Abstract
Due to their spectroscopic properties porphyrins are of special interest for a variety of applications, ranging from drug development or targeting to material sciences and chemical and biological sensors. Since chemical syntheses are limited in terms of regio- and stereoselective functionalization of porphyrins, a biosynthetic approach with tailored enzyme catalysts offers a promising alternative. In this paper, we describe assembly of the entire heme biosynthetic pathway in a three-plasmid system and overexpression of the corresponding genes with Escherichia coli as a host. Without further optimization, this approach yielded remarkable porphyrin production levels, up to 90 μmol/liter, which is close to industrial vitamin B12 production levels. Different combinations of the genes were used to produce all major porphyrins that occur as intermediates in heme biosynthesis. All these porphyrin intermediates were obtained in high yields. The product spectrum was analyzed and quantified by using high-performance liquid chromatography. Intriguingly, although protoporphyrin IX could be produced at high levels, overexpressed Bacillus subtilis ferrochelatase could not convert this substrate appreciably into heme. However, further investigation clearly revealed a high level of expression of the ferrochelatase and a high level of activity in vitro. These results may indicate that heme has a regulatory impact on the iron uptake of E. coli or that the ferrochelatase is inactive in vivo due to an incompatible enzyme interaction.
Porphyrins have long been of interest for biomedical applications. Drugs based on porphyrins have been or are currently being developed for treatment of cancer, psoriasis, and virus infections, for gene regulation therapies, and, more recently, for drug targeting (2, 7, 13, 36). Other innovative porphyrin applications are in material science and chemistry (3, 15, 21, 33, 34, 38). Expanded porphyrins (20) with increased numbers of π-electrons, which absorb light at wavelengths greater than 630 nm, are of particular interest for biomedical laser applications, such as photodynamic treatment of cancer (13). Metalloporphyrins have applications in electrocatalysis, as electrodes in fuel cells, and as chemical sensors (5, 12, 14, 15).
Porphyrins can be chemically synthesized by either total synthesis or functional derivatization of heme or chlorophyll derivatives. However, many regio- and stereoselective functionalizations of porphyrins cannot be achieved chemically. Furthermore, chemical synthesis of porphyrin derivatives usually requires several steps that result in low overall yields, which makes porphyrins very expensive compounds.
Alternatively, a biosynthetic approach involving stereo- and regioselective enzyme catalysts may be used to obtain relatively high yields of natural porphyrins, while mutagenesis and directed evolution may yield enzymes that produce porphyrins with unnatural functionalizations. Studies of natural porphyrins have so far been focused on elucidating the biochemistry and genetics of porphyrin biosynthesis in cells (4, 8) rather than on engineering recombinant cells for the production of porphyrins. Heme biosynthesis is the most extensively studied porphyrin pathway (reviewed in references 4 and 8). As shown in Fig. 1, tetrapyrrole biosynthesis starts with the synthesis of aminolevulinic acid (ALA). Two molecules of ALA are then condensed to form porphobilinogen (PBG). In turn, four molecules of PBG are condensed to form 1-hydroxymethylbilane, which is subsequently cyclized to form uroporphyrinogen III (urogen III), in which ring D undergoes inversion. Direct coupling of these two enzymatic steps prevents spontaneous formation of the urogen I isomer, which is not a substrate of most subsequent biosynthetic enzymes. Urogen III is the common precursor of all tetrapyrroles in living cells at which major branching of the biosynthetic pathways occurs (4, 8). The next step in heme biosynthesis involves the decarboxylation of urogen III to form coproporphyrinogen III (coprogen III), which is followed by oxidation and decarboxylation reactions to synthesize protoporphyrin IX. Finally, insertion of a ferrous iron ion into protoporphyrin IX produces protoheme IX.
FIG. 1.
Assembled pathway for porphyrin biosynthesis in engineered E. coli. Abbreviations: Succinyl-CoA, succinyl coenzyme A; Gly, glycine; HMB, 1-hydroxymethylbilane; Uro III, uroporphyrin III; Copro III, coproporphyrin III; Proto IX, protoporphyrin IX; Uro I, uroporphyrin I; PCP, pentacarboxyporphyrin; Copro I, coproporphyrin I. All porphyrins were derived from the corresponding porphyrinogens. *Ox indicates that there was spontaneous oxidation during and after extraction from E. coli transformants.
In this paper, we describe for the first time overproduction of different porphyrins and porphyrinogens in Escherichia coli by systematic extension of a heme biosynthetic pathway assembled from eight genes obtained from various microorganisms. These genes were cloned on plasmids belonging to different incompatibility groups to enable modular pathway assembly. The overproduced porphyrins and porphyrinogens may serve as starter structures for the production of novel, unnatural porphyrins, whereas the genes may be altered to encode enzymes with new catalytic activities. A particular gene of the assembled pathway may be modified at will, while the native chromosomal copy ensures that there is unaffected functional heme biosynthesis for essential metabolic processes.
Below, for the sake of conciseness, we use the notation commonly adopted for operons to describe combinations of heme biosynthetic genes. Thus, hemAB means any combination of hemA and hemB, not necessarily an operon.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
Strains and plasmids used in this study are listed in Table 1. E. coli JM109 was used as a host both for cloning and for assembly of the porphyrin-heme overproduction pathways. Plasmids pUCmod (31), pACmod (31), and pBBR1MCS-2 (22), which confer carbenicillin, chloramphenicol, and kanamycin resistance, respectively, were used to construct porphyrin-heme biosynthetic pathways, as described below. Bacillus subtilis and E. coli were routinely grown in Luria-Bertani (LB) medium. For E. coli, the medium was supplemented with carbenicillin (100 μg/ml), chloramphenicol (50 μg/ml), and kanamycin (30 μg/ml) when appropriate. Rhodobacter capsulatus was grown in Rhodospirillacae medium as suggested by the Deutsche Sammlung von Mikroorganismen und Zellkulturen, and Synechocystis was grown in BG-11 medium as suggested by the American Type Culture Collection.
TABLE 1.
Plasmids and strains used in this study
| Strain or plasmid | Relevant properties or genotype | Source or referencea |
|---|---|---|
| Strains | ||
| E. coli K-12 strain JM109 | recA1 supE44 endA1 hsdR17 (rk− mk+) gyrA96 relA1 thi Δ(lac-proAB) [F′ traD36 proAB+ lacIqlacZΔM15] | 37 |
| R. capsulatus ATH 2.3.1 | Wild type | DSMZ |
| Synechocystis sp. strain PCC6803 | Wild type | ATCC |
| B. subtilis 168 | Wild type | ATCC |
| Plasmids | ||
| pUCmod | Cloning vector, constitutive lac promoter, Amp | 31 |
| pACmod | Cloning vector, Tcr Cmr | 31 |
| pBBR1MCS-2 | Cloning vector, Kmr | 22 |
| pUCmod-hemA | hemA gene from R. capsulatus | This study |
| pUCmod-hemB | hemB gene from E. coli | This study |
| pUCmod-hemC | hemC gene from E. coli | This study |
| pUCmod-hemD | hemD gene from E. coli | This study |
| pUCmod-hemE | hemE gene from Synechocystis | This study |
| pUCmod-hemF | hemF gene from E. coli | This study |
| pUCmod-hemY | hemY gene from B. subtilis | This study |
| pUCmod-hemH | hemH gene from B. subtilis | This study |
| pACmod-hemA | Constitutively expressed R. capsulatus hemA | This study |
| pACmod hemAB | Constitutively expressed R. capsulatus hemA and E. coli hemB | This study |
| pACmod-hemABD | Constitutively expressed R. capsulatus hemA and E. coli hemB and hemD | This study |
| pACmod-hemABC | Constitutively expressed R. capsulatus hemA and E. coli hemB and hemC | This study |
| pACmod-hemABCD | Constitutively expressed R. capsulatus hemA and E. coli hemB, hemC, and hemD | This study |
| pBBR-hemE | Constitutively expressed Synechocystis hemE | This study |
| pBBR-hemF | Constitutively expressed E. coli hemF | This study |
| pBBR-hemEF | Constitutively expressed Synechocystis hemE and E. coli hemF | This study |
DSMZ, Deutsche Sammlung von Mikroorganismen and Zellkulturen; ATCC, American Type Culture Collection.
DNA manipulations.
Standard DNA manipulations were performed as described previously (29). Products from Qiagen (Hilden, Germany) were used for isolation of genomic and plasmid DNA and to purify DNA fragments from agarose gels. Restriction enzymes, T4 DNA ligase, and Vent DNA polymerase were obtained from New England Biolabs (Beverly, Mass.). Klenow polymerase was obtained from Takara (Shiga, Japan). Taq DNA polymerase in buffer A was obtained from Promega (Madison, Wis.). Oligonucleotide primers were obtained from either Genemed Synthesis (South San Francisco, Calif.) or Integrated DNA Technologies (Coralville, Iowa). DNA sequencing was performed by the Advanced Genetic Analysis Center at the University of Minnesota.
Plasmid construction. (i) Initial cloning of heme biosynthetic genes.
Eight heme biosynthetic genes were amplified (by using Taq DNA polymerase) from chromosomal DNA from R. capsulatus (hemA), E. coli (hemB, hemC, hemD, and hemF), Synechocystis sp. (hemE), and B. subtilis (hemY and hemH) (Table 1). The GenBank accession numbers are as follows: X53309 for hemA, D85613 for hemB, X12614 for hemC and hemD, D64006 for hemE, X75413 for hemF, and M97208 for hemY and hemH. All genes were amplified from the corresponding genomic DNA by using primers that had been designed to generate XbaI and NotI restriction sites near the ends of the amplification products and a Shine-Dalgarno sequence upstream of the coding region, respectively. The PCR products were cloned into pUCmod, a derivative of pUC19 obtained by replacing a DNA stretch encompassing the lac operator through the lacZα gene with a new multiple cloning site (XbaI, SmaI, EcoRI, NcoI, and NotI) (31). This vector facilitated constitutive expression of cloned genes when it was provided with a Shine-Dalgarno sequence. The integrity of the cloned genes was verified by DNA sequence analysis.
(ii) Assembly of heme biosynthetic genes on pACmod and pBBR1MCS-2.
The cloned genes with their lac promoters were reamplified (by using Vent DNA polymerase) from the corresponding pUCmod plasmids with primers containing suitable restriction sites and were assembled in pACmod (a pACYC184 derivative obtained by removing the XbaI site) (31) or pBBR1MCS-2 (22) as described below.
The hemA-containing PCR product was subcloned into pACmod by using HindIII (5′ end) and BamHI (3′ end) sites to construct pACmod-hemA. The hemB gene was amplified to produce a PCR product with a BamHI site (5′ end) and a ClaI site (3′ end). This PCR product was digested with only BamHI (leaving a blunt end at the other side) and was ligated to the pACmod-hemA vector band that was prepared by digestion with SalI, end filling with the Klenow polymerase, and subsequent digestion with BamHI. This construction yielded pAC-hemAB, in which hemA and hemB were contained on a ∼2.5-kb ClaI fragment. The BamHI site was then removed by digestion, end filling, and religation, yielding pACmod-hemABΔBam. This procedure yielded a new, third ClaI site that was protected by overlapping dam methylation. A PCR product carrying the hemD gene and restriction sites for BamHI (5′ end) and XhoI site (3′ end) was subcloned into BamHI- and SalI-digested pACmod, resulting in pACmod-hemD. The ∼2.5-kb ClaI fragment from pACmod-hemABΔBam, containing the hemA and hemB genes, was subcloned into the ClaI site of pACmod-hemD to generate pACmod-hemABD. Plasmid pACmod-hemABC was constructed in two stages. First, the hemC gene was subcloned as a HindIII (5′ end)-BamHI (3′ end) fragment into pACmod. Then the hemA- and hemB-containing ClaI fragment from pACmod-ABΔBam was cloned in the ClaI site. Plasmid pACmod-hemABCD was constructed by cloning the hemC gene as a BamHI fragment in pACmod-hemABD. The hemE gene was subcloned into the XhoI and HindIII sites of pBBR1MCS-2. Similarly, the hemF gene was subcloned into the EcoRI and BamHI sites of pBBR1-MCS2 to construct pBBR1-hemF. Plasmid pBBR1-hemEF was constructed by subcloning the hemF gene into pBBR1-hemE with the EcoRI and BamHI sites.
Overproduction and analysis of porphyrinogens and porphyrins.
Various combinations of plasmids were transformed into E. coli. A three-plasmid system was used throughout to ensure similar culture conditions. All transformants were cultivated for 48 h at 30°C to lower the copy number of pUCmod (23) in 50 ml of LB medium containing carbenicillin (100 μg/ml), chloramphenicol (50 μg/ml), and kanamycin (30 μg/ml). Each culture was processed as follows. After cultivation, 200 mg of DEAE-Sephadex A-25 (Pharmacia Fine Chemicals, Uppsala, Sweden) was added to the culture to adsorb secreted or released porphyrin(ogen)s (27). The bacterial cells and the DEAE resin were pelleted by centrifugation (4,000 × g, 20 min). The pellet was extracted with 50 ml of an organic solvent (acetone-dimethyl formamide-6 N HCl, 10:1:2 [vol/vol/vol])) at 40°C. During this procedure, all porphyrinogens were converted to the corresponding porphyrins by contact with atmospheric oxygen. Fifty microliters of the extract was applied to an octyldecyl silane column (Zorbax RX-C18; 150 by 4.6 mm; 5 μm; Agilent Technologies, Palo Alto, Calif.) by using an Agilent high-performance liquid chromatography (HPLC) system equipped with a photodiode array detector. A previously described mobile-phase system was used (26), with minor modifications. Briefly, the mobile phase consisted of two solvent mixtures, solvent A (100 g of ammonium acetate per liter and 125 ml of acetonitrile, adjusted to pH 5.16 with glacial acetic acid) and solvent B (methanol-acetic acid, 10:1 [vol/vol]) (26). Analytes were eluted at a flow rate of 1 ml/min. The program used consisted of an isocratic elution step with solvent A (5 min), followed by a linear gradient from 0 to 100% solvent B in 30 min. Solvent B was then maintained at a concentration of 100% for 5 min. The system was equilibrated for another 5 min with 100% solvent A before the next sample injection. The mass spectra of various porphyrins and heme were obtained by mass spectrometry (LCQ; ThermoFinnigan, San Jose, Calif.) coupled with an electrospray ionization interface. The spray voltage was 4.0 kV, and the heated capillary temperature was 225°C. The amounts of individual porphyrins and heme were determined by using authentic porphyrins and heme purchased from Porphyrin Products Inc. (Logan, Utah) and ICN Biomedicals Inc. (Aurora, Ohio), respectively.
B. subtilis ferrochelatase activity was assayed essentially as described by Camadro and Labbe (6). However, palmitic acid and Tween 80 were omitted as B. subtilis ferrochelatase is a soluble enzyme (18). Cell extracts were made from three assembled pathways: (i) cells with hemA through hemH (combination 10 in Table 2), (ii) cells with hemA through hemF (combination 9 in Table 2), and (iii) cells with no overexpressed heme biosynthetic genes (combination 1 in Table 2). The latter two cell extracts served as (negative) controls. Reaction buffer without cell extract served as a third control. All transformants were grown for 48 h at 30°C, harvested by centrifugation (4,000 × g, 15 min), and resuspended in 50 mM Tris-HCl buffer (pH 7.6). The cells were disrupted by sonication. Ferrochelatase activity was monitored by time-dependent measurement of fluorescence at room temperature (excitation at 410 nm and emission at 632 nm) by using a Gemini XS spectrofluorometer from Molecular Devices (Sunnyvale, Calif.).
TABLE 2.
Porphyrin accumulation in E. coli transformants overexpressing different combinations of heme biosynthetic genesa
| Combination | Heme biosynthetic genes overexpressed on:
|
Concn (μmol/liter)b
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| pACmod | pBBR1-MCS2 | pUCmod | Uropophyrin I | Uroporphyrin III | Pentacarboxy-porphyrin | Coproporphyrin I | Coproporphyrin III | Protoporphyrin | Heme | Total | |
| 1 | NDc | ND | ND | ND | ND | Trd | 0.2 ± 0.0 | 0.2 ± 0.0 | |||
| 2 | A | 3.3 ± 0.8 | 0.6 ± 0.2 | ND | 0.3 ± 0.0 | 2.0 ± 0.1 | 0.6 ± 0.5 | 0.9 ± 0.1 | 7.7 ± 1.6 | ||
| 3 | A, B | 4.0 ± 1.8 | 0.6 ± 0.3 | ND | 0.1 ± 0.0 | 0.9 ± 0.2 | 0.6 ± 0.1 | 0.4 ± 0.1 | 6.7 ± 2.4 | ||
| 4 | A, B, D | 0.8 ± 0.1 | 1.8 ± 0.1 | ND | 0.1 ± 0.0 | 1.0 ± 0.3 | 1.1 ± 0.1 | 0.5 ± 0.1 | 5.3 ± 0.7 | ||
| 5 | A, B, C | 14.4 ± 0.2 | ND | 1.1 ± 0.2 | 0.5 ± 0.1 | ND | Tr | 0.2 ± 0.1 | 16.2 ± 0.5 | ||
| 6 | A, B, C, D | 7.2 ± 1.8 | 41 ± 10 | ND | ND | 1.6 ± 0.3 | 0.2 ± 0.1 | 0.4 ± 0.3 | 49 ± 12 | ||
| 7 | A, B, C | E | 8.2 ± 0.1 | ND | 2.2 ± 0.1 | 1.0 ± 0.1 | 0.2 ± 0.0 | Tr | 0.2 ± 0.1 | 11.7 ± 0.4 | |
| 8 | A, B, C, D | E | ND | ND | ND | 3.0 ± 0.2 | 47 ± 4 | 0.4 ± 0.2 | 0.4 ± 0.1 | 51 ± 4 | |
| 9 | A, B, C, D | E, F | ND | ND | ND | 0.3 ± 0.1 | 0.7 ± 0.2 | 82 ± 6 | 2.4 ± 0.5 | 85 ± 7 | |
| 10 | A, B, C, D | E, F | H | ND | ND | ND | 0.3 ± 0.1 | 1.0 ± 0.2 | 84 ± 8 | 3.3 ± 0.3 | 89 ± 9 |
| 11 | A, B, C, D | Y | 2.0 ± 0.2 | 7.2 ± 0.6 | ND | ND | 1.4 ± 0.3 | Tr | 0.1 ± 0.1 | 11 ± 1 | |
| 12 | A, B, C, D | F | Y | 0.7 ± 0.1 | 4.8 ± 0.1 | ND | ND | 2.6 ± 0.1 | 0.5 ± 0.1 | 0.3 ± 0.1 | 9.3 ± 0.4 |
| 13 | A, B, C, D | E | Y | 1.3 ± 0.3 | 0.2 ± 0.1 | ND | ND | 37 ± 2 | Tr | 0.7 ± 0.0 | 39 ± 2 |
| 14 | A, B, C | E | Y | 7.0 ± 0.7 | ND | 4.7 ± 0.6 | 2.1 ± 0.4 | 1.8 ± 0.3 | ND | 0.2 ± 0.1 | 16 ± 2 |
| 15 | A, B, C, D | E, F | Y | 0.7 ± 0.3 | 0.4 ± 0.1 | ND | 1.5 ± 0.2 | 21 ± 3 | 28 ± 4 | 1.7 ± 0.4 | 52 ± 8 |
E. coli cells were cultured in LB media containing chloramphenicol, kanamycin, and carbenicillin for 48 h at 30°C.
The values are means ± standard deviations for three experiments.
ND, not detected.
Tr, trace (less than 0.1 μmol/liter).
RESULTS
Modular system for porphyrin(ogen) overproduction.
A modular system was designed for overproduction of porphyrins and porphyrinogens in E. coli. The system is based on three compatible replicons belonging to different incompatibility groups. Each of the plasmids carries one or several heme biosynthetic genes cloned from various bacteria (Table 1). Most genes were obtained from E. coli. Genes from other organisms were selected for various reasons, such as the availability of X-ray data, different substrate specificities, or anticipated production levels; these reasons are specified for the individual genes below. All heme-specific genes in our pathways are constitutively expressed from a lac promoter. Thus, regulation at the level of transcription and translation is (largely) avoided. Overproduction of a particular porphyrin (or porphyrinogen) can be achieved by choosing the appropriate combination of plasmids. We used this system to systematically extend our pathway (Fig. 1) and analyze the products formed. A three-plasmid system, with the appropriate empty control plasmids if necessary, was used throughout to ensure that the growth conditions were similar.
Porphyrin(ogen) overproduction.
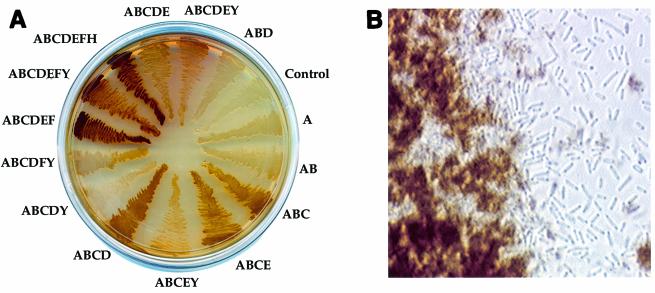
E. coli transformants overexpressing R. capsulatus hemA developed a slightly brown color compared to control cells after overnight growth at 30°C both on LB agar plates and in liquid culture due to the accumulation of porphyrins. However, development of a strong reddish color of E. coli cells both on agar plates and in liquid culture was observed only when the first three genes (hemA, hemB, and hemC) were overexpressed together. Furthermore, the color and its intensity depended strongly on the type of porphyrin produced, and color continued to develop after cells reached the stationary phase. An LB agar plate with E. coli transformants harboring various plasmid combinations is shown in Fig. 2A. E. coli cells overexpressing at least the first five genes of the assembled heme pathway (hemA to hemE) (Fig. 1) accumulated large amounts of porphyrins in the culture medium, resulting in partial precipitation of porphyrins (Fig. 2B).
FIG. 2.
(A) LB agar plate with E. coli transformants harboring various combinations of heme biosynthetic genes after 48 h of growth at 30°C. The letters indicate heme biosynthetic genes that were overproduced in the E. coli strains. The control did not overexpress hem genes. See text for details. (B) Microscope image of a liquid culture of E. coli cells carrying hemA through hemF. The cells were grown in LB medium for 48 h at 30°C. Note the brown porphyrin precipitate surrounding the bacteria.
Systematic extension of an engineered heme biosynthetic pathway.
Tetrapyrrole production starts with biosynthesis of the precursor ALA. Two routes for ALA synthesis are known: from succinyl coenzyme A and glycin (the C4 pathway) and from tRNAGlu (the C5 pathway). The C4 pathway is found in animals, fungi, nonphotosynthetic eukaryotes, and members of the α subclass of the Proteobacteria. Most bacteria (including E. coli) and plants use the C5 pathway (28). We decided to use the C4 route for ALA biosynthesis in our pathway for the following reasons. (i) Only one heme-specific gene, hemA, is required for ALA synthesis with the C4 pathway, while two genes (gtrA and hemL) are needed in the C5 pathway (28) (The designation gtrA in the C5 pathway replaces the former designation hemA, as the hemA genes of the C4 and C5 pathways encode unrelated enzymes [28].) (ii) The use of tRNAGlu for synthesizing ALA has been found to have an adverse effect on protein synthesis (25).
Overexpression of the R. capsulatus hemA gene in recombinant E. coli cells resulted in overproduction of porphyrins (Table 2) due to conversion of ALA by chromosomally encoded heme biosynthetic enzymes.
The E. coli enzyme 5-aminolevulinic acid dehydratase, also known as PBG synthase (encoded by hemB), is the second enzyme in our engineered pathway. It converts two ALA molecules to PBG with concomitant release of a water molecule. Coexpression of hemA and hemB did not result in higher porphyrin production than overexpression of hemA alone (cf. combinations 2 and 3 in Table 2).
The enzyme 1-hydroxymethylbilane synthase (or PBG deaminase), encoded by hemC, catalyzes the tetramerization of PBG to the linear product 1-hydroxymethylbilane, the immediate precursor of urogens I and III. The physiologically relevant urogen III isomer is formed in the presence of the next enzyme of the heme biosynthetic pathway, urogen III synthase (encoded by hemD) (4, 8). Cooverexpression of hemA through hemD results, as expected, in the production of mainly the urogen III isomer. In the absence of urogen III synthase spontaneous cyclization of 1-hydroxymethylbilane leads to the formation of the urogen I isomer (4, 8). Overexpression of hemA, hemB, and hemC led to formation of mainly the urogen I isomer (Table 2), despite the presence of the chromosomal hemD gene.
The next enzyme in our pathway, urogen decarboxylase (encoded by hemE), is derived from Synechocystis sp. This enzyme accepts both urogen I and urogen III as substrates (19, 32) and converts them to the corresponding coprogens. A significant amount of an intermediate with five carboxyl groups accumulated as a result of incomplete urogen I decarboxylation (Table 2). Coprogen III is converted to protoporphyrinogen (protogen) IX by the action of a coprogen III oxidase (CPO). The oxygen-dependent enzyme in our pathway is encoded by the E. coli hemF gene. When overexpressed, as in our pathway, the enzyme further oxidizes protogen IX to the fully conjugated product protoporphyrin IX (Table 2).
Although no enzyme is required for conversion of protogen IX to protoporphyrin IX in our pathway (as this reaction is catalyzed by CPO when the enzyme is overproduced), we incorporated the enzyme protogen IX oxidase (PPO) from B. subtilis (encoded by hemY). This enzyme has the peculiar ability not only to convert protogen IX to protoporphyrin IX but also to convert coprogen III to coproporphyrin III (11). Incorporation of this enzyme into the assembled pathway resulted in competition between coprogen III oxidase and protogen IX oxidase for the substrate coprogen III, thus leading to accumulation of both coproporphyrin III and protoporphyrin IX (cf. combinations 9 and 15 in Table 2). The accumulation of coproporphyrin III is remarkable, as this compound is not considered to be an intermediate in natural porphyrin synthesis (in contrast to coprogen III).
The last enzyme in our pathway is the ferrochelatase from B. subtilis (the hemH gene product). The enzyme from B. subtilis was chosen since its crystal structure has been determined (1). Ferrochelatase incorporates a ferrous iron ion into protoporphyrin IX, which yields protoheme IX (heme) (Table 2).
Production levels.
The porphyrin(ogen) contents of liquid cultures of the various transformants were analyzed by HPLC and mass spectrometry. As described in Materials and Methods, during sample preparation all porphyrinogens were converted to the corresponding porphyrins by contact with atmospheric oxygen, as we could not detect any porphyrinogens by mass spectrometry. Table 2 shows the porphyrin production resulting from various combinations of plasmids. All E. coli transformants listed in Table 2 grew to similar optical densities at 600 nm (about 3.5) after 48 h of cultivation at 30°C, regardless of the overexpressed heme genes. Porphyrin peaks were identified by their spectroscopic properties, molecular weights, and relative retention times on HPLC. Two typical HPLC profiles are shown in Fig. 3. In most cases, the major porphyrin present in the samples was the one formed by the action of the last overexpressed gene in the pathway. An exception was seen with combinations 7 and 14, where uroporphyrin I and 5-carboxyporphyrin accumulated (see below). High levels of expression of the individual proteins were observed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (data not shown). Although the expression levels varied, no significant accumulation of intermediates was observed, indicating that enzyme activities were not limiting. The only exception was some accumulations of uro- and coproporphyrin I resulting from nonenzymatic cyclization of hydroxymethylbilane even when the hemD gene was present; these accumulations were probably due to lower levels of expression of hemD than of hemC. In many cases, small quantities of other porphyrins were also present due to the activities of chromosomally encoded heme biosynthetic enzymes (e.g., combinations 1 to 6). The total production of porphyrins varied considerably, from 5 μM to almost 90 μM, but it was always considerably higher with the assembled overproduction pathway than in the control (E. coli JM109 carrying plasmids without heme biosynthetic genes) (Table 2).
FIG. 3.
HPLC profiles of porphyrin extracts from E. coli cells overexpressing hemA, hemB, and hemC (combination 5 in Table 2) (solid line) and hemA, hemB, hemC, and hemD (combination 6 in Table 2) (dotted line). Insets A and B show magnifications of the elution profiles of uroporphyrin isomers I and III and coproporphyrin isomers I and III, respectively. The separated porphyrins were identified as uroporphyrin I (λmax = 503, 537, 567, and 618 nm; [M+H]+ at m/z = 831.4) (peak 1), uroporphyrin III (λmax = 503, 537, 567, and 618 nm; [M+H]+ at m/z = 831.4) (peak 2), 5-carboxyporphyrin (λmax = 504, 534, 565, and 617 nm; [M+H]+ at m/z = 699.4) (peak 3), coproporphyrin I (λmax = 498, 532, 566, and 618 nm; [M+H]+ at m/z = 655.4) (peak 4), coproporphyrin III (λmax = 498, 532, 566, and 618 nm; [M+H]+ at m/z = 655.4) (peak 5), heme (λmax = 502 [α], 628 [β], and 536 [shoulder] nm; [M]+ at m/z = 616.3) (peak 6), and protoporphyrin IX (λmax = 505 539, 574, and 628 nm; [M+H]+ at m/z = 563.4) (peak 7).
The overexpression of hemA led to a significant (nearly 40-fold) increase in the amount of porphyrins formed. Cooverexpression of hemB did not further enhance porphyrin production, but introduction of the hemC and hemD genes increased the production of porphyrins an additional two- to sevenfold. In all cases, however, the total porphyrin production was lower in combinations with the pACmod-hemABC plasmid than in combinations with the pACmod-hemABCD plasmid. This was, at least in part, due to the lower stability of the former plasmid. For both plasmids, some white colonies always appeared on plates amidst a majority of orange-red colonies. Relatively more white colonies were seen for the pACmod-hemABC plasmid. Analysis of plasmid DNA of white colonies (data not shown) revealed both truncated plasmids and intact plasmids that apparently had one or more inactivated genes.
Gene combinations in which plasmids pACmod-hemABCD and pUCmod-hemY were present (combinations 11, 12, 13, and 15 in Table 2) produced dramatically less porphyrins (about 30 to 40%) than combinations without the latter plasmid produced (combinations 6, 8, 9, and 10 in Table 2). This effect appeared to be more profound in the absence of hemE (compare combinations 11 and 12 with combination 6 in Table 2).
As expected, a combination of plasmids pACmod-hemABCD and pUCmod-hemE resulted in the formation of large quantities of coproporphyrin III. Uroporphyrin III was no longer detected. The total amount of porphyrins formed appeared not to be affected by the presence of urogen III decarboxylase (cf. combinations 6 and 8 in Table 2). In contrast, when plasmids pACmod-hemABC and pUCmod-hemE were combined, uroporphyrin I was still the main product, although smaller amounts of coproporphyrin I and pentacarboxyporphyrin were formed as well, reflecting the lower activity of the enzyme towards urogen I (Table 2).
Coexpression of hemF, coding for the E. coli CPO, resulted in an additional 70 to 80% increase in porphyrin production (compare combinations 9 and 10 with combination 8 in Table 2). The major product, protoporphyrin IX, was largely released into the medium, where it precipitated (Fig. 2B).
Surprisingly, the presence of the ferrochelatase (the product of hemH) in our pathway did not lead to significant accumulation of heme (Table 2), although the hemH gene was highly expressed (data not shown) and ferrochelatase activity could be demonstrated in vitro (Fig. 4) by using a lysate of an E. coli transformant harboring plasmids pACmod-hemABCD, pBBR1-hemEF, and pUCmod-hemH.
FIG. 4.
Assay for zinc chelatase activity of B. subtilis ferrochelatase expressed in E. coli cells. Zinc chelatase activity was assayed by using a cell extract of E. coli cells overexpressing hemA through hemH (combination 10 in Table 2) [▪]). Extracts without overexpressed hemH (combination 9 in Table 2 [○] and empty vector combination 1 in Table 2 [•]) were used as controls. A control without cell extract was also included (▿). See text for details. r.f.u., relative fluorescence units.
DISCUSSION
We developed a versatile system for tailored (over)production of each of the major porphyrins that occur as intermediates in heme biosynthesis. The relevant heme biosynthetic genes were cloned on several plasmids that could be combined at will to favor overproduction of a particular porphyrin. Each gene was placed under control of a constitutive promoter to circumvent the regulatory circuitry at transcriptional and translational levels that governs porphyrin (and heme) biosynthesis in native systems.
Production levels.
The main point of regulation of heme biosynthesis is at the level of ALA production. This is true for both the C4 route and the C5 route to ALA production. In our system, we cloned the R. capsulatus hemA gene under control of a constitutive promoter on a medium-copy-number plasmid (a pACYC derivative). This construct allowed total porphyrin production of up to 90 μM when it was combined with additional overexpressed heme biosynthetic genes. Previously, researchers placed the Rhodobacter sphaeroides hemA gene under control of an inducible lac promoter on a pUC18 derivative to investigate ALA production in E. coli (35). Full expression of the hemA gene was found, and 180 μM ALA was present in extracts from cells grown on LB medium (35). This allowed production of 23 μM porphyrin (eight ALA molecules are used to synthesize one porphyrin molecule), which is significantly less than the amount that we found. However, large amounts of ALA can accumulate extracellularly (30). Indeed, 15 mM extracellular ALA was produced by E. coli transformed with the hemA gene from Bradyrhizobium japonicum when the organism was grown on LB medium supplemented with 90 mM succinate and 30 mM glycine (9). Even more ALA (20 mM) was secreted when the medium was also supplemented with 30 mM levulinic acid (to inhibit ALA dehydratase) (9). Since ALA secretion must compete with conversion into porphyrins, it follows that the latter process is not very efficient, possibly due to regulatory effects in native heme biosynthetic pathways. Consequently, porphyrin production via our engineered pathway (without most regulatory controls, at least at the level of transcription and translation) may greatly benefit from further optimization of the growth medium and thus allow the production in recombinant E. coli of large quantities of porphyrin compounds. However, the levels of production (up to 90 μM, roughly 50 mg/liter) obtained with our engineered E. coli cells without further optimization of cultivation conditions are on the same order of magnitude as commercial levels of production reported for vitamin B12 (100 to 300 mg/liter) when engineered microbial strains are used (24).
B. subtilis PPO.
There have been contradictory reports concerning B. subtilis PPO. This enzyme has been found to be a cytoplasmic enzyme (11) and a membrane-bound enzyme (16, 17). The B. subtilis PPO has been reported (16) to be very toxic to E. coli when large amounts are produced. In contrast, we found no adverse effects when the hemY gene was constitutively expressed from a lac promoter on a high-copy-number plasmid (the pUC19 derivative pUCmod). The B. subtilis PPO accepts both coprogen III and protogen IX as substrates, producing coproporphyrin III and protoporphyrin IX, respectively (11), although there is some controversy regarding the kinetic data for coprogen III oxidation versus protogen IX oxidation (10, 16). Our experiments confirmed that the B. subtilis PPO converts coprogen III to coproporphyrin III. We did not find significant accumulation of coproporphyrin III (there was only 0.7 μmol/liter) when hemA through hemF were combined (combination 9 in Table 2), but a large amount (21 μmol/liter) was formed (at the expense of protoporphyrin IX) when the hemY gene was added (combination 15 in Table 2). Coproporphyrin III is considered not to be a physiological intermediate in most heme biosynhetic pathways, but it has been suggested that this compound functions as an intermediate in B. subtilis (17). Accumulation of coproporphyrin III in combination 15 but not in combination 9 further confirms that the E. coli CPO can use coprogen III but not coproporphyrin III as a substrate.
Our experiments revealed another intriguing property of the B. subtilis PPO: the ability to decrease porphyrin production levels. Lower porphyrin production levels may be achieved either by diminished biosynthesis or by breakdown of porphyrin (precursors). The production of porphyrins is most affected when only hemA through hemD are present. Thus, PPO is likely to interfere with urogen synthesis, perhaps by forming unfavorable interactions with the early heme biosynthetic enzymes in the absence of the enzymes further down the pathway.
B. subtilis ferrochelatase.
Although high levels of protoporphyrin IX could be produced (up to 85 μM), only a small amount of this compound was converted to heme. Cooverexpression of the ferrochelatase gene (hemH) did not lead to any appreciable increase in heme production. This observation suggested that the ferrochelatase might not be active in vivo. However, the enzyme was expressed at high levels (data not shown) and displayed activity in vitro (Fig. 4). In principle, this paradox may be explained by a lack of iron. However, the E. coli strains were grown in rich medium; thus, it is unlikely that iron availability was limiting, except if iron transport into the cell was perhaps regulated by the intracellular concentration of free heme.
Alternatively, the ferrochelatase may be active in vitro but not in vivo. Such a discrepancy has recently been described for a B. subtilis ferrochelatase mutation that was fully functional in vitro but could not support wild-type levels of B. subtilis growth (27). The mutant had an amino acid substitution (S54A) on the surface of the protein. The mutation not only caused slower growth but also led to coproporphyrin III accumulation, whereas accumulation of protoporphyrin IX might be expected. Coproporphyrin III accumulation suggests that there is an interaction among the last three enzymes of the heme biosynthetic pathway. Such an interaction requires a compatible set of enzymes. In our system only two enzymes are used, E. coli CPO (which oxidizes coprogen III to protogen IX and protogen IX to protoporphyrin IX) and the B. subtilis ferrochelatase. Thus, the two enzymes may not be compatible, and a distinct PPO may be required for efficient substrate channeling in vivo. However, in vitro no inhibitory effect of the upstream heme biosynthetic enzymes on the ferrochelatase activity was observed.
In summary, we demonstrated that E. coli can be engineered to produce large quantities of different porphyrin structures. The modular plasmid system developed for porphyrin pathway assembly should enable us to modify the catalytic functions of individual biosynthetic genes by rational and/or in vitro evolutionary approaches to create pathways for the production of novel unnatural functionalized porphyrins. For production of metalloporphyrins in E. coli, further studies are under way to identify the rate-limiting factors that govern metal incorporation into overproduced porphyrins.
Acknowledgments
This research was supported by the National Institutes of Health (grant NIH/1R01-GM65471-01) and by the David and Lucile Packard Foundation (grant 2001-18996).
REFERENCES
- 1.Al-Karadaghi, S., M. Hansson, S. Nikonov, B. Jonsson, and L. Hederstedt. 1997. Crystal structure of ferrochelatase: the terminal enzyme in heme biosynthesis. Structure 5:1501-1510. [DOI] [PubMed] [Google Scholar]
- 2.Aviezer, D., S. Cotton, M. David, A. Segev, N. Khaselev, N. Galili, Z. Gross, and A. Yayon. 2000. Porphyrin analogues as novel antagonists of fibroblast growth factor and vascular endothelial growth factor receptor binding that inhibit endothelial cell proliferation, tumor progression, and metastasis. Cancer Res. 60:2973-2980. [PubMed] [Google Scholar]
- 3.Balasubramanian, T., J. P. Strachan, P. D. Boyle, and J. S. Lindsey. 2000. Rational synthesis of beta-substituted chlorin building blocks. J. Org. Chem. 65:7919-7929. [DOI] [PubMed] [Google Scholar]
- 4.Battersby, A. R. 2000. Tetrapyrroles: the pigments of life. Nat. Prod. Rep. 17:507-526. [DOI] [PubMed] [Google Scholar]
- 5.Birnbaum, E. R., M. W. Grinstaff, J. A. Labinger, J. E. Bercaw, and H. B. Gray. 1995. On the mechanism of catalytic alkene oxidation by molecular oxygen and halogenated iron porphyrins. J. Mol. Catal. A 104:L119-L122. [Google Scholar]
- 6.Camadro, J. M., and P. Labbe. 1988. Purification and properties of ferrochelatase from the yeast Saccharomyces cerevisiae. Evidence for a precursor form of the protein. J. Biol. Chem. 263:11675-11682. [PubMed] [Google Scholar]
- 7.Cavaleiro, J. A. S., M. G. P. M. Neves, A. C. Tome, A. M. S. Silva, M. A. F. Faustino, P. S. Lacerda, and A. M. G. Silva. 2000. Porphyrin derivatives: synthesis and potential applications. J. Heterocycl. Chem. 37:527-534. [Google Scholar]
- 8.Chadwick, D. E., and K. Ackrill (ed.). 1994. The Biosynthesis of the tetrapyrrole pigments, vol. 180. John Wiley & Sons, New York, N.Y.
- 9.Choi, C., B.-S. Hong, H.-C. Sung, H.-S. Lee, and J.-H. Kim. 1999. Optimization of extracellular 5-aminolevulinic acid production from Escherichia coli transformed with ALA synthase gene of Bradyrhizobium japonicum Biotechnol. Lett. 21:551-554. [Google Scholar]
- 10.Corrigall, A. V., K. B. Siziba, M. H. Maneli, E. G. Shephard, M. Ziman, T. A. Dailey, H. A. Dailey, R. E. Kirsch, and P. N. Meissner. 1998. Purification of and kinetic studies on a cloned protoporphyrinogen oxidase from the aerobic bacterium Bacillus subtilis. Arch. Biochem. Biophys. 358:251-256. [DOI] [PubMed] [Google Scholar]
- 11.Dailey, T. A., P. Meissner, and H. A. Dailey. 1994. Expression of a cloned protoporphyrinogen oxidase J. Biol. Chem. 269:813-815. [PubMed] [Google Scholar]
- 12.D'Amico, A., C. Di Natale, R. Paolesse, A. Macagnano, and A. Mantini. 2000. Metalloporphyrins as basic material for volatile sensitive sensors. Sensors Actuators B 65:209-215. [Google Scholar]
- 13.Dougherty, T. J., C. J. Gomer, B. W. Henderson, G. Jori, D. Kessel, M. Korbelik, J. Moan, and Q. Peng. 1998. Photodynamic therapy J. Natl. Cancer Inst. 90:889-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grinstaff, M. W., M. G. Hill, E. R. Birnbaum, W. P. Schaefer, J. A. Labinger, and H. B. Gray. 1995. Structures, electronic properties, and oxidation-reduction reactivity of halogenated iron porphyrins. Inorg. Chem. 34:4896-4902. [Google Scholar]
- 15.Grinstaff, M. W., M. G. Hill, J. A. Labinger, and H. B. Gray. 1994. Mechanism of catalytic oxygenation of alkanes by halogenated iron porphyrins. Science 264:1311-1313. [DOI] [PubMed] [Google Scholar]
- 16.Hansson, M., and L. Hederstedt. 1994. Bacillus subtilis HemY is a peripheral membrane protein essential for protoheme IX synthesis which can oxidize coproporphyrinogen III and protoporphyrinogen IX. J. Bacteriol. 176:5962-5970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hansson, M., M. C. U. Gustafsson, C. G. Kannangara, and L. Hederstedt. 1997. Isolated Bacillus subtilis HemY has coproporphyrinogen III to coproporphyrin III oxidase activity. Biochim. Biophys. Acta 1340:97-104. [DOI] [PubMed] [Google Scholar]
- 18.Hansson, M., and L. Hederstedt. 1994. Purification and characterisation of a water-soluble ferrochelatase from Bacillus subtilis Eur. J. Biochem. 220:201-208. [DOI] [PubMed] [Google Scholar]
- 19.Jackson, A. H., H. A. Sancovich, A. M. Ferramola, N. Evans, D. E. Games, S. A. Matlin, G. H. Elder, and S. G. Smith. 1976. Macrocyclic intermediates in the biosynthesis of porphyrins. Phil. Trans. R. Soc. Lond. B 273:191-206. [DOI] [PubMed] [Google Scholar]
- 20.Jasat, A., and D. Dolphin. 1997. Expanded porphyrins and their heterologs. Chem. Rev. 97:2267-2340. [DOI] [PubMed] [Google Scholar]
- 21.Karpishin, T. B., M. W. Grinstaff, S. Komar-Panicucci, G. McLendon, and H. B. Gray. 1994. Electron transfer in cytochrome c depends upon the structure of the intervening medium. Structure 2:415-422. [DOI] [PubMed] [Google Scholar]
- 22.Kovach, M. E., P. H. Elzer, D. S. Hill, G. T. Robertson, M. A. Farris, R. M. Roop, 2nd, and K. M. Peterson. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175-176. [DOI] [PubMed] [Google Scholar]
- 23.Lin-Chao, S., W. T. Chen, and T. T. Wong. 1992. High copy number of the pUC plasmid results from a Rom/Rop-suppressible point mutation in RNA II. Mol. Microbiol. 6:3385-3393. [DOI] [PubMed] [Google Scholar]
- 24.Martens, J. H., H. Barg, M. J. Warren, and D. Jahn. 2002. Microbial production of vitamin B12. Appl. Microbiol. Biotechnol. 58:275-285. [DOI] [PubMed] [Google Scholar]
- 25.Nakayashiki, T., K. Nishimura, R. Tanaka, and H. Inokuchi. 1995. Partial inhibition of protein synthesis accelerates the synthesis of porphyrin in heme-deficient mutants of Escherichia coli. Mol. Gen. Genet. 249:139-146. [DOI] [PubMed] [Google Scholar]
- 26.Ng, J. C., L. Qi, and M. R. Moore. 2002. HPLC measurement of harderoporphyrin in the harderian glands of rodents as a biomarker for sub-lethal or chronic arsenic exposure. Toxicol. Lett. 133:93-101. [DOI] [PubMed] [Google Scholar]
- 27.Olsson, U., A. Billberg, S. Sjovall, S. Al-Karadaghi, and M. Hansson. 2002. In vivo and in vitro studies of Bacillus subtilis ferrochelatase mutants suggest substrate channeling in the heme biosynthesis pathway. J. Bacteriol. 184:4018-4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Panek, H., and M. R. O'Brian. 2002. A whole genome view of prokaryotic haem biosynthesis. Microbiology 148:2273-2282. [DOI] [PubMed] [Google Scholar]
- 29.Sambrook, J., and D. W. Russell (ed.). 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 30.Sasaki, K., M. Watanabe, and T. Tanaka. 2002. Biosynthesis, biotechnological production and applications of 5-aminolevulinic acid. Appl. Microbiol. Biotechnol. 58:23-29. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt-Dannert, C., D. Umeno, and F. H. Arnold. 2000. Molecular breeding of carotenoid biosynthetic pathways. Nat. Biotechnol. 18:750-753. [DOI] [PubMed] [Google Scholar]
- 32.Straka, J. G., and J. P. Kushner. 1983. Purification and characterization of bovine hepatic uroporphyrinogen decarboxylase. Biochemistry 22:4664-4672. [DOI] [PubMed] [Google Scholar]
- 33.Sutton, J. M., and R. W. Boyle. 2001. First synthesis of porphyrin-phthalocyanine heterodimers with a direct ethynyl linkage. Chem. Commun. 19:2014-2015. [DOI] [PubMed] [Google Scholar]
- 34.Sutton, J. M., O. J. Clarke, N. Fernandez, and R. W. Boyle. 2002. Porphyrin, chlorin, and bacteriochlorin isothiocyanates: useful reagents for the synthesis of photoactive bioconjugates. Bioconjug. Chem. 13:249-263. [DOI] [PubMed] [Google Scholar]
- 35.van der Werf, M. J., and J. G. Zeikus. 1996. 5-Aminolevulinate production by Escherichia coli containing the Rhodobacter sphaeroides hemA gene. Appl. Environ. Microbiol. 62:3560-3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whitaker, C. J., S. H. Battah, M. J. Forsyth, C. Edwards, R. W. Boyle, and E. K. Matthews. 2000. Photosensitization of pancreatic tumour cells by delta-aminolaevulinic acid esters. Anticancer Drug Des. 15:161-170. [PubMed] [Google Scholar]
- 37.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]
- 38.Zimmerman, S. C., M. S. Wendland, N. A. Rakow, I. Zharov, and K. S. Suslick. 2002. Synthetic hosts by monomolecular imprinting inside dendrimers. Nature 418:399-403. [DOI] [PubMed] [Google Scholar]