Abstract
To elucidate the regulation and limiting factors in the glycosylation of secreted proteins, the mpg1 and dpm1 genes from Trichoderma reesei (Hypocrea jecorina) encoding GTP:α-d-mannose-1-phosphate guanyltransferase and dolichyl phosphate mannose synthase (DPMS), respectively, were overexpressed in T. reesei. No significant increases were observed in DPMS activity or protein secretion in dpm1-overexpressing transformants, whereas overexpression of mpg1 led to a twofold increase in GDP-mannose (GDPMan) levels. GDPMan was effectively utilized by mannnosyltransferases and resulted in hypermannosylation of secreted proteins in both N and O glycosylation. Overexpression of the mpg1 gene also increased the transcription of the dpm1 gene and DPMS activity. Our data indicate that the level of cellular GDPMan can play a major regulatory role in protein glycosylation in T. reesei.
The filamentous fungus Trichoderma reesei (Hypocrea jecorina) is known for its high protein secretory capabilities. Some strains secrete up to 40 g of protein per liter (5). Most of the secreted proteins are highly glycosylated cellulases with both N- and O-glycans. O mannosylation has been reported to be necessary for secretion of the proteins (19, 24) and to be influenced in vivo by choline and Tween 80 added to the culture medium. The same compounds have been reported to affect the activity of dolichyl phosphate mannose synthase (DPMS) in this fungus (14), the key enzyme in the O glycosylation pathway (13). Overexpression of the Saccharomyces cerevisiae DPM1 gene encoding DPMS in T. reesei elevated the enzyme activity twofold and resulted in an increased level of protein secretion. The secreted proteins were glycosylated to the same extent as in the control strain, although their amount was up to sevenfold higher (17).
We have isolated the dpm1 gene encoding DPMS from T. reesei and tried to analyze the DPMS activity by overexpression of the gene in S. cerevisiae (18). The overexpression did not result in an increase in DPMS activity. The reason for this could be that the DPMS protein from T. reesei belongs to the human group of the Dpm1 proteins. In humans the enzyme requires two other subunits (Dpm2p and Dpm3p) to be stably expressed in the endoplasmic reticulum membranes. This finding is in contrast to the S. cerevisiae DPMS, which does not require additional protein subunits for full activity. Human Dpm3p subunit is associated with Dpm2p via its N-terminal domain and with Dpm1p via the C-terminal end (22). Dpm3p directly stabilizes Dpm1p and is itself stabilized by Dpm2p. Human DPMS activity is 10-fold higher in the presence of Dpm2p, indicating that this protein plays an important role in the enzymatic reaction.
A number of our earlier data have indicated that the availability of GDP-mannose (GDPMan) might be the rate-limiting factor for protein mannosylation in T. reesei. Thus, we cloned the T. reesei mpg1 gene coding for GDP:α-d-mannose-1-phosphate guanyltransferase (MPGI; EC 2.7.7.13) (16). This enzyme catalyzes the transfer of the mannosyl residue from mannose-1-phosphate to GTP to form GDPMan. The latter is then engaged in the O-mannosylation pathway as a substrate for DPMS but also acts as a donor of mannosyl residues for the elongation of O-linked sugar chains (27). GDPMan also takes part in N glycosylation directly and via dolichyl phosphate mannose (DPM) and in glycosylphosphatidylinositol anchor formation. Expression of the T. reesei mpg1 gene in the temperature-sensitive mutant, dpm1-6, of S. cerevisiae increased the cellular GDPMan concentration and allowed the mutated DPMS to overcome the temperature-sensitive phenotype (16). Overexpression of the yeast Mpg1p was reported to also suppress the alg1 mutation, which affects the elongation of Dol-PP-GlcNAc2 to Dol-PP-GlcNAc2Man in the endoplasmic reticulum of S. cerevisiae (11). These data suggest the interrelation of the enzymes involved in the protein glycosylation pathways.
In the present study we studied the effects of the overexpression of the T. reesei genes dpm1 and mpg1 encoding DPMS and MPGI, respectively, on the efficiency of glycosylation and protein secretion in T. reesei. Overexpression of mpg1 had a significant effect on the activity of mannosyltransferases involved in the elongation of the sugar chains, as well as on the amount of mannose residues in the secreted proteins of T. reesei.
MATERIALS AND METHODS
Strains and growth conditions.
T. reesei QM9414 (7) was used as a recipient strain for transformation. Escherichia coli JM109 was used for plasmid propagation (29). T. reesei was cultivated at 30°C on a rotary shaker (250 rpm) in 2-liter shake flasks containing 1 liter of minimal medium (MM) composed of 1 g of MgSO4 · 7H2O, 6 g of (NH4)2SO4, 10g KH2PO4, 3 g of sodium citrate · 2H2O, trace elements (25 mg of FeSO4 · 7H2O, 2.7 mg of MnCl2 · 4H2O, 6.2 mg of ZnSO4 · 7H2O, and 14 mg of CaCl2 · 2H2O) per liter, with 1% lactose as a carbon source.
Expression of the dpm1 and mpg1 genes in T. reesei.
To increase the expression levels of the homologous dpm1 and mpg1 genes in T. reesei, the genes were introduced into T. reesei under the Aspergillus nidulans gpdA gene promoter GAPDH (glyceraldehyde-3-phosphate dehydrogenase) and trpC (indole-3-glycerol phosphate synthase) terminator by using pAN52-1NotI plasmid (NCBI accession no. Z32697). The complete coding sequences of the T. reesei dpm1 or mpg1 genes were amplified by PCR by using The Expand High Fidelity PCR System (Boehringer Mannheim). The oligonucleotides Dpm1s (5′-GCC CCT ACA AAG AGC TCC AAT-3′) and Dpm1r (5′-TCA GAC CTT GAG CCA CAG GGA AAA-3′) were used for dpm1 gene amplification. For mpg1 gene amplification, Mpg1s (5′-AAG GGA CTT ATT CTT GTC GGC-3′) and Mpg1r (5′-TCA CAT AAT GAT GGC GGG AAC-3′) were used as the forward and reverse primers, respectively. The pAN521N plasmid was cut between the promoter and the terminator by using BamHI, the sticky ends were blunted with mung bean nuclease (Promega), and the PCR product (dpm1 or mpg1) was ligated to the plasmid. The hygromycin B resistance marker cassette was added to the expression plasmid by sticky-end ligation to the NotI site. The resulting plasmids were used for transformation of T. reesei QM9414 by protoplast transformation (21). Transformants were selected for hygromycin B resistance on MM plates containing hygromycin B at 75 μg/ml. The transformants obtained were then cultivated in liquid MM for DNA preparation.
Molecular biology methods.
Chromosomal DNA was isolated from T. reesei by using the Invitrogen Easy-DNA kit. Total RNA was isolated by using the single-step method described by Chomczynski and Sacchi (1). Other molecular biological techniques were performed according to standard protocols (25).
For Northern analysis, 20 μg of total RNA was loaded onto agarose gels, blotted, and hybridized with the 1-kb XhoI/EcoRI fragment of dpm1 or the 1.1-kb BamHI fragment of mpg1. A control hybridization was performed with a 1.9-kb fragment of the T. reesei act1 (actin-encoding) gene. The radioactive probes were prepared by using [α-32P]dATP and the Amersham Megaprime DNA labeling system according to the standard Amersham protocol. The levels of the dpm1 and mpg1 mRNA were normalized against act1 mRNA. Quantification of the 32P signals was performed by using the ImageQuant program.
Biochemical techniques.
The saccharides bound to the proteins isolated from the T. reesei culture filtrates were assayed by the phenol-sulfuric acid procedure (3). Secreted proteins were precipitated with 2 volumes of ethanol washed twice with 70% ethanol and resuspended in distilled water. The calibration curve was prepared with d-mannose. Protein concentrations were estimated according to the method of Lowry et al. (20).
Determination of carbohydrate content.
Glycoproteins from the T. reesei culture filtrates were precipitated with 2 volumes of ethanol washed twice with 70% ethanol and then air dried.
Total hydrolysis of glycoproteins was done with 2 M trifluoroacetic acid (TFA) at 100°C with preliminary metanolysis with 1.5 M HCl in anhydrous methanol at 85°C for 16 h (30). Neutral sugars and hexosamines were determined in the hydrolysates by high-performance anion-exchange chromatography with pulsed amperometric detection (HPAEC-PAD) by using a Dionex Series 4500i system. Neutral sugars and hexosamines were eluted with 18 mM NaOH at 1 ml/min.
O-linked sugars were liberated by mild alkaline hydrolysis according to Duk et al. (4). Glycoproteins were slot blotted onto Immobilon-P membrane (Millipore). Membranes were washed overnight in water at 4°C and O-linked sugars hydrolyzed and desalted by using GlycoClean H Cartridges (Glyko, Inc.) and determined by using the DX 500 Chromatography System Dionex with a CarboPac Guard column (2 by 50 mm), followed by a Carbo Pac PA 10 analytical column (2 by 250 mm). Sugars were eluted with 0.2 M NaOH. The sugars not sensitive to alkaline hydrolysis were hydrolyzed with TFA and mannose concentration was assayed as described for O-linked mannose.
Enzyme activity assays. (i) MPGI activity.
T. reesei strains were grown at 30°C, harvested, resuspended in 5 ml of 20 mM morpholinepropanesulfonic acid-NaOH (pH 7.8) containing 5 mM MgCl2, and disrupted by vortexing with 0.5-mm glass beads, and the homogenate was then centrifuged at 4,000 × g for 10 min to remove unbroken cells and cell debris. The resulted supernatant was used as an enzyme source. Synthesis of GDPMan was measured as described previously (16) with 10 μM [14C]mannose-1-phosphate produced by hydrolyzing GDP[14C]Man (Amersham) with snake venom phosphodiesterase (ICN) and ca. 100 μg of the cell extract protein. The reaction was carried out at 37°C for 5 and 10 min. The reaction mixture was then applied to a polyethyleneimine-cellulose thin-layer chromatography plates (Merck) and developed in 0.3 M LiCl. Radioactive products were detected by autoradiography and identified by comparison to the mobility of GDP[14C]Man and [14C]Man-1-phosphate. The MPGI activity was quantified by scraping the radioactive spots from the plates and then resuspending them into a BioCare scintillation cocktail, and the radioactivity was measured in a scintillation counter.
The hydrolytic activity of MPGI was measured in the presence of 10 μM GDP[14C]Man and 100 μg of enzyme fraction, with incubation at 37°C for 30 min and 1 h. The reaction products were detected by thin-layer chromatography as described above.
(ii) DPMS activity.
Control and transformed strains of T. reesei were grown at 30°C, harvested, and resuspended in 25 ml of 150 mM Tris-HCl buffer (pH 7.4) containing 15 mM MgCl2 and 9 mM 2-mercaptoethanol. The cells were homogenized in a Beadbeater with 0.5-mm glass beads, and the homogenate was then centrifuged at 4,000 × g for 10 min to remove unbroken cells and cell debris. The supernatant liquid was centrifuged for 1 h at 50,000 × g. DPMS activity was measured in the pelleted membrane fraction by incubation with GDP[14C]Man (specific activity, 288 Ci/mol [Amersham]) and 5 ng of dolichyl phosphate (DolP) according to the method of Kruszewska et al. (14, 15, 18).
(iii) Protein O-mannosyltransferase activity.
Protein O-mannosyltransferase activity was measured by using the membrane fractions as an enzyme source and as an acceptor of mannosyl residues. First, mannosyl residue was transferred from GDP[14C]Man via DPM in the presence of 10 mM MgCl2 for 2 h. To activate the elongation of the O-linked sugar chain, the reaction mixture was supplemented with 10 mM MnCl2 after 1 h, and the reaction was continued for an additional 1 h. Radioactivity was measured in the protein fraction. The elongation of the sugar chain was calculated as a difference between radioactive mannose bound to the protein in the presence of MnCl2 (first mannosyl residue and the elongation) and the mannose transferred in the presence of MgCl2 (only the first mannosyl residue was transferred) (13, 26).
Immunodetection of DPMS.
Total membrane fraction use for activity assay was also examined for the concentration of the DPMI protein by immunostaining with monoclonal antibody to S. cerevisiae Dpm1p (Molecular Probes, Eugene, Oreg.). Membrane proteins (200 μg) were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis on a 10% polyacrylamide gel and then transferred to the Immobilon-P membrane (Millipore), and Trichoderma DPMI protein was detected by immunological reaction with S. cerevisiae Dpm1 antibody. Immunoreactive material was detected by using an anti-mouse immunoglobulin G secondary antibody conjugated to alkaline phosphatase (Sigma).
Determination of the intracellular pool of GDPMan.
T. reesei strains were cultivated in MM containing lactose as a carbon source. After 200 h of cultivation mycelia (in duplicate for each strain) were harvested, disrupted with glass beads, and extracted twice with 50 ml of 50% ethanol-10 mM ammonium phosphate (pH 3.5). Recovery of GDPMan was calculated by using radioactive GDPMan as an internal standard. The GDPMan content was determined by HPAEC-PAD by using the Dionex DX 500 system, equipped with the electrochemical detector ED40 and a CarboPac PA1 analytical column (9 by 250 mm). Samples (10 ml) were analyzed by applying a gradient from 0 to 0.25 M sodium acetate in 0.04 M NaOH for 35 min and a flow rate of 1.5 ml/min. The following pulse potentials and durations were used for detection: E1 = 0.40 V (t1 = 400 ms), E2 = 1.0 V (t2 = 200 ms), and E3 = 0.25 V (t3 = 400 ms). GDPMan from yeast (Sigma) was used to calibrate the column, and l-fucose (Sigma) was applied as an internal standard.
Quantification of fungal dry weight.
Fungal dry weight was quantified by filtering culture samples through G1 sintered glass funnels, washing the biomass with a threefold volume of tap water, and drying the samples to constant weight at 110°C.
RESULTS
Construction of T. reesei strains overexpressing the homologous dpm1 or mpg1 genes.
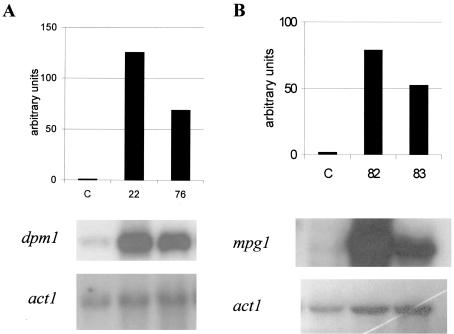
T. reesei dpm1 and mpg1 genes were cloned under the constitutive promoter of the gpdA gene (encoding GAPDH) from A. nidulans (10). The expression constructs were transformed into the T. reesei strain QM9414, and the transformants were selected on the basis of their resistance to hygromycin B. Genomic DNA was isolated from the transformed strains, digested with EcoRI, and analyzed by Southern blotting for the presence of additional copies of the dpm1 or mpg1 gene integrated into the genome. Two dpm1 (JSK00/22 and JSK00/76) and two mpg1 (JSK00/82 and JSK00/83) transformants, carrying additional copies of the corresponding gene, were then analyzed by Northern hybridization for a higher amount of the corresponding transcripts and compared to the parental strain (Fig. 1). The amounts of the dpm1 or mpg1 mRNAs were clearly increased in the respective transformants.
FIG. 1.
Transcription of the dpm1 gene in the dpm1 transformants JSK00/22 (column 22) and JSK00/76 (column 76) (A) and of the mpg1 gene in the mpg1 transformants JSK00/82 (column 82) and JSK00/83 (column 83) (B) compared to the host strain QM9414 (column C) analyzed after 144 h of culture in lactose-based medium. The columns show the signal intensity in arbitrary units after normalization with the actin probing. The signal intensities between panels A and B cannot be compared. Northern hybridizations with the dpm1, mpg1, and act1 probes are shown at the bottom of each panel.
Effects of the overexpression of the dpm1 gene.
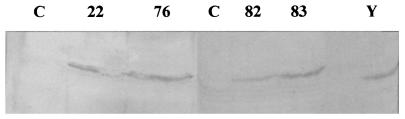
The DPMS enzyme encoded by the dpm1 gene catalyzes the formation of DPM from GDPMan and DolP. To determine whether the increased transcription of the dpm1 gene results in increased DPMS activity in T. reesei, we isolated membrane fractions from the transformants JSK00/22 and JSK00/76 and from the nontransformed host QM9414 as a control, and then we measured DPMS activity. We found no significant increases in the DPMS activity in the transformants (data not shown). Immunodetection of the DPMI protein in the membrane fractions from the two transformants showed a significant increase in the amount of the protein compare to the host strain (Fig. 2). No changes were seen in the growth rates or amounts of secreted protein in the dpm1-overexpressing transformants compared to the host (data not shown).
FIG. 2.
Immunodetection of DPMI protein from dpm1 transformants (JSK00/22 and JSK00/76 [lanes 22 and 76, respectively]) and mpg1 transformants (JSK00/82 and JSK00/83[lanes 82 and 83, respectively]) and the host strain (QM9414 [lane C]) with S. cerevisiae anti-Dpm1 protein antibody. An S. cerevisiae membrane fraction was used as a positive control (yeast [lane Y]).
Effects of the overexpression of the mpg1 gene on the glycosylation pathway activities.
MPGI encoded by the mpg1 gene is responsible for GDPMan production in the cell. The two mpg1 transformants with 50-fold (JSK00/83)- and 80-fold (JSK00/82)-increased mpg1 transcript levels were examined for GDPMan levels, DPMS, and mannosyl transferase activities.
The GDPMan concentrations measured in the cell extracts were up to 137% higher in the transformant JSK00/82 and 18% higher in JSK00/83 compared to the control strain (Table 1). Guanyltransferase ability to synthesize and hydrolyze GDPMan was also measured for the transformants and control strain in vitro in cell extracts. The results showed higher synthesis of GDPMan for both transformants compared to the control strain (Table 2). Overexpression of mpg1 gene also strengthen the hydrolytic activity of guanyltransferase measured in vitro (Table 3). Elevated amounts of GDPMan could be utilized by DPMS or by the sugar chain elongating mannosyltransferases in the protein O-glycosylation pathway. Consequently, the membrane fractions of the two mpg1 transformants and the control strain were isolated, and the DPMS activities were measured. The DPMS activities (means ± standard deviations) obtained from five parallel cultures cultures grown ca. 200 h on lactose were 71 ± 4, 92 ± 5, and 81 ± 3 pmol/mg of protein/5 min for strains QM914, JSK00/82, and JSK00/83, respectively. This analysis showed moderate increases in the DPMS activity: a 30% increase in the transformant JSK00/82 and a 14% increase in JSK00/83. Western blotting analysis of the DPMI protein showed detectable amounts in the membrane fractions of the JSK00/82 and JSK00/83 stains.
TABLE 1.
GDPMan levels in transformants JSK00/82 and JSK00/83 overexpressing mpg1 and in the control host strain QM9414a
| Strain | GDPMan level (μg/g [dry weight] of fungus)
|
|
|---|---|---|
| Expt 1 | Expt 2 | |
| QM9414 | 12 | 15 |
| JSK00/82 | 29 | 35 |
| JSK00/83 | 14 | 18 |
Values from two independent experiments are shown; data were analyzed after 200 h of growth in lactose medium.
TABLE 2.
Synthesis of GDPMan (MPGI activity) measured in cell extracts of transformants JSK00/82 and JSK00/83 overexpressing mpg1 and of the control host strain QM9414a
| Strain | Mean MPGI activity (pmol of GDPMan/mg of protein) ± SD at:
|
|
|---|---|---|
| 5 min | 10 min | |
| QM9414 | 98 ± 11 | 109 ± 10 |
| JSK00/82 | 153 ± 9 | 222 ± 11 |
| JSK00/83 | 121 ± 13 | 199 ± 13 |
Values from three independent experiments are shown; data were analyzed after 200 h of growth in lactose medium. The activity is expressed as picomoles of GDPMan synthesized per 1 mg of protein for 5 or 10 min.
TABLE 3.
Hydrolytic activity of MPGI measured in cell extracts of transformants JSK00/82 and JSK00/83 overexpressing mpg1 and of the control host strain QM9414a
| Strain | Mean % hydrolyzed GDPMan to Man-1-phosphate/100 μg of protein ± SD at:
|
|
|---|---|---|
| 30 min | 1 h | |
| QM9414 | 22 ± 3 | 45 ± 3 |
| JSK00/82 | 30 ± 1 | 66 ± 4 |
| JSK00/83 | 35 ± 2 | 67 ± 2 |
Values from three independent experiments are shown; data were analyzed after 200 h of growth in lactose medium. The activity is expressed as a percentage of the radioactivity found as Man-1-phosphate.
In O mannosylation, DPM is used as the donor of the first mannosyl residue linked to OH-serine/threonine in the glycosylated protein. The O-linked sugar chain is then elongated by mannosyltransferases by using GDPMan as a substrate (26). The transfer of the first mannosyl residue requires magnesium or manganese cations, but for the elongation reactions manganese is obligatory (9, 26). To analyze these activities in the T. reesei transformants overexpressing mpg1 and to distinguish between the activities of protein mannosyltransferases and the transferases responsible for mannosyl chain elongation, the transferase reaction was carried out in the presence of magnesium for 2 h or in the presence of magnesium for 1 h, and then manganese cations were added to activate the elongation reactions (26). The addition of the first mannosyl residue showed a lower activity in the JSK00/82 transformant and a higher activity in the JSK00/83 transformant than in the parental strain. Clear changes were observed in the activity of the transferases elongating the O-linked sugar chains (Table 4). Their activity was about five times higher in both of the transformants compared to the control.
TABLE 4.
Activity of mannosyltransferases transferring the first mannosyl residue or involved in the chain elongation of O-glycans by the T. reesei host strain QM9414 and the transformants JSK00/82 and JSK00/83 overexpressing mpg1 from cultures grown for 200 h on lactosea
| Strain | Mean mannosyltransferase activity (pmol/mg of protein/2 h) ± SD
|
||
|---|---|---|---|
| Total incorporation (Mg2+, Mn2+) | Transfer of the first mannosyl residue (Mg2+) | Sugar chain elongation | |
| QM9414 | 94 ± 3 | 72 ± 3 | 22 ± 2 |
| JSK00/82 | 172 ± 4 | 56 ± 3 | 116 ± 4 |
| JSK00/83 | 206 ± 4 | 102 ± 4 | 104 ± 3 |
The data are presented as the mean ± the standard deviation from five separate cultures. The data show values for the transfer of mannosyl residues (picomoles) from GDP[14C]Man to milligrams of endogenous protein in the membrane fractions in the presence of either Mg2+ or Mg2+ followed by Mn2+, as well as the total amount of mannose incorporated into the protein fraction.
Secretion and glycosylation of extracellular proteins in the mpg1-overproducing strains.
Our earlier studies have shown a correlation between DPMS activity and protein secretion (14, 17). mpg1 overexpression only slightly influenced the DPMS activity. Protein concentration in the culture supernatants of the transformants were measured at 24-h intervals and then were compared to the protein quantities secreted by the control strain. The growth of both transformants was quantified as dry weight of mycelia and compared to the host strain. There were no changes in the growth rate or protein secretion between the transformants and the parental strain (data not shown). Subsequently, we determined the glycosylation degree of the proteins secreted to the medium (Table 5). Total sugar concentrations (measured by the phenol method and HPLC) were 77 and 48% higher for JSK00/82 and JSK00/83, respectively, compared to the control strain. To answer the question of whether the mpg1 overexpression influences O mannosylation, we liberated the O-linked sugars by mild alkaline hydrolysis, hydrolyzed sugar chains, and determined mannose concentrations. The mannose concentrations were elevated by 71 and 193% for JSK82/00 and JSK83/00, respectively (Table 6). The mannose content in the carbohydrates resistant to mild alkaline treatment, N-glycans, was also increased by 55 and 11%, respectively, for strains JSK00/82 and JSK00/83 (Table 6).
TABLE 5.
Total carbohydrates bound to extracellular secreted proteins in the host strain QM9414 and the mpg1-overexpressing transformants JSK00/82 and JSK00/83 after 200 h of growth in lactose medium
| Strain | Total carbohydrates bound
|
|
|---|---|---|
| Amt (μg of sugar/mg of protein) | % of control | |
| QM9414 | 400 | 100 |
| JSK00/82 | 710 | 177 |
| JSK00/83 | 590 | 148 |
TABLE 6.
Mannose bound to extracellular secreted proteins in the host strain QM9414 and in the mpg1-overexpressing transformants JSK00/82 and JSK00/83a
| Strain | Amt (μg of mannose/mg of protein) of mannose bound
|
|
|---|---|---|
| O linked | N linked | |
| QM9414 | 52; 60 | 190; 208 |
| JSK00/82 | 102; 90 | 312; 306 |
| JSK00/83 | 150; 178 | 221; 223 |
Data are shown from two independent experiments (values are separated by semicolons) from cultures on lactose medium after 200 h of growth. N-linked mannose was released by TFA from proteins after β-elimination of O-linked mannose.
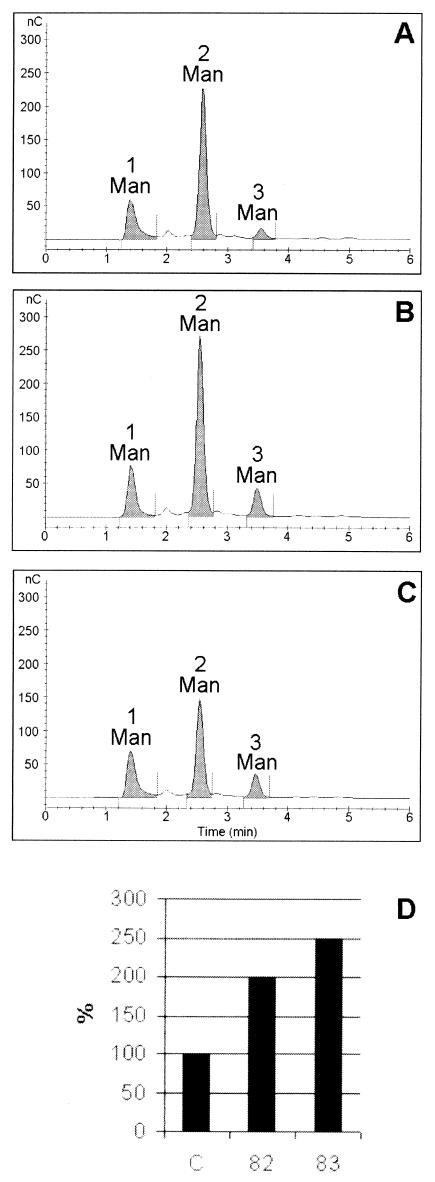
It could be assumed that effect on the degree of O glycosylation caused by the overexpression of mpg1 could be due either to increased elongation of the carbohydrate chains or to glycosylation of additional sites that normally do not carry a carbohydrate chain. To analyze the length of the O-linked sugars, the secreted proteins were applied on Immobilon-P membrane, carbohydrates were released from the proteins by mild alkaline treatment, and the sugars were subjected to HPLC analysis. The analysis revealed in all of the strains the same amount of single mannose residues, a main peak of Man2 and a lower amount of Man3. The proportions of Man2 to Man3 in the parental and the transformed strains were, however, 10/1, 5/1 and 4/1 for the sugars liberated from the proteins of the control strain, strain JSK00/82, and strain JSK00/83, respectively. This indicates that more of the longer, Man3, sugar chain was found on the proteins of the transformants (Fig. 3). These results could confirm the higher activity of mannosyltransferases elongating the sugar chain observed in vitro and indirectly suggest the presence of the higher amount of GDPMan in the cell.
FIG.3.
HPAEC of carbohydrates after β-elimination of O-linked carbohydrates from secreted proteins of the control strain QM9414 (A) and of the mpg1-overexpressing transformants JSK00/82 (B) and JSK00/83 (C). Man, single mannose residue; Man2, disaccharide; Man3, trisaccharide. (D) The relative amounts of Man3 to Man2 in the control strain QM9414 (column C) and in the transformants JSK00/82 (column 82) and JSK00/83 (column 83). The quantity on the y axis is expressed in nanocoulombs.
Expression of the dpm1 gene in strains transformed with the mpg1 gene.
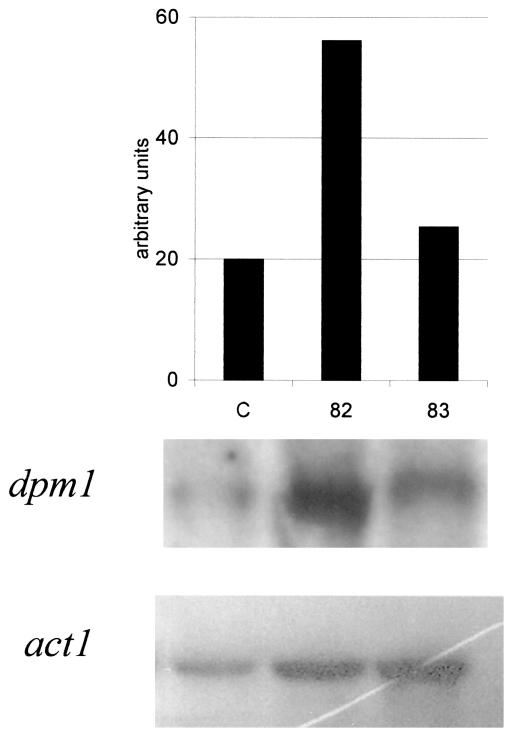
To learn whether the observed changes in DPMS activity and the extent of glycosylation of secreted proteins in the mpg1-overproducing strain were generated at the transcriptional or posttranscriptional level, we carried out Northern blot analyses of the strains JSK00/82, JSK00/83, and the host QM9414 for dpm1 mRNA. The level of the dpm1 transcript was elevated in JSK00/82 nearly threefold relative to the control (Fig. 4). In JSK00/83 the level was only slightly higher than in the control, which could be due to experimental variation. Immunodetection of DPMI protein in membrane fractions of the transformants revealed elevated amounts of the protein in JSK00/82 and JSK00/83 strains compared to the control (Fig. 2). The difference in dpm1 mRNA levels and DPMI protein concentration between the two transformants could come from the expression levels of the transformed mpg1 constructs (Fig. 1B) and the GDPMan levels (Table 1) in these strains.
FIG. 4.
Northern analysis of dpm1 expression in the mpg1-overexpressing transformants JSK00/82 (column 82) and JSK00/83 (column 83), and in the control strain QM9414 (column C). See the legend for Fig. 1 for more information.
DISCUSSION
Our results indicated that the overexpression of the homologous dpm1 gene, encoding DPMS, did not lead to increased DPMS activity in T. reesei, although the DPMI protein concentration increased. This finding contrasts with previous results concerning overexpression of the yeast DPM1 gene, which resulted in a >2-fold-increased DPMS activity in T. reesei (17). Expression of the T. reesei dpm1 gene in Schizosaccharomyces pombe resulted in a >4-fold-increased DPMS activity (18). These results could be explained by the fact that the T. reesei and S. pombe DPMS enzymes appear to belong to the human group of the DPMS proteins (2, 18) unlike the S. cerevisiae enzyme (2). The increased DPMS activity observed in the S. pombe cells expressing T. reesei dpm1 suggests that in this fungus the Dpm2 and Dpm3 subunits could be in excess relative to native Dpm1p and form enzymatic complexes with the T. reesei DPMI subunit (18). In contrast, one may speculate that the shortage of the DPMII and DPMIII subunits in T. reesei would not allow the formation of more of the active DPMS enzyme complex despite of the surplus of the DPMI subunit encoded by the overexpressed dpm1 gene.
Increased glycosylation of secreted proteins was the most spectacular effect observed in T. reesei strains overexpressing mpg1 gene. A higher mannose concentration was observed in both O- and N-glycans. Synthesis of the glycans required intensive production of GDPMan and elevated activity of O- and N-glycosylation pathways.
Overexpression of the mpg1 gene coding for the enzyme synthesizing GDPMan increased the cellular level of the GDPMan in both transformants, but an especially high concentration was found in JSK00/82 strain and only a slightly higher concentration was found in JSK00/83. Higher activity of guanyltransferase in the transformed strains was also confirmed by in vitro studies. Since the GDPMan is an intermediate in the glycosylation processes and in glycosylphosphatidylinositol anchor synthesis, it is more difficult to draw conclusions about its production from the measurement of its concentration than from its effects on the final reactions in protein glycosylation. Similar results for glycosylation were observed for the JSK00/82 and JSK00/83 strains since the GDPMan concentration in the latter strain was only 18% higher. Intensive glycosylation required elevated the activity of enzymes engaged in the processes, i.e., DPMS and mannosyltransferases. DPMS activity was increased by 30% in strain JSK00/82 and slightly (14%) in strain JSK00/83. This is in accordance with our earlier results, which showed that overexpression of the T. reesei mpg1 gene in the dpm1-6 mutant of S. cerevisiae could restore its temperature-sensitive phenotype (16), suggesting that intracellular substrate concentrations play a meaningful role in DPMS activity in vivo. The increased DPMS activities should therefore be caused by increased amounts of the enzyme. The increased DPMS activity was accompanied by an increased dpm1 transcript level, especially in the JSK00/82 mpg1-overexpressing strain, which makes additional synthesis of the DPMI protein possible. Western blotting analysis revealed a higher concentration of DPMI protein in the JSK00/82 and JSK00/83 strains compared to the control strain.
Since a simple increase in dpm1 mRNA and DPMI protein does not ensure an increase in DPMS activity, as demonstrated here, one must speculate that the overexpression of mpg1 resulting in increased dpm1 mRNA also increases expression of the putative equivalents of the human subunits Dpm2p and Dpm3p in T. reesei. It is possible that this upregulation of transcription could be caused by the elevated GDPMan levels, with GDPMan serving as a substrate for the DPMS complex.
GDPMan is also efficiently utilized by mannosyltransferases elongating the O-linked sugar chain. The mannosyltransferase activity measured in vitro was five times higher for both strains regardless of the GDPMan level in the transformant cells. Results observed in vivo, i.e., the amount of mannose linked to the secreted proteins and the longer sugar chains observed in the proteins secreted by the transformants, clearly confirmed the findings of the in vitro studies. Our observations also suggested that the GDPMan is used for the elongation of the O-linked sugars rather than for glycosylation of the normally nonglycosylated serine or threonine residues.
The importance of the O glycosylation for the T. reesei cell is difficult to overestimate. It has been reported that inhibition of the N-glycosylation pathway by tunicamycin has no effect on secretion of cellulases but that O glycosylation would be indispensable for protein secretion in T. reesei (19).
The main cellulase of T. reesei, cellobiohydrolase I (CBHI), is secreted at several grams per liter by many industrial strains, and its glycan structures in several strains have been analyzed (6, 8, 12, 23). CBHI has a multidomain structure, the catalytic domain being separated from the cellulose-binding domain by an O-glycosylated linker (6). In the highly secretory strain ALKO 2877, the glycosylated forms of the linker contain from 14 to 26 hexoses (8). Threonines in the linker are completely glycosylated, with at least one and up to three mannoses per site. In the cultivation conditions shown in that paper the main O-linked sugar found on the secreted proteins contained two mannosyl residues, and three mannoses were observed only rarely.
Since glycosylation of the linker strongly affects the relative orientation of the catalytic and cellulose-binding domains (28), O glycosylation also might play an important role in the catalytic activity of cellulases.
The structure of the N-linked saccharides depends on the fungal strain. CBHI from T. reesei RutC-30 contains mammalian-type high-mannose structures with outer-branch phosphodiester-mannose linkages (23), whereas the CBHI from T. reesei QM9414 was reported to have only single N-acetylglucosamine residues linked to the catalytic domain in the particular culture conditions studied (12). These differences in glycosylation levels and glycan structures found between strains (and culture conditions) are likely to be caused by differences in the activity of the individual components of the glycosylation machinery. Our results suggest that the MPGI activity and the GDPMan levels of the cell would be able to play a significant role in determining these variations in glycosylation of secreted proteins in T. reesei.
Acknowledgments
This work was supported by the State Committee for Scientific Research (KBN), Warsaw, Poland, under project 6P04B00621 (J.S.K.). The financial support of the Centre for Excellence in Molecular Biotechnology is also acknowledged.
REFERENCES
- 1.Chomczynski, P., and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidinum thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156-159. [DOI] [PubMed] [Google Scholar]
- 2.Colussi, P. A., C. H. Taron, J. C. Mach, and P. Orlean. 1997. Human and Saccharomyces cerevisiae dolichol phosphate mannose synthases represent two classes of the enzyme, but both function in Schizosaccharomyces pombe. Proc. Natl. Acad. Sci. USA 94:7873-7878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dubois, M., K. A. Gilles, J. K. Hamilton, P. A. Robers, and F. Smith. 1956. Colorimetric method for determination of sugar and related substrates. Anal. Biochem. 28:350-356. [Google Scholar]
- 4.Duk, M., M. Ugorski, and E. Lisowska. 1997. β-Elimination of O-glycans from glycoproteins transferred to Immobilon P membranes: method and some applications. Anal. Biochem. 253:98-102. [DOI] [PubMed] [Google Scholar]
- 5.Durand, H., M. Clanet, and G. Tiraby. 1988. Genetic improvement of Trichoderma reesei for large scale cellulase production. Enzyme Microbiol. Technol. 10:341-345. [Google Scholar]
- 6.Fagerstam, L. G., L. G. Pettersson, and J. A. Engström. 1984. The primary structure of a β-1,4-glucan cellobiohydrolase from the fungus Trichoderma reesei QM9414. FEBS Lett. 167:309-315. [Google Scholar]
- 7.Harman, G. E., and C. P. Kubicek. 1998. Enzymes, biocontrol and commercial applications, p. 129-163. In E. G. Harman and C. P. Kubicek (ed.), Trichoderma and Glocladium, vol. 2. Taylor and Francis, Ltd., London, United Kingdom.
- 8.Harrison, M. J., A. S. Nouwens, D. R. Jardine, N. E. Zachara, A. A. Gooley, H. Nevalainen, and N. H. Packer. 1998. Modified glycosylation of cellobiohydrolase I from a high cellulase-producing mutant strain of Trichoderma reesei. Eur. J. Biochem. 256:119-127. [DOI] [PubMed] [Google Scholar]
- 9.Haselbeck, A., and W. Tanner. 1983. O glycosylation in Saccharomyces cerevisiae is initiated at the endoplasmic reticulum. FEBS Lett. 158:335-338. [DOI] [PubMed] [Google Scholar]
- 10.Hirano, T., T. Sato, K. Yaegashi, and H. Enei. 2000. Efficient transformation of the edible basidiomycete Lentinus edodes with a vector using a glyceraldehyde-3-phosphate dehydrogenase promoter to hygromycin B resistance. Mol. Gen. Genet. 263:1047-1052. [DOI] [PubMed] [Google Scholar]
- 11.Janik, A., J. Kruszewska, U. Lenart, M. Sosnowska, and G. Palamarczyk. 1999. Overexpression of α-d-mannose-1-phosphate guanyltransferase encoding gene restores the viability of the Saccharomyces cerevisiae mutants affected in early steps of glycoconjugate formation, p. 35-42 In H. J. Gilbert, G. J. Davies, B. Henrissat, and B. Svensson (ed.), Recent advances in carbohydrate bioengineering. Royal Society of Chemistry, Cambridge, United Kingdom.
- 12.Klarskov, K., K. Piens, J. Ståhlberg, P. B. Hoi, J. van Beeumen, and M. Claeyssens. 1997. Cellobiohydrolase I from Trichoderma reesei: identification of an active-site nucleophile and additional information on sequence including glycosylation pattern of the core protein. Carbohydr. Res. 304:143-153. [DOI] [PubMed] [Google Scholar]
- 13.Kruszewska, J., R. Messner, C. P. Kubicek, and G. Palamarczyk. 1989. O glycosylation of proteins by membrane fraction of Trichoderma reesei QM9414. J. Gen. Microbiol. 135:301-307. [Google Scholar]
- 14.Kruszewska, J., G. Palamarczyk, and C. P. Kubicek. 1990. Stimulation of exoprotein secretion by choline and Tween 80 in Trichoderma reesei QM9414 correlates with increased activity of dolichol phosphate mannose synthase. J. Gen. Microbiol. 136:1293-1298. [Google Scholar]
- 15.Kruszewska, J., C. P. Kubicek, and G. Palamarczyk. 1994. Modulation of mannosylphosphodolichol synthase and dolichol kinase activity in Trichoderma reesei related to protein secretion. Acta Biochim. Pol. 41:331-339. [PubMed] [Google Scholar]
- 16.Kruszewska, J. S., M. Saloheimo, M. Penttilä, and G. Palamarczyk. 1998. Isolation of a Trichoderma reesei cDNA encoding GDP-α-d-mannose-1-phosphate guanyltransferase involved in early steps of protein glycosylation. Curr. Genet. 33:445-450. [DOI] [PubMed] [Google Scholar]
- 17.Kruszewska, J., A. H. Butterweck, W. Kurztkowski, A. Migdalski, C. P. Kubicek, and G. Palamarczyk. 1999. Overexpression of the Saccharomyces cerevisiae mannosylphosphodolichol synthase-encoding gene in Trichoderma reesei results in an increased level of protein secretion and abnormal cell ultrastructure. Appl. Environ. Microbiol. 65:2382-2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kruszewska, J. S., M. Saloheimo, A. Migdalski, P. Orlean, M. Penttilä, and G. Palamarczyk. 2000. Dolichol phosphate mannose synthase from the filamentous fungus Trichoderma reesei belongs to the human and Schizosaccharomyces pombe class of the enzyme. Glycobiology 10:983-991. [DOI] [PubMed] [Google Scholar]
- 19.Kubicek, C. P., T. Panda, G. Schreferl-Kunar, R. Messner, and F. Gruber. 1987. O-linked—but not N-linked—glycosylation is necessary for secretion of endoglucanase I and II by Trichoderma reesei. Can. J. Microbiol. 33:698-703. [Google Scholar]
- 20.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 21.Mach, R. L., M. Schindler, and C. P. Kubicek. 1994. Transformation of Trichoderma reesei based on hygromycin B resistance using homologous expression signals. Curr. Genet. 25:567-570. [DOI] [PubMed] [Google Scholar]
- 22.Maeda, Y., S. Tanaka, J. Hino, K. Kangawa, and T. Kinoshita. 2000. Human dolichol-phosphate-mannose synthase consists of three subunits: DPM1, DPM2, and DPM3. EMBO J. 11:2475-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maras, M., A. de Bruyn, J. Schraml, P. Hersewijn, M. Claeyssens, W. Fiers, and R. Contreras. 1997. Structural characterisation of N-linked oligosaccharides from cellobiohydrolase I secreted by filamentous fungus Trichoderma reesei RUTC 30. Eur. J. Biochem. 245:617-625. [DOI] [PubMed] [Google Scholar]
- 24.Messner, R., and C. P. Kubicek. 1988. Intracellular precursors of endo-β-1,4-glucanases in Trichoderma reesei. FEMS Microbiol. Lett. 50:227-232. [Google Scholar]
- 25.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed., vol. 1, p. 7.37-7.52. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 26.Sharma, C. B., P. Babczinski, L. Lehle, and W. Tanner. 1974. The role of dolicholmonophosphate in glycoprotein biosynthesis in Saccharomyces cerevisiae. Eur. J. Biochem. 46:35-41. [DOI] [PubMed] [Google Scholar]
- 27.Strahl-Bolsinger, S., M. Gentzch, and W. Tanner. 1999. Protein O-mannosylation. Biochim. Biophys. Acta. 1426:297-307. [DOI] [PubMed] [Google Scholar]
- 28.Williamson, G., N. J. Belshaw, and M. P. Williamson. 1992. O-glycosylation in Aspergillus glucoamylase: conformation and role in binding. Biochem. J. 282:423-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]
- 30.Zdebska, E., and J. Koscielak. 1999. A single-sample method for determination of carbohydrate and protein contents in glycoprotein bands separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Anal. Biochem. 275:171-179. [DOI] [PubMed] [Google Scholar]