5.3 ± 4.8 CFU/g. The geometric log mean density of E. coli in these algae was lower, 3.99 CFU/g, and the median was 3.72 CFU/g. The highest E. coli counts were found in attached Cladophora: log 6.2/g in epilithic algae at North Beach. Attached algae at Waukegan Municipal Beach and Washington Park Beach also had higher concentrations than local unattached algae. A Kruskal-Wallis test showed no significant difference in means among attached, floating, and stranded algae (P = 0.067). High variance and small sample size made examination of these differences difficult. Algae collected from beaches of South Manitou Island, Platte Bay, 63rd Street Beach, and Bradford Beach had relatively low E. coli counts (Fig. 2A).
FIG. 2.
Log mean concentrations (± 1 standard error) of E. coli (A) and enterococci (B) in Cladophora collected from 10 Lake Michigan beaches in Wisconsin (WI), Illinois (IL), Indiana (IN), and Michigan (MI).
Concentrations of enterococci in Cladophora averaged 4.8 ± 4.5 log CFU/g. As with E. coli concentrations, geometric mean and median concentrations of enterococci were much lower (2.3 and 2.1 log CFU/g), suggesting nonnormal distribution of bacteria among samples. The highest enterococci concentrations were found in floating algae at North Beach (6.0 log CFU/g). Very low counts of enterococci were recovered from algae collected at South Manitou Island and Good Harbor Bay; also, fewer enterococci were recovered at Bradford Beach (Fig. 2B). In general, concentrations of enterococci were higher along southern Lake Michigan, particularly at North Beach, Waukegan Municipal Beach, Illinois State Park Beach, and Washington Park Beach. Median enterococcus counts for stranded, floating, and attached algae were 1.9, 2.1, and 4.97 log CFU/g. The Kruskal-Wallis statistical test implied that concentrations of enterococci in attached algae were significantly higher than those in floating or stranded algae (P = 0.025). Variation in indicator bacteria among beaches is difficult to explain due to limited sample size.
Algal E. coli was correlated with enterococci in southern Lake Michigan beaches (P < 0.001, R2 = 0.73, n = 17) but not northern beaches (P = 0.892, n = 16). In general, Cladophora of southern Lake Michigan tended to have higher concentrations of E. coli and enterococci. Lake water followed similar trends (19, 32, 34).
Sand versus lake water.
Transect sampling suggested that patterns of E. coli concentrations in water, beach sand, and Cladophora (floating and stranded) were similar at Washington Park Beach and 63rd Street Beach. In the combined data, E. coli counts in floating algae were significantly higher than in stranded algae, sand, or water, and stranded algae had more E. coli than either sand or water (P ≤ 0.05). Algal E. coli counts from Washington Park Beach were higher (5.3 ± 4.7 log CFU/g) than counts from 63rd Street Beach (4.7 ± 3 9 log CFU/g) (P < 0.006), even though 63rd Street Beach historically had higher water E. coli counts (Chicago Park District and LaPorte Health Department, Indiana, unpublished data). E. coli in stranded algae and adjacent water were correlated (P = 0.024, n = 6), but the small sample size makes this inference tenuous.
E. coli concentrations in floating algae and lake water at Washington Park Beach were significantly correlated (P = 0.004, R2 = 0.72, n = 9). Similarly, there was a significant relationship between water temperature (that ranged between 6 and 23°C during June to November) and E. coli concentrations in floating algae (P = 0.004, R2 = 0.41, n = 18). The highest E. coli density occurred during the middle of summer. It is noteworthy that algal E. coli concentrations remained relatively stable during most of the summer; the log mean E. coli concentration from June 24 through September 13 was 4.0 ± 0.33 CFU/g. By October, algal E. coli density was below detection, coinciding with a drop in water temperature, even though Cladophora still looked healthy.
E. coli persistence in Cladophora mats.
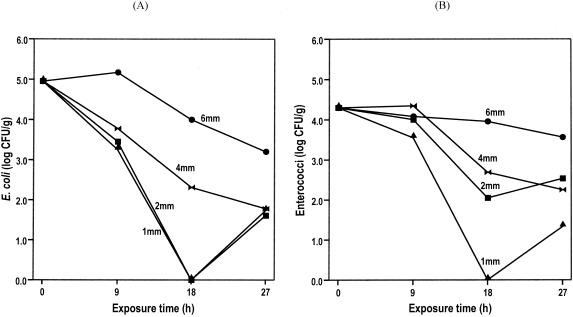
While there was generally an exponential decline in E. coli over a 27-h sunlight exposure period, only a modest population loss occurred in the first 9 h, even in the 1-mm-thick mat (Fig. 3A). Without replication, it is difficult to generalize, but there is a preliminary suggestion of an increase in density in the 6-mm mat over the first 9 h, coincident with a mean algal high temperature of 48°C. E. coli counts in 1- and 2-mm mats quickly declined; in 1- to 4-mm-thick mats, the counts remained at about 2 log CFU even after 27 h of exposure. Mats of 6-mm thickness were much more resistant to depopulation, maintaining their density at almost 4 log after 27 h.
FIG. 3.
Responses of E. coli (A) and enterococci (B) within Cladophora mats that were exposed to direct sunlight for four consecutive days. Algal mats of various thicknesses were retrieved and analyzed after 9, 18, and 27 h of exposure.
Enterococcal persistence in Cladophora mats.
Enterococci appeared to be as vulnerable as E. coli to exposure at algal thickness of 1 mm (Fig. 3B). Enterococci in 2-, 4-, and 6-mm mats remained at about 4 log CFU/g during the first 9 h. For mat thicknesses of 2 and 4 mm, enterococcal counts declined by approximately 2 log in 18 h of exposure but then remained steady thereafter. Enterococci densities in the 6-mm mat were essentially unaffected throughout the exposure time.
Indicator bacterial survival and growth potential in sunlight-exposed and refrigerated Cladophora mats.
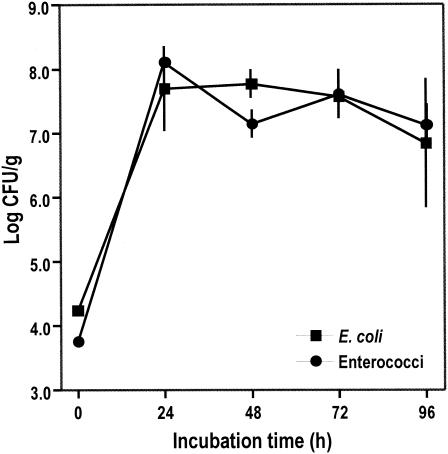
Concentrations of both E. coli and enterococci increased by approximately 4 log in 24 h following rewetting of the dried Cladophora mat (Fig. 4). During the next 72 h, counts of these bacteria remained stable (enterococci) or declined only slightly (E. coli). When the experiment was terminated after 96 h, concentrations of E. coli and enterococci were still in excess, by 2 log, of their initial numbers. These results suggest that E. coli and enterococci could persist for long periods in the sun-dried and subsequently refrigerated Cladophora mats; the residual bacteria in the dried algae could readily multiply upon hydration and incubation at 35°C.
FIG. 4.
Response of E. coli and enterococci (± 1 standard error) in rehydrated, unaugmented, and unseeded Cladophora, which had been sun dried (for 28 h) and subsequently stored at 4°C for over 6 months.
DISCUSSION
Associations between Cladophora and microbial communities are not well understood, although some research has presented evidence of a relationship between Cladophora and bacilliform bacteria (27). The cell wall of Cladophora provides a suitable attachment and grazing surface for many other organisms, such as diatoms, protozoa, mollusks, rotifers, and young crayfish (8, 27, 31), and links between bacteria and algae have been found frequently in aquatic environments (3, 13, 18, 21). The findings of this study are significant because it is perhaps the first to demonstrate the presence of fecal indicator bacteria, E. coli and enterococci, on Cladophora.
In spite of a limited number of studies that suggest that indicator bacteria (E. coli and enterococci) can multiply in nature (1, 7, 11, 14, 29), there remain a number of ecological questions regarding such growth. Commonly cited potential limiting factors include interspecific competition, predation, and nutrient limitation (6, 10). The present study demonstrates that Cladophora harbors high densities of E. coli and enterococci relative to water and beach sand and that the indicator bacteria in Cladophora are ubiquitous and perhaps even independent of point sources. Further, the experiments show that E. coli and enterococci can survive for extended periods (over 6 months) in the algal mat and quickly multiply when moisture is returned. Thus, Cladophora stranded on the beach is a potential source of these indicator bacteria whether the algal mat is dry or remains moist or whether it has been exposed to sunlight or buried in the sand. These observations demonstrate that Cladophora provides both the minimal habitat and nutrient source for survival and possibly growth of E. coli and enterococci.
The explanation for the occurrence of bacteria in floating and attached algae is less intuitive. Perhaps Cladophora is so rich in nutrients and biofilm habitat that indicator bacteria can maintain populations despite obvious interspecific pressures from resident organisms, such as periphyton and grazers (8, 20, 27, 31). Regardless, the persistence and survival of indicator bacteria in Cladophora under natural conditions seems to depend on a variety of factors (predation, sunlight, and temperature) (10). Since E. coli and enterococci survived for over 6 months in sun-dried and refrigerated Cladophora, perhaps other factors (competition, predation, and sunlight) were responsible for the gradual disappearance of E. coli and enterococci in naturally occurring Cladophora by October.
Our findings clearly suggest that Cladophora can be a secondary habitat for indicator bacteria that could potentially influence water quality in affected Great Lakes swimming areas. The long-term survival of E. coli and enterococci in Cladophora mats also has important ecological and public health implications. Masses of floating Cladophora, as a result of wave action, can release indicator bacteria and elevate their levels in the water. Also, algal mats washed onto beach sand may get buried in the sand by wave action or human activities, where they are protected from sunlight and desiccation. Here, indicator bacteria may multiply due to available nutrients from the decomposing mats; in turn, the beach sand can serve as a source of indicator bacteria for the nearshore water, especially when waves resuspend buried mats. Previously, studies have shown that pathogenic bacteria (e.g., vibrios) are often associated with algae (17). It is possible that Cladophora provides a niche for pathogenic bacteria.
While the case for natural multiplication needs further validation, Cladophora can be a reservoir for E. coli and enterococci in Lake Michigan. To understand the ecological and environmental implications of the present findings, more laboratory studies are necessary. These might include (i) in vitro studies showing the range of tolerance and growth potential of subject bacteria under a variety of environmental conditions (insolation, desiccation, and temperature), (ii) a thorough investigation of the genomic and phenotypic relationships of algae and ambient bacteria to investigate clonality or source-sink relationships further, (iii) noninvasive sterilization and inoculation of algae using wild and lab strains to discover intrinsic growth potential, maximum carrying capacity, and associated limiting factors, (iv) high-resolution microscopic studies of algal thalli and biofilm to further understand the physical association of algae and indicator bacteria, and (v) more investigations of the health implications of these findings.
Acknowledgments
The first and last authors contributed equally to this research.
We thank Douglas Wilcox and Eric Garza, U.S. Geological Survey, for their critical review of the manuscript. Paul Murphy, National Park Service, and Julie Kinzelman, Racine Health Department, helped in collecting Cladophora samples. Special thanks go to Melanie Fowler, U.S. Geological Survey, who helped in the initial stages of this research.
Footnotes
This article is contribution 1244 of the USGS Great Lakes Science Center.
REFERENCES
- 1.Anderson, S. A., S. J. Turner, and G. D. Lewis. 1997. Enterococci in the New Zealand environment: implications for water quality monitoring. Water Sci. Technol. 35:325-331. [Google Scholar]
- 2.Ashbolt, N. J., M. R. Dorsch, and B. Banens. 1997. Blooming E. coli, what do they mean? In D. Kay and C. Fricker (ed.), Coliforms and E. coli, problem or solution? The Royal Society of Chemistry, Cambridge, England.
- 3.Barbeyron, T., and Y. Berger. 1989. Commensal bacteria living with two multicellular marine algae: Antithamnion plumula (Ellis) Thuret and Cladophora rupestris (L.) Kützing (Linne), Kützing. Phenotypic characterization. Cah. Biol. Mar. 30:361-374. [Google Scholar]
- 4.Beaudeau, P., N. Tousset, F. Bruchon, A. Lefevre, and H. D. Taylor. 2001. In situ measurement and statistical modelling of Escherichia coli decay in small rivers. Water Res. 35:3168-3178. [DOI] [PubMed] [Google Scholar]
- 5.Blum, J. L. 1982. Colonization and growth of attached algae at the Lake Michigan water line. J. Great Lakes Res. 8:10-15. [Google Scholar]
- 6.Byappanahalli, M. N. 2000. Assessing the persistence and multiplication of fecal indicator bacteria in Hawaii soil environment. Ph.D. thesis. University of Hawaii at Manoa, Honolulu.
- 7.Byappanahalli, M. N., and R. S. Fujioka. 1998. Evidence that tropical soil environment can support the growth of Escherichia coli. Water Sci. Technol. 38:171-174. [Google Scholar]
- 8.Chilton, E. W., R. L. Lowe, and K. M. Schurr. 1986. Invertebrate communities associated with Bangia atropurpurea and Cladophora glomerata in western Lake Erie. J. Great Lakes Res. 12:149-153. [Google Scholar]
- 9.Clesceri, L. S., A. E. Greenberg, and A. D. Eaton (ed.). 1998. Standard methods for the examination of water and wastewater, 20th ed. American Public Health Association, Washington, D.C.
- 10.Crane, S. R., and J. A. Moore. 1986. Modeling enteric bacterial die-off: a review. Water Air Soil Pollut. 27:411-439. [Google Scholar]
- 11.Desmarais, T. R., H. Solo-Gabriele, and C. J. Palmer. 2002. Influence of soil on fecal indicator organisms in a tidally influenced subtropical environment. Appl. Environ. Microbiol. 68:1165-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dodds, W. K., and D. A. Gudder. 1992. The ecology of Cladophora. J. Phycol. 28:415-427. [Google Scholar]
- 13.Fisher, M., L. W. Wilcox, and L. E. Graham. 1998. Molecular characterization of epiphytic bacterial communities on charophycean green algae. Appl. Environ. Microbiol. 64:4384-4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujioka, R. S., and M. N. Byappanahalli. 2001. Microbial ecology controls the establishment of fecal bacteria in tropical soil environment, p. 273-283. In K. H. T. Matsuo, S. Takizawa, and H. Satoh (ed.), Advances in water and wastewater treatment technology: molecular technology, nutrient removal, sludge reduction and environmental health. Elsevier, Amsterdam, The Netherlands.
- 15.Fujioka, R. S., C. Sian-Denton, M. Borja, J. Castro, and K. Morphew. 1999. Soil: the environmental source of Escherichia coli and enterococci in Guam's streams. J. Appl. Microbiol. Symp. Suppl. 85:83S-89S. [DOI] [PubMed]
- 16.Hardina, C. M., and R. S. Fujioka. 1991. Soil: the environmental source of Escherichia coli and enterococci in Hawaii's streams. Environ. Toxicol. Water Qual. 6:185-195. [Google Scholar]
- 17.Islam, M. S., B. S. Drasar, and D. J. Bradley. 1989. Attachment of toxigenic Vibrio cholerae O1 to various freshwater plants and survival with filamentous green alga Rhizoclonium fontanum. J. Trop. Med. Hyg. 92:396-401. [PubMed] [Google Scholar]
- 18.Kaplan, L. A., and T. L. Bott. 1989. Diel fluctuations in bacterial activity on streambed substrata during vernal algal blooms: effects of temperature, water chemistry and habitat. Limnol. Oceanogr. 34:718-733. [Google Scholar]
- 19.Kinzelman, J., C. Ng, E. Jackson, S. Gradus, and R. Bagley. 2003. Enterococci as indicators of Lake Michigan recreational water quality: comparison of two methodologies and their impacts on public health regulatory events. Appl. Environ. Microbiol. 69:92-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marks, J. C., and M. E. Power. 2001. Nutrient induced changes in the species composition of epiphytes on Cladophora glomerata Kutz (Chlorophyta). Hydrobiologia 450:187-196. [Google Scholar]
- 21.Matsuo, Y., M. Suzuki, H. Kasai, Y. Shizuri, and S. Harayama. 2003. Isolation and phylogenetic characterization of bacteria capable of inducing differentiation in the green alga Monostroma oxyspermum. Environ. Microbiol. 5:25-35. [DOI] [PubMed] [Google Scholar]
- 22.Muller, T., A. Ulrich, E. M. Ott, and M. Muller. 2001. Identification of plant-associated enterococci. J. Appl. Microbiol. 91:268-278. [DOI] [PubMed] [Google Scholar]
- 23.Mundt, J. O. 1963. Occurrence of enterococci in animals and wild environment. Appl. Microbiol. 11:141-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Natural Resources Defense Council. 2002. Testing the waters 2002: a guide to water quality at vacation beaches. [Online.] http://www.nrdc.org/water/oceans/ttw/exesum.asp.
- 25.Oragui, J. I., and D. D. Mara. 1983. Investigation of the survival characteristics of Rhodococcus coprophilus and certain fecal indicator bacteria. Appl. Environ. Microbiol. 46:356-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oshiro, R., and R. Fujioka. 1995. Sand, soil, and pigeon droppings: sources of indicator bacteria in the waters of Hanauma Bay, Oahu, Hawaii. Water Sci. Technol. 31:251-254. [Google Scholar]
- 27.Rex, L. L., B. H. Rosen, and J. C. Kingston. 1982. A comparison of epiphytes on Bangia atropurpurea (Rhodophyta) and Cladophora glomerata (Chlorophyta) from northern Lake Michigan. J. Great Lakes Res. 8:164-168. [Google Scholar]
- 28.Rivera, S. C., T. C. Hazen, and G. A. Toranzos. 1988. Isolation of fecal coliforms from pristine sites in a tropical rainforest. Appl. Environ. Microbiol. 54:513-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Solo-Gabriele, H., M. A. Wolfert, T. R. Desmarais, and C. J. Palmer. 2000. Sources of Escherichia coli in a coastal subtropical environment. Appl. Environ. Microbiol. 66:230-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stevenson, R. J., and E. F. Stoermer. 1982. Seasonal abundance patterns of diatoms on Cladophora in Lake Huron. J. Great Lakes Res. 8:169-183. [Google Scholar]
- 31.Taft, C. E. 1975. History of Cladophora in the Great Lakes, p. 5-16. In H. Shear and D. E. Konasewich (ed.), Cladophora in the Great Lakes. Great Lakes Research Advisory Board, International Joint Commission Regional Office, Windsor, Ontario, Canada.
- 32.Whitman, R., M. Fowler, D. Shively, and M. Byappanahalli. 2002. Distribution and characterization of E. coli within the Dunes Creek watershed, Indiana Dunes State Park. Report to the Indiana Department of Natural Resources. U.S. Geological Survey, Porter, Ind.
- 33.Whitman, R. L., A. V. Gochee, W. A. Dustman, and K. J. Kennedy. 1995. Use of coliform bacteria in assessing human sewage contamination. Nat. Areas J. 15:227-233. [Google Scholar]
- 34.Whitman, R. L., T. G. Horvath, M. L. Goodrich, M. B. Nevers, M. J. Wolcott, and S. K. Haack. 2001. Characterization of E. coli levels at 63rd Street Beach. Report to the City of Chicago, Department of the Environment and the Chicago Park District. U.S. Geological Survey, Porter, Ind.
- 35.Whitman, R. L., M. B. Nevers, and P. J. Gerovac. 1999. Interaction of ambient conditions and fecal coliform bacteria in southern Lake Michigan waters: monitoring program implications. Nat. Areas J. 19:166-171. [Google Scholar]