Abstract
Plasmid pSt04 of Streptococcus thermophilus contains a gene encoding a protein with homology to small heat shock proteins (A. Geis, H. A. M. El Demerdash, and K. J. Heller, Plasmid 50:53-69, 2003). Strains cured from the shsp plasmids showed significantly reduced heat and acid resistance and a lower maximal growth temperature. Transformation of the cloned shsp gene into S. thermophilus St11 lacking a plasmid encoding shsp resulted in increased resistance to incubation at 60°C or pH 3.5 and in the ability to grow at 52°C. A food-grade cloning system for S. thermophilus, based on the plasmid-encoded shsp gene as a selection marker, was developed. This approach allowed selection after transfer of native and recombinant shsp plasmids into different S. thermophilus and Lactococcus lactis strains. Using a recombinant plasmid carrying an erythromycin resistance (Emr) gene in addition to shsp, we demonstrated that both markers are equally efficient in selecting for plasmid-bearing cells. The average transformation rates in S. thermophilus (when we were selecting for heat resistance) were determined to be 2.4 × 104 and 1.0 × 104 CFU/0.5 μg of DNA, with standard deviations of 0.54 × 104 and 0.32 × 104, for shsp and Emr selection, respectively. When we selected for pH resistance, the average transformation rates were determined to be 2.25 × 104 and 3.8 × 103 CFU/0.5 μg of DNA, with standard deviations of 0.63 × 104 and 3.48 × 103, for shsp and Emr selection, respectively. The applicability of shsp as a selection marker was further demonstrated by constructing S. thermophilus plasmid pHRM1 carrying the shsp gene as a selection marker and the restriction-modification genes of another S. thermophilus plasmid as a functional trait.
Plasmids are widely distributed among the mesophilic lactococci, in which they encode several functions that are essential for dairy fermentation, such as lactose and protein metabolism and aroma and exopolysaccharide synthesis. Genes for bacteriophage defense systems, as well as for bacteriocin production and immunity, have also been located on lactococcal plasmids (for a review, see reference 20).
In contrast to lactococcal strains, only a few Streptococcus thermophilus strains carry plasmids, and of these strains most carry a limited number of plasmids that are classified into five homology groups (12, 15, 24, 29). Many plasmids in S. thermophilus are cryptic, since no obvious phenotypic traits appear to be associated with the presence of these plasmids. However, a few S. thermophilus plasmids have recently been described that carry, in addition to genes encoding replication functions, genes encoding small heat shock proteins (24, 28) or restriction-modification enzymes (24, 27). Since plasmids play an important role in the development and application of techniques for the genetic manipulation of key industrial traits in food-grade lactic acid bacteria (2, 14, 17, 25), we wanted to evaluate the potential of some of S. thermophilus plasmids to be used as food-grade cloning plasmids. The term “food-grade” in conjunction with plasmids was first used in 1986 by Herman and McKay (12), who proposed the β-galactosidase of S. thermophilus be used as a food-grade selection marker. Since then, several food-grade vector systems have been described for lactococci and lactobacilli. Whereas some of these systems, such as immunity to bacteriocins (1, 21, 31), resistance to cadmium (18), or the establishment of new metabolic pathways (3), are widely applicable in many strains, others, such as complementation of chromosomal mutations (4, 5, 7, 26), require specific genetic backgrounds in order to offer selective advantages to limited numbers of strains. This is also true for the widely used applications of genes involved in lactose metabolism (10, 11, 18, 19, 30).
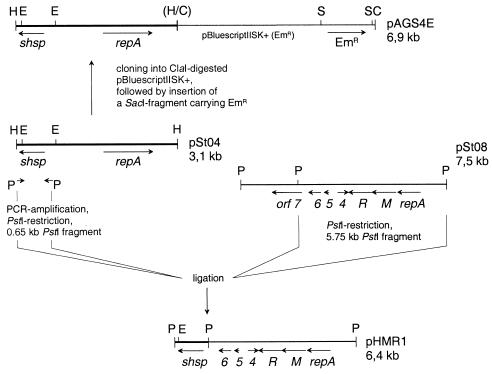
One of the S. thermophilus plasmids that we recently isolated (9a), pSt04, is related to a group of plasmids replicating by a rolling circle mechanism (Fig. 1). According to sequence (9a), pSt04 is 3,119 bp long and contains two open reading frames (ORFs): repA and shsp. The gene product of the shsp gene is highly similar (>90%) to genes encoding small heat shock proteins in other S. thermophilus strains (9a, 24, 28).
FIG. 1.
Plasmids used in the present study. Constructions are described in the text. ORFs (orf) and their directions of transcription are indicated by arrows. Restriction sites are shown for HinPI (H), EcoRI (E), ClaI (C), and SacI (S). Restriction sites no longer accessible are shown in brackets. R, ORF encoding restriction endonuclease; M, ORF encoding methylase; repA, replication protein. Thus far, no tentative function could be assigned to ORF4, -5, and -6, since they do not exhibit similarity to any functionally characterized gene product in the GenBank database.
Another plasmid we recently characterized is pSt08. It encodes a type II restriction-modification system (R/M system) and shares identity up to 60% with lactococcal R/M systems (9a). The activity of the R/M system is indicated by (i) phage resistance of plasmid-bearing cells and (ii) phage sensitivity of plasmid-cured cells (22).
We show here that shsp can efficiently serve in S. thermophilus and Lactococcus lactis as a broadly applicable food-grade selection marker. We verify this application by constructing a plasmid in S. thermophilus carrying shsp and the genes for the pSt08-encoded R/M system, and we successfully transform the plasmid into different S. thermophilus strains by using shsp as a selection marker.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains used are listed in Table 1.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Descriptiona | Source or reference |
|---|---|---|
| Strains | ||
| S. thermophilus | ||
| S4 | Plasmid pSt04 | BAfMb |
| S4-1 | Plasmid-cured S4 | This study |
| St11 | Recipient strain for plasmids | 23 |
| S8-1 | Plasmid-cured S. thermophilus S8 | This study |
| ER1-1 | Plasmid-cured S. thermophilus ER1 | This study |
| An55 | Isolate from yogurt, plasmid free | BAfM |
| HM69 | Isolate from yogurt, plasmid free | BAfM |
| L. lactis MG1363 | Plasmid-cured derivative of NCDO712 | 9 |
| E. coli XL1-Blue | F′::Tn10 proA+B+laclq lacZM15/recA1 endA1 gyrA96 (Nalr) thi hsdR17(rK− mK+) supE44relA1 lac | Stratagene, La Jolla, Calif. |
| Plasmids | ||
| pSt04 | Rolling-circle plasmid; repA+shsp+ | 9a |
| pAGS4E | HinPI-linearized pSt04 cloned into pBluescript II SK(+), linearized with ClaI | This study |
| pSt08 | Rolling-circle plasmid; repA+ type II R/M system | 9a |
| pHMR1 | shsp of pSt04 cloned into a 5.7-kb PstI fragment of plasmid pSt08 carrying repA and the genes for a type II R/M system | This study |
For all S. thermophilus strains the absence of a chromosomal copy of shsp was demonstrated by Southern blotting. Nalr, nalidixic acid resistance.
BAFM, Bundesanstalt for Milchforschung (Federal Dairy Research Centre) laboratory collection.
Plasmid constructions.
Plasmid pAGS4E was constructed by cloning pSt04, linearized with HinP1, into the E. coli vector pBluescript II SK(+), linearized with ClaI. The recombinant plasmid pAGS4 was subsequently marked by an erythromycin resistance (Emr) gene derived from pE194 and cloned into the SacI site of the vector. The physical structure of pAGS4E was verified by restriction analysis and partial nucleotide sequencing.
For construction of pHRM1, the shsp gene of plasmid pSt04 was amplified by PCR with primers with PstI extensions at their 5′ ends. After restriction and subsequent purification by agarose gel electrophoresis, the amplified fragment was ligated to a 5.7-kb PstI restriction fragment obtained from plasmid pSt08 carrying the replication functions and the genes for a type II R/M system (unpublished data). The physical structure of pHRM1 was verified by restriction analysis and partial DNA sequencing.
Plasmid curing.
For plasmid curing the protoplast method of Gasson (8) was used with some modifications. S. thermophilus cells, grown at 42°C overnight, were inoculated into LTM17 medium supplemented with 40 mM d,l-threonine. They were incubated at 40°C until an optical density at 620 nm (OD620) of 0.6 to 0.7 was reached. Then, 4 ml of the cells were harvested by centrifugation at 14,000 × g for 1 min. The cell sediment was washed once with protoplast buffer (40 mM ammonium acetate, 0.5 M sucrose, 0.25% gelatin [pH 6.5]) and then suspended in 0.5 ml of the same buffer containing 2.5 mg of lysozyme and 1 mg of mutanolysin. Incubation was at 37°C for 45 to 60 min. When the formation of protoplasts, followed by analysis by phase-contrast microscopy, was complete, the protoplasts were harvested by centrifugation, washed with protoplast buffer, resuspended in 1 ml of the same buffer, and serially diluted in the same buffer by a factor of up to 107. Dilutions were plated onto GTM17 agar containing 0.5 M sucrose and 0.25% gelatin and then incubated at 40°C for regeneration. Colonies appearing after about 4 days were checked for the absence of plasmid.
Transformation protocols.
An optimized protocol for electroporation of S. thermophilus, followed by selection for thermoresistance as determined by the presence of shsp, was developed. To prepare competent S. thermophilus cells, 200 ml of Elliker broth (6) supplemented with 1 to 2% glycine and 10 to 20 mM MgCl2 (depending on the strain) was inoculated with 1% of an overnight culture grown in Elliker broth. Cells were grown to an OD620 of 0.5 to 0.6 at 40°C. Cells were harvested by centrifugation at 10,000 rpm/min and 4°C for 15 to 20 min. The medium was carefully discarded. Cells were washed with ice-cold electroporation buffer (272 mM sucrose, 1 mM EDTA, 7 mM HEPES, 20 mM MgCl2, 20 mM glycine, 15% glycerol [pH 6.5]), incubated on ice for 20 min, and centrifuged for 15 min. Sedimented cells were washed another two times with electroporation buffer and were then resuspended in the same buffer at a final OD620 of 2 to 2.5. Cells were frozen in aliquots of 100 μl in liquid nitrogen and stored at −80°C.
When we selected for Emr, 0.2 to 3 μg of plasmid DNA and 100 to 200 μl of thawed cells were carefully mixed in a precooled Eppendorf tube. Incubation at 4°C (on ice) for 15 min was followed by a heat shock at 42°C for 60 to 90 s. The mixture was immediately transferred to ice after the heat shock and incubated for 1 to 5 min. It was then transferred to a precooled electroporation cuvette. After a single pulse at 21 kV/cm in an Eppendorf 2510 electroporator, regeneration medium (HJL medium [30 g of tryptone, 10 g of yeast extract, 5 g of KH2PO4, 2 g of beef extract, 5 g of lactose, and 1 liter of distilled water] supplemented with 0.025 mM MgCl2 and 30 ng of erythromycin/ml) (13) was immediately added. For phenotypic expression incubation was at 42°C for 4 to 5 h. Aliquots were mixed with 4 ml of soft agar, plated on LTM17 or GTM17 plus 3 μg of erythromycin/ml, and incubated at 42°C anaerobically for 2 days. Control transformations without added plasmid usually yielded no or only very few colonies when undiluted samples were plated.
The optimized protocol for electrotransformation, followed by selection for heat resistance, was as follows. First, 0.5 μg of plasmid DNA was thoroughly mixed with 80 to 100 μl of competent cells in Eppendorf tubes, and the mixture was kept on ice for 20 to 30 min. After transfer of the mixture to a precooled gene pulser cuvette, electroporation was done at 21 kV/cm in an Eppendorf 2510 electroporator. Cells were then diluted with 1 ml of regeneration medium (13), incubated at 42°C for 3 h, collected by centrifugation, and resuspended in Belliker broth (Elliker medium plus 1% beef extract) without sugar, followed by incubation at 50°C for 45 to 60 min. Cells were spread on LTM17 plates (16) and incubated at 50°C for 2 days, followed by incubation at 42°C for 1 day. Control transformations without added plasmid usually yielded a number of colonies corresponding to the number of false-positive CFU in samples with plasmid added.
Electroporation of L. lactis strains was done at 1.25 kV/cm and 25 μF in a Bio-Rad GenePulser. The pulse controller was set at 200 Ω. After electroporation, the cells were diluted with 1 ml of regeneration medium, followed by incubation at 30°C for 1 h. Thereafter, the incubation temperature was first increased to 37°C for 45 min and finally to 42°C for another 45 min. Cells were plated on GM17 plates (32) or GM17 plates containing 5 μg of erythromycin/ml. Incubation of the plates was for 1 day at 41°C, followed by incubation for another day at 37°C.
Nucleotide sequence accession numbers.
Nucleotide sequences of pSt04 and pSt08 are available under EMBL accession numbers AJ242477 and AJ239049, respectively.
RESULTS AND DISCUSSION
Presence of a plasmid encoding shsp increases thermoresistance and pH resistance of S. thermophilus.
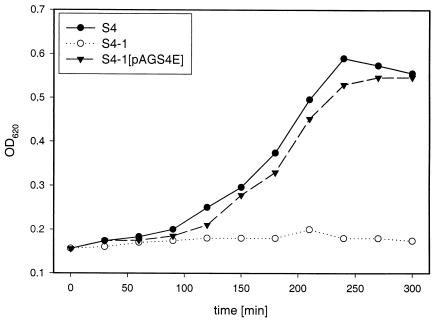
To evaluate the applicability of the shsp gene as a selection marker, the growth of S. thermophilus strains S4, its plasmid-free derivative S4-1, and S4-1(pAGS4E) was tested at elevated temperatures between 42 and 52°C. Plasmid pAGS4E (Fig. 1) was constructed to allow parallel selection either for Emr or for shsp-induced heat tolerance. Figure 2 shows that curing of plasmid pSt04 resulted in a loss of ability to grow at 52°C, demonstrating that pSt04 was responsible for the growth phenotype at an elevated temperature. This was further proven by the fact that no difference was observed between strains S4 and S4-1(pAGS4E).
FIG. 2.
Effect of shsp on growth at elevated temperatures. S. thermophilus cultures were grown at 42°C to early log phase and then shifted to 52°C. Growth at 52°C in Belliker broth was monitored for S. thermophilus S4, plasmid-cured strain S4-1, and S4-1 transformed with pAGS4E. The experiment was repeated three times, and typical results are shown.
Sensitivity to elevated temperatures was further assayed with strains S4 and S4-1. Incubation at 60°C for 60 min resulted in an inactivation of ca. 90% of S4-1 cells, while >50% of the S4 cells survived this treatment. After 120 min, ca. 99% of the S4-1 cells but only ca. 65% of S4 cells were inactivated (data not shown). These data correspond to those of O'Sullivan et al. (24), who demonstrated that carrying plasmid pCI65st, encoding two genes for small heat shock proteins, confers on S. thermophilus cells some degree of resistance to elevated temperatures.
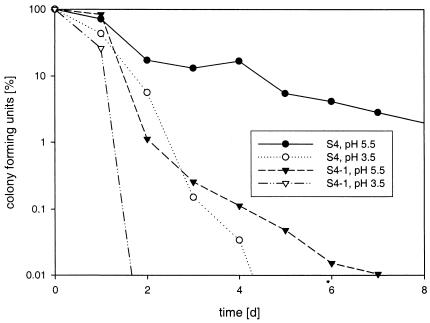
In addition to protection against elevated temperatures, the presence of pAGS4E encoding shsp proved to protect cells against a low pH. When incubated at a pH 5.5 or pH 3.5, a pH-dependent decrease in viable counts was observed, which was much higher with shsp mutant cells than with wild-type shsp cells (Fig. 3). The extent of inactivation increased when the temperature was raised from 4°C (data not shown) to room temperature (Fig. 3). The data suggest that shsp is involved not only in heat resistance but also in pH resistance. Thus, it may be more appropriate to address shsp as a stress rather than a heat shock protein.
FIG. 3.
Survival of S. thermophilus S4 and its plasmid-cured derivative S4-1 during storage at 20°C in Belliker broth at different pH values. S. thermophilus cultures were grown in pH 7-buffered medium to late log phase, harvested, and transferred to Belliker broth adjusted to pH 5.5, pH 4.5, and pH 3.5. At the incubation times indicated, the numbers of CFU were determined after dilution and plating on LTM17-agar plates. The experiment was repeated three times, and typical results are shown.
Optimized shsp selection methods for transformation of S. thermophilus.
Plasmid pAGS4E carries selection markers for Emr and shsp. It is therefore an ideal plasmid for comparing transformation efficiencies when selecting for one of the two markers. The key steps in selecting for shsp-based heat resistance of S. thermophilus St11 after electrotransformation are: (i) phenotypic expression for 3 h at 42°C in regeneration medium; (ii) incubation at the selective temperature of 52°C for 45 to 60 min in Belliker broth without sugar; and (iii) incubation on LTM17 agar plates at 52°C for 2 days, followed by incubation at 42°C for another day. For different strains, the selective temperature may have to be varied between 48 and 52°C (data not shown).
In three independent experiments, transformation rates of 2.9 × 104, 2.1 × 104, and 3.3 × 104 CFU per 0.5 μg of DNA, respectively, were determined in S. thermophilus St11 when we selected for shsp. When we selected for Emr, transformation rates of 1.2 × 104, 0.8 × 104, and 1.4 × 104 CFU/0.5 μg of DNA were determined in the same samples. From every individual assay 100 colonies were picked and screened for the presence of pAGS4E by agarose gel electrophoresis. pAGS4E was found to be present in 74 to 100% (erythromycin selection) and 77 to 93% (shsp selection) of the colonies, indicating that the number of false-positive transformants was comparable with both methods. The corrected average values of transformation rates (CFU minus false-positive ones) were 2.4 × 104 and 1.0 × 104 CFU/0.5 μg of DNA, with standard deviations of 0.54 and 0.32 × 104 for shsp and Emr selection, respectively. From these experiments it appears that shsp serves as a selection marker that is at least as suitable as Emr in S. thermophilus.
In addition to an elevated temperature, a low pH was also used as a selective condition for shsp cells. The preparation of competent cells and electroporation were identical to those for the temperature analysis described above. Thereafter, cells were incubated for 30 min at 42°C in sugar-free Belliker medium (pH 3.5). After a plating step, cells were incubated at 42°C for 2 days. The results obtained were comparable to those obtained with the temperature-based selection method. In five independent experiments, transformation rates of between 2.1 × 104 and 3.3 × 104 CFU/0.5 μg of DNA were observed in S. thermophilus St11. Between 55 and 89% of the colonies were demonstrated to contain plasmid pAGS4E. Again, control transformations with identical samples selected for Emr yielded transformation rates of between 1.4 × 103 and 9.2 × 103 CFU/0.5 μg of DNA. A total of 42 to 92% of the colonies were shown to contain plasmid pAGS4E. The average transformation rates, corrected for the number of colonies not bearing a plasmid, were 2.25 × 104 and 3.8 × 103 CFU/0.5 μg of DNA, with standard deviations of 0.63 × 104 and 3.48 × 103 for shsp and Emr selection, respectively.
Both transformation methods were successfully applied for the transformation of native plasmid pSt04 into S. thermophilus S4-1. The transformation rates were comparable to those for the transformation of pAGS4E into S. thermophilus St11 (data not shown).
By applying the shsp-based selection to an analysis of elevated temperature resistance, pAGS4E was successfully transformed into seven different strains of S. thermophilus: S4-1, St11, S8, S8-1, ER1-1, An55, and HM69. Furthermore, the shsp-based selection not only worked in thermophilic but also in mesophilic lactic acid bacteria. The three mesophilic L. lactis strains IL-1403, Bu2-60, and MG1363 were successfully transformed with pAGS4E by using the shsp selection scheme for elevated temperature resistance. Transformation efficiencies of pAGS4E DNA (average of five experiments), corrected for the number of colonies not bearing a plasmid, for the three strains IL-1403, Bu2-60, and MG1363 were 1.8 × 104, 2.0 × 104, and 2.4 × 104 CFU/0.5 μg of DNA for shsp selection and 6.9 × 103, 3.8 × 103, and 6.6 × 103 CFU/0.5 μg of DNA, respectively, for Emr selection. However, as described in Materials and Methods, the selective temperature had to be reduced to 42°C for the mesophilic L. lactis strains.
Construction of a food-grade plasmid conferring bacteriophage resistance on S. thermophilus.
To verify the applicability of the shsp gene as selection marker plasmid and to demonstrate that shsp-based selection was efficient enough to allow construction to be made in S. thermophilus, pHMR1 was constructed by combining the shsp gene of pSt04 with the replication and restriction-modification genes and the replicon of pSt08 (Fig. 1). Plasmid pSt08 had been shown to confer phage resistance to S. thermophilus S8 since the efficiency of plating of bacteriophage 8/S8 increased by a factor of ca. 160 upon curing of the plasmid (9a). It had further been shown by curing experiments that pSt08 did not confer heat resistance to the cells and that transformation of pAGS4E into pSt08-bearing, as well as pSt08-free, cells resulted in resistance of both strains to an elevated temperature (22). When pHMR1 was transformed into plasmid-cured strain S. thermophilus S8-1, the efficiency of plating of phage 8/S8 was reduced by a factor of ca. 190. Plasmid pHMR1 in addition has been transformed into six different S. thermophilus strains (S4-1, St11, S8-1, ER1-1, An55, and HM69) with efficiencies ranging between 1.5 × 101 transformants/0.5 μg of DNA for ER1-1 to 2.5 × 104 for HM69. These results clearly demonstrated that shsp can be used as a selection marker in constructions of food-grade plasmids. Furthermore, our construction proves that shsp is indeed the gene responsible for resistance to elevated temperatures. It should be pointed out that the combination of shsp with genes encoding a restriction-modification enzyme system is not unique: pCI65st, a 6.5-kb plasmid from S. thermophilus ND1-6, is a natural plasmid that carries two nearly identical hsp genes in addition to a gene encoding a component of a type 1 restriction system (24).
Conclusions.
The small heat shock gene shsp present on S. thermophilus plasmid pSt04 confers to cells resistance to elevated temperatures and low pH conditions. These properties allow shsp to be applied as selection marker in transformations. The advantages of shsp as selection marker are that (i) it is equally efficient as the Emr marker, a frequently applied selection marker in lactic streptococci; (ii) it does not require strains with specifically altered genetic information; (iii) it is a rather small gene of just ca. 480 bp and therefore does not overly contribute to the size of cloning vectors to be constructed; (iv) it provides an additional opportunity to select for maintenance of more than one plasmid within the same cell; and (v) it is a food-grade selection marker for S. thermophilus and other lactic acid bacteria applied in food fermentations.
Acknowledgments
H.A.M.E.D. is thankful to the Egyptian Government and Suez Canal University (Egypt) for providing 4 years of scholarship support.
The excellent technical assistance of Veronika Wind is gratefully acknowledged. We thank also the staff members of the Institute for Microbiology of FDRC, Kiel, Germany.
REFERENCES
- 1.Allison, G. E., and T. R. Klaenhammer. 1996. Functional analysis of the gene encoding immunity to lactacin F, lafI, and its use as a Lactobacillus-specific, food-grade genetic marker. Appl. Environ. Microbiol. 62:4450-4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biswas, I., A. Gruss, S. D. Ehrlich, and E. Maguin. 1993. High-efficiency inactivation and replacement system for gram-postive bacteria. J. Bacteriol. 175:3625-3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boucher, I., M. Parrot, H. Gaudreau, C. P. Champagne, C. Vadeboncoeur, and S. Moineau. 2002. Novel food-grade plasmid vector based on melibiose fermentation for the genetic engineering of Lactococcus lactis. Appl. Environ. Microbiol. 68:6152-6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bron, P. A., M. G. Benchimol, J. Lambert, E. Palumbo, M. Deghorain, J. Delcour, W. M. De Vos, M. Kleerebezem, and P. Hols. 2002. Use of the alr gene as a food-grade selection marker in lactic acid bacteria. Appl. Environ. Microbiol. 68:5663-5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dickely, F., D. Nilsson, E. B. Hansen, and E. Johansen. 1995. Isolation of Lactococcus lactis nonsense suppressors and construction of a food-grade cloning vector. Mol. Microbiol. 15:839-847. [DOI] [PubMed] [Google Scholar]
- 6.Elliker, P. R., A. W. Anderson, and G. Hannesson. 1956. An agar culture medium for lactic acid streptococci and lactobacilli. J. Dairy Sci. 89:1611-1612. [Google Scholar]
- 7.Fu, X., and J. G. Xu. 2000. Development of a chromosome-plasmid balanced lethal system for Lactobacillus acidophilus with thyA gene as selective marker. Microbiol. Immunol. 44:551-556. [DOI] [PubMed] [Google Scholar]
- 8.Gasson, M. J. 1980. Production, regeneration and fusion of protoplasts in lactic streptococci. FEMS Microbiol. Lett. 9:99-102. [Google Scholar]
- 9.Gasson, M. J. 1983. Plasmid complements of Streptococcus lactis NCDO712 and other lactic streptococci after protoplast-induced curing. J. Bacteriol. 178:6508-6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9a.Geis, A., H. A. M. El Demerdash, and K. J. Heller. 2003. Sequence analysis and characterization of plasmids from Streptococcus thermophilus. Plasmid 50:53-69. [DOI] [PubMed] [Google Scholar]
- 10.Gosalbes, M. J., C. D. Esteban, J. L. Galan, and G. Perez-Martinez. 2000. Integrative food-grade expression system based on the lactose regulon of Lactobacillus casei. Appl. Environ. Microbiol. 66:4822-4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hashiba, H., R. Takiguchi, K. Jyoho, and K. Aoyama. 1992. Establishment of a host-vector system in Lactobacillus helveticus with β-galactosidase activity as a selection marker. Biosci. Biotechnol. Biochem. 56:190-194. [DOI] [PubMed] [Google Scholar]
- 12.Herman, R. E., and L. L. McKay. 1985. Isolation and partial characterization of plasmid DNA from Streptococcus thermophilus. Appl. Environ. Microbiol. 50:1103-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hogg, D. M., and G. R. Jago. 1970. Extraction of the 260 nm absorbing material from group N Streptococcus: a method for estimating cell growth. J. Dairy Res. 37:199-202. [Google Scholar]
- 14.Hughes, B. F., and L. L. McKay. 1991. Deriving phage-insensitive lactococci using a food-grade vector encoding phage and nisin resistance. J. Dairy Sci. 75:914-923. [Google Scholar]
- 15.Janzen, T., J. Kleinschmidt, H. Neve, and A. Geis. 1992. Sequencing and chractererization of pST1, a cryptic plasmid from Streptococcus thermophilus. FEMS Microbiol. Lett. 95:175-180. [DOI] [PubMed] [Google Scholar]
- 16.Krusch, U., H. Neve, B. Luschei, and M. Teuber. 1987. Characterization of virulent bacteriophages of Streptococcus salivarius subsp. thermophilus by host specificity and electron microscopy. Kieler Milchwirtschaftl. Forsch. Ber. 39:155-167. [Google Scholar]
- 17.Leenhouts, K., G. Buist, A. Bolhuis, A. Berger, J. Kiel, I. Mierau, G. Venema, and J. Kok. 1996. A general system for generating unlabeled gene replacements in bacterial chromosomes. Mol. Gen. Genet. 253:217-224. [DOI] [PubMed] [Google Scholar]
- 18.Liu, C. Q., V. Leelawatcharamas, M. L. Harvey, and N. W. Dunn. 1996. Cloning vectors for lactococci based on a plasmid encoding resistance to cadmium. Curr. Microbiol. 33:35-39. [DOI] [PubMed] [Google Scholar]
- 19.MacCormick, C. A., H. G. Griffin, and M. J. Gasson. 1995. Construction of a food-grade host/vector system for Lactococcus lactis based on the lactose operon. FEMS Microbiol. Lett. 127:105-109. [DOI] [PubMed] [Google Scholar]
- 20.McKay, L. L., and K. A. Baldwin. 1990. Applications for biotechnologes: present and future improvements in lactic acid bacteria. FEMS Microbiol. Rev. 87:3-14. [DOI] [PubMed] [Google Scholar]
- 21.Mills, S., A. Coffey, L. O'Sullivan, D. Stokes, C. Hill, G. F. Fitzgerald, and R. P. Ross. 2002. Use of lacticin 481 to facilitate delivery of the bacteriophage resistance plasmid, pCBG104 to cheese starters. J. Appl. Microbiol. 92:238-246. [DOI] [PubMed] [Google Scholar]
- 22.Mohamed, H. A. M. I. 2002. New techniques for food bio/technology: development of food-grade vectors for improvement of thermophilic starter culture performance. Ph.D. thesis. University of Kiel, Kiel, Germany.
- 23.Mollet, B., J. Knol, B. Poolman, O. Marciset, and M. Delley. 1993. Directed genomic integration, gene replacement, and integrative gene expression in Streptococcus thermophilus. J. Bacteriol. 175:4315-4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Sullivan, T., D. van Sinderen, and G. Fitzgerald. 1999. Structural and functional analysis of pCI65st, a 6.5-kb plasmid from Streptococcus thermophilus ND1-6. Microbiology 145:127-134. [DOI] [PubMed] [Google Scholar]
- 25.Pascalle, G. G., A. de Ruyter, P. Oscar, C. Willco, and M. W. deVos. 1997. Food-grade controlled lysis of Lactcococcus lactis for accelerated cheese ripening. Nat. Biotechnol. 15:976-979. [DOI] [PubMed] [Google Scholar]
- 26.Posno, M., P. T. Heuvelmans, M. J. van Giezen, B. C. Lokman, R. J. Leer, and P. H. Pouwels. 1991. Complementation of the inability of Lactobacillus strains to utilize d-xylose with d-xylose catabolism-encoding genes of Lactobacillus pentosus. Appl. Environ. Microbiol. 57:2764-2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Solaiman, D. K. Y., and G. A. Somkuti. 1991. A type II restriction endonuclase of Streptococcus thermophilus ST117. FEMS Microbiol. Lett. 80:75-80. [DOI] [PubMed] [Google Scholar]
- 28.Somkuti, G. A., D. K. Y. Soliman, and D. H. Steinberg. 1998. Structural and functional properties of the hsp16.4-bearing plasmid pER341 in Streptococcus thermophilus. Plasmid 40:61-72. [DOI] [PubMed] [Google Scholar]
- 29.Somokuti, G. A., and D. A. Steinberg. 1986. Distribution and analysis of plasmid in Streptococcus thermophilus. J. Indust. Microbiol. 1:157-163. [Google Scholar]
- 30.Takala, M., J. Saris, and H. Tynkkynen. 2003. Food-grade host/vector expression system for Lactobacillus casei based on complementation of plasmid-associated phospho-β-galactosidase gene lacG. Appl. Microbiol. Biotechnol. 60:564-570. [DOI] [PubMed] [Google Scholar]
- 31.Takala, T. M., and P. E. Saris. 2002. A food-grade cloning vector for lactic acid bacteria based on the nisin immunity gene nisI. Appl. Microbiol. Biotechnol. 59:467-471. [DOI] [PubMed] [Google Scholar]
- 32.Terzaghi, B. E., and W. E. Sandine. 1975. Improved medium for lactic streptococci and their bacteriophages. Appl. Microbiol. 29:807-813. [DOI] [PMC free article] [PubMed] [Google Scholar]