Abstract
Deinococcus geothermalis is an extremely radiation-resistant thermophilic bacterium closely related to the mesophile Deinococcus radiodurans, which is being engineered for in situ bioremediation of radioactive wastes. We report that D. geothermalis is transformable with plasmids designed for D. radiodurans and have generated a Hg(II)-resistant D. geothermalis strain capable of reducing Hg(II) at elevated temperatures and in the presence of 50 Gy/h. Additionally, D. geothermalis is capable of reducing Fe(III)-nitrilotriacetic acid, U(VI), and Cr(VI). These characteristics support the prospective development of this thermophilic radiophile for bioremediation of radioactive mixed waste environments with temperatures as high as 55°C.
The bacterium Deinococcus geothermalis (13) is remarkable not only for its extreme resistance to ionizing radiation but also for its ability to grow at temperatures as high as 55°C (13) and in the presence of chronic irradiation (8). The organism was isolated by Ferreira et al. (13) from hot springs together with Deinococcus murrayi. Both bacteria are moderately thermophilic and belong to the bacterial family Deinococcaceae (4, 7, 22), currently comprised of seven distinct nonpathogenic radiation-resistant species, of which Deinococcus radiodurans strain R1 is the best characterized (4). Advances in genetic engineering for D. radiodurans (9-12, 29) were a stimulus for its genome sequencing (17, 33), annotation (22), and proteomic (18) and transcriptome (19) analyses. The other deinococcal species have been reported as nontransformable or have not yet been tested for transformability by chromosomal or plasmid DNA and have been left unexplored by recombinant DNA technologies. Other genetic approaches including conjugation and protoplast fusion have not been successful in the Deinococcaceae (16).
A present genetic engineering goal for D. radiodurans is its development for bioremediation of U.S. Department of Energy (DOE) mixed radioactive environmental waste sites left over from nuclear weapons production during the Cold War (21, 25, 27, 28). These sites contain immense volumes of waste (3 × 106 m3) that include radionuclides, heavy metals, and toxic organic compounds and have contaminated 40 million cubic meters of soil and 4 trillion liters of groundwater since 1946 (1, 21, 25, 27, 28). While there has been significant progress in engineering D. radiodurans for remediation of radioactive DOE waste environments (5, 8, 15), prospective treatment of contaminated sites with engineered D. radiodurans will be limited to temperatures below 39°C, its maximum growth temperature. However, there is a need to develop bioremediating bacteria that are resistant to both radiation and high temperatures because of the existence of thermally insulated contaminated environments where temperatures are elevated by the decay of long-lived radionuclides (e.g., 137Cs and 90Sr) (1). For example, soil columns beneath at least 67 radioactive leaking tanks at DOE's Hanford Site in south-central Washington State have been contaminated and have recorded temperatures as high as 70°C at depths of greater than 18 m (1). Since D. geothermalis and D. murrayi are both radiation resistant and thermophilic, they have become desirable targets for genetic development of bioremediating strains similar to those developed for D. radiodurans (5, 8, 15) but capable of survival and growth at higher temperatures. Given the need to develop bioremediating bacteria for treatment of radioactive high-temperature waste environments, D. geothermalis and D. murrayi were tested for their transformability with the autonomously replicating Escherichia coli-D. radiodurans shuttle plasmid pMD66 (9), which expresses kanamycin (KAN) and tetracycline (TET) resistance in D. radiodurans and additionally expresses ampicillin resistance in E. coli.
pMD66 and its numerous derivatives (9-12) have been used successfully to functionally express cloned genes in D. radiodurans growing under chronic irradiation. Examples include the mer operon of E. coli (5), which encodes Hg(II) resistance and reduction, and the Pseudomonas operon todC1C2BA (15), which encodes partial degradation of toluene. The present work shows that D. geothermalis is capable of expressing Hg(II)-reducing functions cloned in pMD66 at elevated temperatures and under chronic radiation and, like D. radiodurans (14), is naturally capable of reducing a variety of other metal contaminants present in DOE waste sites.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and transformation.
D. radiodurans R1 (ATCC BAA-816) (33), D. geothermalis DSM11300, and D. murrayi DSM11303 were grown in TGY broth (1% Bacto Tryptone, 0.1% glucose, 0.5% Bacto Yeast Extract) (Difco) or minimal medium (MM) (see Table 2) (32). Liquid cultures were inoculated at ∼5 × 106 cells/ml. For solid medium, Bacto Agar (Difco) or Noble agar (Difco) was added to TGY or MM, respectively, to 1.5% (wt/vol). D. radiodurans was grown at 32°C, and D. geothermalis and D. murrayi were grown at 37°C or at higher temperatures as indicated. E. coli was grown in Luria-Bertani medium at 37°C (9). pMD66 (9) (purified from E. coli) encodes ampicillin resistance (Apr), Kmr, and Tetr in E. coli and Kmr and Tetr in D. radiodurans. When pMD66 is prepared from E. coli, the plasmid transforms D. radiodurans with low efficiency (∼50 transformants/μg) (9). However, when the same plasmid is purified from D. radiodurans, it transforms wild-type D. radiodurans with efficiencies as high as 106 transformants/μg (9). Plasmid transformation-restriction in D. radiodurans (9), therefore, distinguishes between the sources of plasmids. When purified from E. coli, the plasmid is called pMD66, and when purified from D. radiodurans, it is called pMD68 (9). The situation for a derivative (pMD300) of pMD66 encoding chloramphenicol resistance (Cmr) is analogous (10). pMD300 purified from E. coli encodes Cmr in E. coli and D. radiodurans and when purified from D. radiodurans is called pMD308 (10). Expression of heterologous genes and antibiotic resistance markers cloned into pMD66-type vectors is driven by two different deinococcal constitutive promoters (P1 and P2) (e.g., see Fig. 2A) that are active on autonomous plasmids in D. radiodurans or when integrated into D. radiodurans chromosomes (11, 12). pMD66-type plasmids contain a deinococcal origin of replication (dORI); an E. coli origin of replication (eORI); and resistance genes including aphA (encoding Kmr), bla (encoding Apr), and/or the mer operon (encoding mercury resistance) (3, 5).
TABLE 2.
Growth characteristics of D. radiodurans and D. geothermalis grown in mma
| Substratec | Growth of strainb
|
|
|---|---|---|
| D. radiodurans | D. geothermalis | |
| Fructose + C,H,L,A,M,P | +++ | +++ |
| Fructose + Met | +++ | +++ |
| Fructose −aa +(NH4)2SO4 | − | +++ |
| Fructose −NAD +Met | − | +++ |
| α-Ketoglutarate | − | ++ |
| Succinate | − | ++ |
| Fumarate | − | ++ |
| Oxaloacetate | + | ++ |
| Malate | − | + |
Deinococcal cells were grown in deinococcal MM (32) at 32°C. Deinococcal MM contained the indicated Embden-Meyerhof-Parnas substrate (2 mg/ml), NAD (1 μg/ml), methionine (Met) at 50 μg/ml, phosphate buffer (20 mM, pH 7.5), CaCl2 (0.18 mM), and MgSO4 (0.8 mM), and Mn2+ (5.4 μM MnCl2) was added as the only transition metal cation.
Growth on substrate: +++, good; ++, moderate; +, poor; −, absent.
Abbreviations: Fructose + C,H,L,A,M,P, fructose plus Cys, His, Lys, Asp, Met, and Pro, each at 50 μg/ml. +Met, only methionine added at 50 μg/ml. −aa, no amino acids added. +(NH4)2SO4, ammonium sulfate added to a final concentration of 15 mM. −NAD, no NAD added.
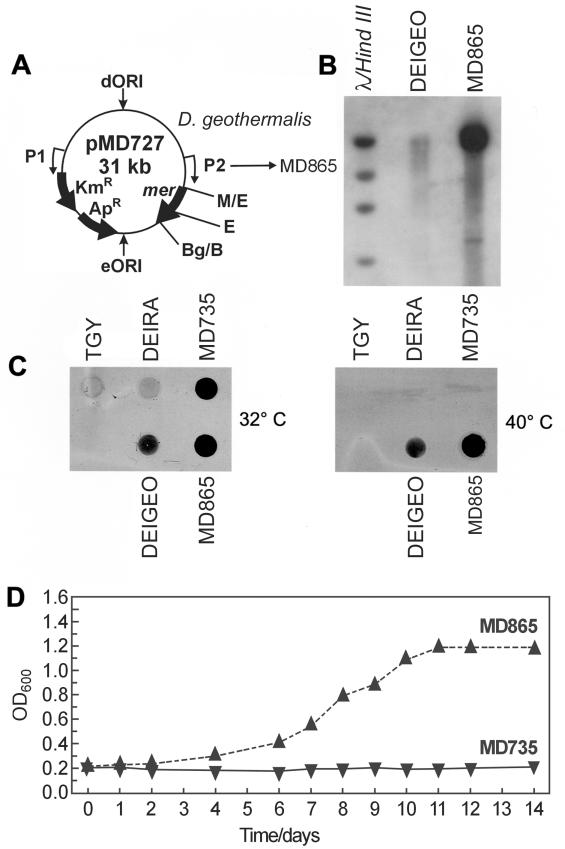
FIG.2.
Construction and characterization of Hg(II)-resistant-reducing D. geothermalis. (A) pMD727 (5) was transformed into D. geothermalis, giving strain MD865. (B) Southern blot hybridization of EcoRI-digested total DNA from D. geothermalis (wild type, mer negative) and MD865 (D. geothermalis/mer+) with a radiolabeled mer probe. pMD727 contains a unique EcoRI (E) site. Molecular size standards: λ/HindIII, as in Fig. 1A and B. Wild-type strain abbreviations are as in Fig. 1. (C) Hg(0) volatilization assays at 32 and 40°C for D. geothermalis, D. radiodurans, MD865 and MD735 (D. radiodurans/mer+), and TGY (growth medium, no cells). (D) Growth curves for MD865 and MD735 in TGY plus 50 μM merbromin [Hg(II)] at 50°C.
The selective drug concentrations for deinococci were 8 μg of KAN per ml, 2.5 μg of TET per ml, and 3 μg of chloramphenicol per ml. For E. coli, antibiotics were added to Luria-Bertani medium as follows: KAN (50 μg/ml), TET (25 μg/ml), and chloramphenicol (30 μg/ml). Transformation of deinococci was by a CaCl2-dependent technique described previously for D. radiodurans (16) but with the following modifications. Exponential cultures of deinococci were resuspended at 108 cells per ml in TGY broth-0.1 M CaCl2-glycerol (20:8:3, vol/vol/vol). For transformation, 100 μl of the cell suspension and 5 μl of water containing various amounts of transforming DNA were added. The cell mixture was held on ice for 15 min and then incubated at 32°C for 30 min with gentle agitation. TGY (0.9 ml) was then added, and the mixture was incubated at 32°C (for D. radiodurans) or 37°C (for D. geothermalis) for 16 h with aeration before being plated on drug-selected agar.
Irradiation.
Growth of cells in the presence of chronic irradiation, 50 Gy/h (137Cs Gammacell 40 irradiation unit [Atomic Energy of Canada Limited]), was carried out as described previously (5, 8, 15). For high-level acute irradiation exposures, early-stationary-phase deinococcal cultures (optical density at 600 nm [OD600] of 0.9 corresponds to ∼108 CFU/ml) were irradiated without change of broth on ice at 10 kGy/h (60Co Gammacell irradiation unit [J. L. Shepard and Associates; Model 109]). For the deinococcal species under investigation, three independent cell cultures and irradiation treatments of the same kind were performed. Following exposure to the indicated doses, cell suspensions were appropriately diluted and assayed for viability by plate assay on rich (TGY) medium (9). Viability data were used to construct survival curves with standard deviations according to conventional formats (9, 24). The effect of chronic exposure to gamma radiation and Hg(II) on the growth of engineered D. geothermalis was determined using TGY agar plates with and without 30 μM merbromin [Hg(II)] (5). Plates were spotted with ∼105 cells and following plate inoculation were placed into the 137Cs irradiator (50 Gy/h) for incubation at 50°C for 5 days.
DNA isolation and manipulation.
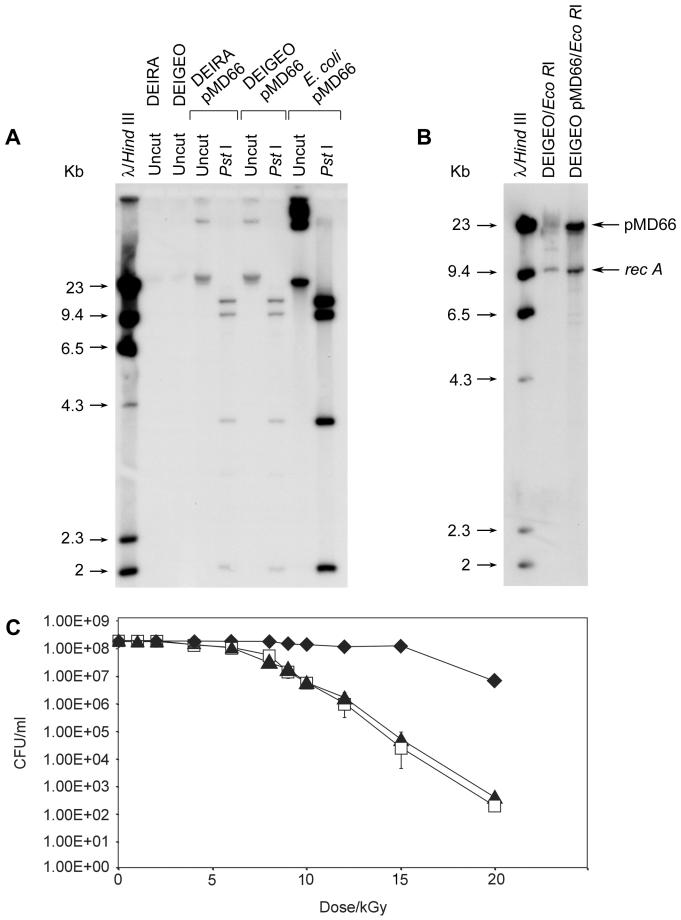
Isolation of plasmid DNA and total DNA from E. coli, D. radiodurans, and D. geothermalis; use of enzymatic reagents; gel electrophoresis; plasmid rescue in E. coli; radiolabeling of DNA; hybridization; washing of blots; and autoradiography were performed as previously described (9-12). For Fig. 1B, D. geothermalis (wild-type) and D. geothermalis/pMD66 total DNA preparations were digested with EcoRI. The blot was double hybridized with a 1.5-kb XbaI genomic recA (D. radiodurans) probe and a 1.5-kb EcoRI-Bpu10I probe of pBR322 that is specific to pMD66.
FIG.1.
Transformation of D. geothermalis with pMD66 and resistance of pMD66-transformed D. geothermalis to acute gamma radiation. (A) D. geothermalis/pMD66. Total DNA from the indicated strains was uncut or digested with PstI before electrophoresis, blotting, and probing of the blot with a whole-plasmid radiolabeled pMD66 probe. Abbreviations: DEIRA, D. radiodurans; DEIGEO, D. geothermalis. (B) The copy number of pMD66 in D. geothermalis/pMD66 is about threefold higher than its chromosomal copy number. (C) Survival of D. geothermalis/pMD66 following acute gamma radiation. Symbols: open squares, D. geothermalis plated on TGY at 37°C; solid triangles, D. geothermalis/pMD66 plated on TGY-KAN at 37°C; solid diamonds, D. radiodurans plated on TGY at 32°C.
Mercury volatilization assay.
Cells were pregrown to an OD600 of 0.5 in the presence of 20 μM merbromin [Hg(II)] as described previously (5). Cells of each strain were harvested, washed twice in fresh medium lacking Hg(II), and concentrated to an OD600 of 2.0 in fresh medium, followed by the inoculation of 107 cells of each into 200 μl of medium containing 30 μM merbromin contained in 300-μl wells of a microplate, respectively. The plates were covered with a sheet of X-ray film, held together with a weight, and incubated in the dark at 32 or 40°C. Following exposure for 14 h, the films were developed.
Metal reduction by D. geothermalis.
The native metal reduction capabilities of D. radiodurans have been examined previously (14). The protocols for examining metal reduction by D. geothermalis are essentially identical to those used for D. radiodurans (6, 14, 30, 31) but at higher temperatures. The ability of D. geothermalis to reduce Fe(III) (as Fe-nitrilotriacetic acid [NTA]) was examined in cultures containing 10 mM lactate in basal medium at 45°C. For the experiment with Cr(VI) and U(VI), cultures were incubated in TGY at 40°C.
RESULTS
Transformation of D. geothermalis and D. murrayi.
Plasmid transformation of D. geothermalis by using pMD66/68 was successful (Table 1), with stable introduction proven by hybridization with a pMD66 probe (Fig. 1A). D. geothermalis was also transformable with the Cmr-encoding D. radiodurans plasmid pMD300/308 (data not shown). While the transformation efficiencies were much lower for D. geothermalis than for D. radiodurans, irrespective of the source of plasmid (Table 1), this was not a problem because the transforming DNA could be prepared and used in bulk. Electroporation as an alternative method of transformation was previously shown not to be effective for D. radiodurans (M. J. Daly, unpublished data), where the low electroporation efficiencies were attributed to the unusually thick cell wall structures of deinococci. Figure 1B shows the assessment of the copy number of pMD66 in D. geothermalis relative to the chromosomal content by comparing the hybridization of two similarly sized nonhomologous probes to total DNA prepared from D. geothermalis/pMD66. The recA probe (1.5 kb, derived from D. radiodurans) is a reporter of chromosome copy number, and the pMD66 probe (1.5 kb, derived from pBR322) is specific to the plasmid. The hybridization signal for the recA probe was determined by densitometry to be about one-third of the intensity arising from the pMD66 probe, suggesting that pMD66 exists in multiple copies in D. geothermalis.
TABLE 1.
Transformation of pMD66/68 into D. geothermalis, D. radiodurans and E. coli
| No. of transformants/μg of DNA for recipienta:
|
|||
|---|---|---|---|
| D. geothermalis | D. radiodurans | E. coli | |
| Plasmid sourceb | |||
| pMD66 (purified from E. coli) | 1 × 101 ± 2 × 101 | 1 × 102 ± 8 × 101 | 9 × 104 ± 3 × 103 |
| pMD68 (purified from D. radiodurans) | 5 × 101 ± 2 × 101 | 8 × 105 ± 1.5 × 104 | 4 × 105 ± 2.1 × 104 |
| pMD66 (purified from D. geothermalis) | 5 × 102 ± 29 × 101 | 4 × 105 ± 7 × 103 | 4 × 102 ± 25 × 101 |
Kmr transformants per microgram of plasmid purified from the indicated strains.
pMD66, pMD68, and pMD66-D. geothermalis have identical restriction maps (Fig. 1A).
Stability of D. geothermalis/pMD66.
Deinococcal sequences in pMD66/68 are derived exclusively from D. radiodurans strain SARK (9, 16), which has no detectable homology to D. radiodurans strain R1 or D. geothermalis (Fig. 1A). To test whether pMD66 is uniformly retained and repaired in D. geothermalis following acute irradiation, D. geothermalis/pMD66 was assessed for survival following various exposures to ionizing radiation and recovery on TGY, or on TGY supplemented with KAN as a marker for the presence of pMD66 (Fig. 1C). The survival of D. geothermalis/pMD66 plated on TGY-KAN was indistinguishable from that found for wild-type D. geothermalis on TGY. Plasmid rescue in E. coli from total DNA purified from a culture of D. geothermalis/pMD66 following recovery from 12 kGy showed that, of 1,000 Kmr E. coli colonies rescued, 100% were also Tetr and Apr, supporting the idea that irradiation-induced mutations and deletions are rare in D. geothermalis, as is the case in D. radiodurans (9, 11). These results show that pMD66 is retained in D. geothermalis without alteration following high-dose irradiation and recovery and is repaired with similar efficiency to its chromosomes.
Construction and characterization of Hg(II)-resistant D. geothermalis.
The complete E. coli Hg(II) resistance (mer) operon (4.2 kb, encoding six proteins) (3, 5) has previously been functionally expressed in D. radiodurans by using a pMD66 derivative, pMD727 (5) (Fig. 2A). In D. radiodurans, all six mer genes are necessary for reduction of Hg(II) to Hg(0). pMD727 was successfully transformed into D. geothermalis (Fig. 2B), yielding strain MD865. This construction placed the mer genes under the control of a constitutive D. radiodurans promoter (P2, Fig. 2A), and Southern analysis with a radiolabeled probe containing a 1.5-kb EcoRI-BglII fragment from the mer operon showed no significant homology with the D. geothermalis genome (Fig. 2B). Reduction of Hg(II) to volatile elemental Hg(0) by D. geothermalis strain MD865 was examined by testing for mercury volatilization, which causes film darkening (5, 26). Following 14 h of incubation with Hg(II) in a microplate at 32 or 40°C, covered by X-ray film, wild-type D. geothermalis showed modest Hg(0) volatilization. However, strain MD865 (D. geothermalis/mer+) showed substantial Hg(0) volatilization based on film darkening compared to wild-type D. geothermalis at 32 or 40°C (Fig. 2C). MD865 also was resistant to 50 μM Hg(II) during growth at 50°C (Fig. 2D) and displayed luxuriant growth at 50°C in the presence of 50 Gy/h on solid medium containing 30 μM merbromin (data not shown). Wild-type D. geothermalis did not grow in medium containing 30 μM merbromin in the presence or absence of chronic radiation.
Reduction of metals.
D. geothermalis reduced Fe(III)-NTA in the presence of lactate at 30°C (data not shown) and in the presence of lactate or pyruvate at 45°C (Fig. 3A). At 40°C D. geothermalis rapidly reduced Cr(VI) in TGY cultures under both aerobic and anaerobic conditions (Fig. 3B). AQDS (anthraquinone-2,6-disulfonate) is a quinone-containing organic compound that can be utilized as an electron acceptor for respiration and growth by a variety of dissimilatory metal-reducing bacteria (20). As an electron acceptor, AQDS is reduced to the corresponding dihydroquinone (AH2DS) (20). Reduction of U(VI) by D. geothermalis at 40°C occurred only in the presence of AQDS (Fig. 3C). These results are very similar to the reduction capabilities reported for D. radiodurans at lower temperatures (14).
FIG. 3.
Metalloreduction by wild-type D. geothermalis. (A) Fe(III)-NTA reduction coupled to oxidation of organic substrates at 45°C in the absence of oxygen. (B) Cr(VI) reduction in aerobic or anaerobic conditions as measured by loss of Cr(VI) from solution at 40°C. (C) Reduction of U(VI) in the presence or absence of AQDS as measured by loss of U(VI) at 40°C.
Growth characteristics of D. geothermalis.
D. geothermalis was tested for its amino acid utilization and growth on various Embden-Meyerhof-Parnas substrates. Table 2 shows that, in the absence of irradiation, growth of D. geothermalis is independent of any amino acids and the bacterium can utilize ammonium sulfate and grow on tricarboxylic acid cycle intermediates. In the presence of chronic irradiation, growth of D. geothermalis is less dependent than that of D. radiodurans on Cys and Met, or other exogenously provided amino acids (data not shown). Therefore, the metabolism of D. geothermalis appears substantially more robust than that in D. radiodurans.
DISCUSSION
D. geothermalis is transformable with autonomous plasmids originally constructed for D. radiodurans. Thus, experimental advances in the genetic management of D. radiodurans over the last decade (5, 8, 24) could facilitate rapid development of D. geothermalis for fundamental and practical objectives. D. geothermalis is a thermophile (13) with substrate utilization-growth characteristics that are distinct from those of D. radiodurans (Table 2). Under nonirradiating conditions, D. geothermalis is not dependent on exogenous amino acids for growth and can utilize ammonium sulfate. These characteristics endow the species with the ability to grow in nutritionally restricted environments that do not support the growth of D. radiodurans (32). D. geothermalis is also able to grow over a broad temperature range extending to 55°C (13) and displays superior growth in the presence of chronic irradiation (50 Gy/h) in nutritionally restricted medium, compared to D. radiodurans. While these characteristics support the idea that D. geothermalis may be a more robust candidate than D. radiodurans for treatment of radioactive waste environments (32), until now there has been no genetic system available to exploit this species.
Our data show that plasmid-based transformation systems developed for D. radiodurans (Fig. 1 and 2) can be used to functionally express cloned genes in D. geothermalis at temperatures as high as 50°C (Fig. 2) and in the presence of chronic irradiation. Plasmids introduced into D. geothermalis are also efficiently expressed following exposure to high-level acute irradiation (Fig. 1C), without any apparent plasmid loss or mutagenesis. The differential hybridization results with a chromosome- and a plasmid-derived probe in MD865 (D. geothermalis/pMD66) (Fig. 1B) support the idea that pMD66 exists in multiple copies in D. geothermalis. The survival of D. geothermalis/pMD66 plated on TGY-KAN was indistinguishable from that found for wild-type D. geothermalis on TGY. As in D. radiodurans, this suggests that multiple identical plasmid copies serve as a substrate for efficient repair by homologous recombination (10). Therefore, these studies establish D. geothermalis and D. radiodurans as the only two extremely radiation-resistant vegetative bacteria that are currently amenable to genetic engineering.
The presence of pMD66 in D. geothermalis as a covalently closed circle was confirmed by plasmid rescue in E. coli (Table 1) (12), and restriction enzyme mapping and Southern analysis confirmed its predicted structure and stability in D. geothermalis (Fig. 1A). When total DNA containing pMD66 was purified from D. geothermalis and transformed back into wild-type D. geothermalis, there was only a small increase in the number of transformants over that with pMD66 purified from E. coli. In contrast, there was a large increase in transformation frequency observed in D. radiodurans with pMD66 purified from D. radiodurans or D. geothermalis over that with pMD66 purified from E. coli (Table 1). Therefore, the plasmid transformation capabilities of D. geothermalis appear to be significantly less than those of for D. radiodurans. While the reasons for this difference are unclear, the fact that pMD66 purified from D. geothermalis could be used to transform D. radiodurans at high efficiency, but not D. geothermalis, suggests that transport of DNA into D. geothermalis is inefficient. Wild-type D. murrayi is naturally resistant to KAN and, therefore, was not tested for transformability with pMD66/68. However, D. murrayi is sensitive to chloramphenicol and could be a suitable host for plasmids encoding Cmr, but we found it to be nontransformable with high concentrations of pMD300/308 (10) purified from E. coli or D. radiodurans and did not investigate this species further.
To demonstrate the utility of D. geothermalis for bioremediation purposes, we introduced the highly characterized Hg(II) resistance operon (mer) of E. coli (3) into D. geothermalis on an autonomously replicating D. radiodurans plasmid (Fig. 2A). Ionic Hg(II) is a prevalent contaminant of radioactive DOE waste sites, where the highest concentration level in contaminated areas has been reported as 10 μM (28). When present in D. radiodurans, the mer operon confers Hg(II) resistance and endows cells with the ability to reduce highly toxic Hg(II) to much less toxic elemental Hg(0) (5). Similarly, we show that strain MD865 (D. geothermalis/mer+) is (i) resistant to the bactericidal effects of ionic Hg(II) at concentrations (50 μM; Fig. 2D) well above the highest concentration reported for Hg(II)-contaminated DOE waste sites, (ii) able to reduce toxic Hg(II) to much less toxic elemental and volatile Hg(0) (Fig. 2C), and (iii) able to functionally express the mer operon in highly irradiating environments (50 Gy/h) at temperatures as high as 50°C. It is notable that the mesophilic E. coli Mer proteins (3) were functional in D. geothermalis growing at 50°C. While mechanisms underlying thermophilicity appear to be complex and currently are not well characterized (23), there is some precedent for the interchangeability of genes from mesophiles and thermophiles. For example, the aspartate aminotransferase gene (aspATSs) of the hyperthermophile Sulfolobus solfataricus has been functionally expressed at mesophilic temperatures in E. coli (2). We believe that numerous other metal resistance functions from other bacteria, specific for other metals, could be cloned into D. geothermalis by this approach.
It was recently shown that under strict anaerobic conditions D. radiodurans can reduce Fe(III)-NTA coupled to the oxidation of lactate to CO2 and acetate (14). D. radiodurans could also reduce U(VI) or Tc(VII) in the presence of AQDS and could directly reduce Cr(VI) in both anaerobic and aerobic conditions (14). The enzymatic reduction of multivalent metals and radionuclides can have a major impact on their solubility and, hence, mobility in the environment. Such changes in solubility make microbial metal reduction a suitable process for immobilizing metals and radionuclides within contaminated environments in situ (8, 25). Localized contaminated sediments and soils at DOE sites can have temperature levels that exceed those that can be tolerated by D. radiodurans. We show that the D. geothermalis suite of metal-reducing capabilities appears to be very similar to that reported in detail for D. radiodurans (14) but functional at higher temperatures (Fig. 3).
We are not aware of expression of any cloned genes in D. geothermalis previous to this report. Our demonstration that plasmids developed for D. radiodurans are functional in D. geothermalis strongly supports the idea that bioremediating gene constructs developed for D. radiodurans could be transferred to D. geothermalis. This could yield metabolically proficient, extremely radiation-resistant, and thermophilic bacteria suitable for the treatment of high-temperature mixed radioactive wastes.
Acknowledgments
This research was funded by U.S. Department of Energy (Office of Biological and Environmental Research) grants DE-FG02-97ER62492 from the Natural and Accelerated Bioremediation Research Program and DE-FG02-01ER63220 from the Genomes to Life Program.
REFERENCES
- 1.Agnew, S. F., and R. A. Corbin. 1998. Analysis of SX farm leak histories: historical leak model. Los Alamos National Laboratory, Los Alamos, N.Mex.
- 2.Arnone, M. I., L. Birolo, M. V. Cubellis, G. Nitti, G. Marino, and G. Sannia. 1992. Expression of a hyperthermophilic aspartate aminotransferase in Escherichia coli. Biochim. Biophys. Acta 1160:206-212. [DOI] [PubMed] [Google Scholar]
- 3.Barrineau, P., P. Gilbert, W. J. Jackson, C. S. Jones, A. O. Summers, and S. Wisdom. 1985. The structure of the mer operon. Basic Life Sci. 30:707-718. [DOI] [PubMed] [Google Scholar]
- 4.Battista, J. R. 1997. Against all odds: the survival strategies of Deinococcus radiodurans. Annu. Rev. Microbiol. 51:203-224. [DOI] [PubMed] [Google Scholar]
- 5.Brim, H., S. C. McFarlan, J. K. Fredrickson, K. W. Minton, M. Zhai, L. P. Wackett, and M. J. Daly. 2000. Engineering Deinococcus radiodurans for metal remediation in radioactive mixed waste environments. Nat. Biotechnol. 18:85-90. [DOI] [PubMed] [Google Scholar]
- 6.Brina, R., and A. G. Miller. 1992. Direct detection of trace levels of uranium by laser-induced kinetic phosphometry. Anal. Chem. 64:1413-1418. [Google Scholar]
- 7.Brooks, B. W., R. G. E. Murray, J. L. Johnson, E. Stackebrandt, C. R. Woese, and G. E. Fox. 1980. Red-pigmented micrococci: a basis for taxonomy. Int. J. Syst. Bacteriol. 30:627-646. [Google Scholar]
- 8.Daly, M. J. 2000. Engineering radiation-resistant bacteria for environmental biotechnology. Curr. Opin. Biotechnol. 11:280-285. [DOI] [PubMed] [Google Scholar]
- 9.Daly, M. J., L. Ouyang, P. Fuchs, and K. W. Minton. 1994. In vivo damage and recA-dependent repair of plasmid and chromosomal DNA in the radiation-resistant bacterium Deinococcus radiodurans. J. Bacteriol. 176:3508-3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daly, M. J., L. Ouyang, and K. W. Minton. 1994. Interplasmidic recombination following irradiation of the radioresistant bacterium Deinococcus radiodurans. J. Bacteriol. 176:7506-7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daly, M. J., and K. W. Minton. 1995. Interchromosomal recombination in the extremely radioresistant bacterium Deinococcus radiodurans. J. Bacteriol. 177:5495-5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daly, M. J., and K. W. Minton. 1996. An alternative pathway of recombination of chromosomal fragments precedes recA-dependent recombination in the radioresistant bacterium Deinococcus radiodurans. J. Bacteriol. 178:4461-4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferreira, A. C., M. F. Nobre, F. A. Rainey, M. T. Silva, R. Wait, J. Burghardt, A. P. Chung, and M. S. da Costa. 1997. Deinococcus geothermalis sp. nov. and Deinococcus murrayi sp. nov., two extremely radiation-resistant and slightly thermophilic species from hot springs. Int. J. Syst. Bacteriol. 47:939-947. [DOI] [PubMed] [Google Scholar]
- 14.Fredrickson, J. K., H. M. Kostandarithes, S. W. Li, A. E. Plymale, and M. J. Daly. 2000. Reduction of Fe(III), Cr(VI), U(VI) and Tc(VII) by Deinococcus radiodurans R1. Appl. Environ. Microbiol. 66:2006-2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lange, C. C., L. P. Wackett, K. W. Minton, and M. J. Daly. 1998. Engineering a recombinant Deinococcus radiodurans for organopollutant degradation in radioactive mixed waste environments. Nat. Biotechnol. 16:929-933. [DOI] [PubMed] [Google Scholar]
- 16.Lennon, E., and K. W. Minton. 1990. Gene fusions with lacZ by duplication insertion in the radioresistant bacterium Deinococcus radiodurans. J. Bacteriol. 172:2955-2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin, J., R. Qi, C. Aston, J. Jing, T. S. Anantharaman, B. Mishra, O. White, M. J. Daly, K. W. Minton, J. C. Venter, and D. C. Schwartz. 1999. Whole genome shotgun optical mapping of Deinococcus radiodurans using genomic DNA molecules. Science 285:1558-1561. [DOI] [PubMed] [Google Scholar]
- 18.Lipton, M. S., L. Pasa-Toliæ, G. A. Anderson, D. J. Anderson, D. L. Auberry, J. R. Battista, M. J. Daly, J. K. Fredrickson, K. K. Hixson, H. Kostandarithes, C. Masselon, L. M. Markillie, R. J. Moore, M. F. Romine, Y. Shen, E. Stritmatter, N. Toliæ, H. R. Udseth, A. Venkateswaran, K. K. Wong, R. Zhao, and R. D. Smith. 2002. Global analysis of the Deinococcus radiodurans proteome using accurate mass tags. Proc. Natl. Acad. Sci. USA 99:11049-11054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu, Y., J. Zhou, M. V. Omelchenko, A. S. Beliaev, A. Venkateswaran, J. Stair, L. Wu, D. K. Thompson, D. Xu, I. B. Rogozin, E. K. Gaidamakova, M. Zhai, K. S. Makarova, E. V. Koonin, and M. J. Daly. 2003. Transcriptome dynamics of Deinococcus radiodurans recovering from ionizing radiation. Proc. Natl. Acad. Sci. USA 100:4191-4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lovley, D. R., E. L. Coates, J. D. Blunt-Harris, E. J. P. Phillips, and J. C. Woodward. 1996. Humic substances as electron acceptors for microbial respiration. Nature 382:445-448. [Google Scholar]
- 21.Macilwain, C. 1996. Science seeks weapons clean-up role. Nature 383:375-379. [Google Scholar]
- 22.Makarova, K. S., L. Aravind, Y. I. Wolf, R. L. Tatusov, K. W. Minton, E. V. Koonin, and M. J. Daly. 2001. Genome of the extremely radiation-resistant bacterium Deinococcus radiodurans viewed from the perspective of comparative genomics. Microbiol. Mol. Biol. Rev. 65:44-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Makarova, K. S., Y. I. Wolf, and E. V. Koonin. 2003. Potential genomic determinants of hyperthermophily. Trends Genet. 19:172-176. [DOI] [PubMed] [Google Scholar]
- 24.Mattimore, V., and J. R. Battista. 1996. Radioresistance of Deinococcus radiodurans: functions necessary to survive ionizing radiation are also necessary to survive prolonged desiccation. J. Bacteriol. 178:633-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCullough, J., T. C. Hazen, S. M. Benson, F. Blaine-Metting, and A. C. Palmisano. 1999. Bioremediation of metals and radionuclides. Office of Biological and Environmental Research, U.S. Department of Energy, Germantown, Md.
- 26.Nakamura, K., and H. Nakahara. 1988. Simplified X-ray film method for detection of bacterial volatilization of Hg chloride by Escherichia coli. Appl. Environ. Microbiol. 54:2871-2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Office of Environmental Management, U.S. Department of Energy. 1996. 1996 baseline environmental management report. Office of Environmental Management, U.S. Department of Energy, Washington, D.C. [Online.] http://www.em.doe.gov/bemr96.
- 28.Riley, R. G., J. M. Zachara, and F. J. Wobber. 1992. Chemical contaminants on DOE lands and selection of contaminant mixtures for subsurface science research. Subsurface Science Program, Office of Energy Research, U.S. Department of Energy, Washington, D.C.
- 29.Smith, M. D., E. Lennon, L. B. McNeil, and K. W. Minton. 1988. Duplication insertion of drug resistance determinants in the radioresistant bacterium Deinococcus radiodurans. J. Bacteriol. 170:2126-2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stookey, L. L. 1970. Ferrozine—a new spectrophotometric reagent for iron. Anal. Chem. 42:779-781. [Google Scholar]
- 31.Urone, P. F. 1955. Stability of colorimetric reagent for chromium, s-diphenylcarbazide, in various solvents. Anal. Chem. 27:1354-1355. [Google Scholar]
- 32.Venkateswaran, A., S. C. McFarlan, D. Ghosal, K. W. Minton, A. Vasilenko, K. S. Makarova, L. P. Wackett, and M. J. Daly. 2000. Physiologic determinants of radiation resistance in Deinococcus radiodurans. Appl. Environ. Microbiol. 66:2620-2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.White, O., J. A. Eisen, J. F. Heidelberg, E. K. Hickey, J. D. Peterson, R. J. Dodson, D. H. Haft, M. L. Gwinn, W. C. Nelson, D. L. Richardson, K. S. Moffet, H. Qin, L. Jiang, W. Pamphile, M. Crosby, M. Shen, J. J. Vamathevan, P. Lam, L. McDonald, T. Utterback, C. Zalewski, K. S. Makarova, L Aravind, M. J. Daly, K. W. Minton, R. D. Fleishmann, K. A. Ketchum, K. E. Nelson, S. Salzberg, J. C. Venter, and C. M. Fraser. 1999. Complete genome sequencing of the radioresistant bacterium Deinococcus radiodurans R1. Science 286:1571-1577. [DOI] [PMC free article] [PubMed] [Google Scholar]