Abstract
Quantitative data on modes of transmission are a crucial element in understanding the ecology of microorganisms associated with animals. We investigated the transmission patterns of a γ-proteobacterium informally known as pea aphid Bemisia-like symbiont (PABS), also known as T-type, which is widely but not universally distributed in natural populations of the pea aphid, Acyrthosiphon pisum. The vertical transmission of PABS to asexual and sexual morphs and sexually produced eggs was demonstrated by a diagnostic PCR-based assay, and the maximum estimated failure rate was 2%. Aphids naturally lacking PABS acquired PABS bacteria administered via the diet, and the infection persisted by vertical transmission for at least three aphid generations. PABS was also detected in two of five aphid honeydew samples tested and in all five siphuncular fluid samples tested but in none of 15 samples of salivary secretions from PABS-positive aphids. However, PABS-negative aphids did not acquire PABS when they were cocultured with PABS-positive aphids; the maximal estimated level of horizontal transmission was 18%. A deterministic model indicated that the force of infection by a horizontal transmission rate of 3% is sufficient to maintain a previously described estimate of the prevalence of PABS-positive aphids (37%), if the vertical transmission rate is 98%. We concluded that PABS infections in A. pisum can be maintained by high vertical transmission rates and occasional horizontal transmission, possibly via the oral route, in the absence of selection either for or against aphids bearing this bacterium.
Individual animals are a transient habitat for populations of biotrophic microorganisms, and only those microbial populations with propagules which disperse to other animals persist beyond the life span of each animal. Consequently, the patterns of transmission are crucial to the ecology of microorganisms associated with animals. Transmission of microorganisms between animal hosts may be vertical (i.e., from a parent animal to its offspring) or horizontal (where the donor and recipient host are not necessarily related) (8, 11). The mode of transmission can be deduced from the following two complementary approaches: ecological, in which the abundance and distribution of microorganisms in different animal tissues, especially the reproductive organs, and the free-living environment are determined (2, 16, 22); and evolutionary, in which the historical incidence of vertical and horizontal transmission is deduced from the level of congruence of the phylogenies of the animal and microorganisms (14, 21, 23). The ecological approach offers accurate information on the prevalence of horizontal and vertical transmission and underlying processes, while the evolutionary approach can identify historical events too rare to be identified by the ecological methods.
Many of the microorganisms associated with animals are commensals that have less than universal prevalence; i.e., they have no detectable significance to the animal host and are borne by some, but not all, members of the host population. The prevalence of these microorganisms is expected to be determined by their transmission dynamics, but despite increasing research interest in commensal microorganisms (1, 12, 17), their transmission patterns are poorly known.
In this study we used an insect-borne bacterium, a γ-proteobacterium known informally as pea aphid Bemisia-like symbiont (PABS) or T-type (5, 25), which has been found in a number of aphid species by PCR-based techniques (25) but has not been brought into culture. Detailed analysis of PABS in one aphid species, Acyrthosiphon pisum, revealed (i) its presence in 37 to 90% of the individuals, with the prevalence varying in different populations sampled, and (ii) no detectable difference between the fitness of aphids bearing the bacterium and the fitness of aphids lacking the bacterium under field conditions (5, 7). PABS is one of a small number of bacteria borne by aphids and known as accessory bacteria or secondary symbionts and are distinguished from the primary symbiont Buchnera sp. by two features: they are generally not universally prevalent, and they are not required by the insect for normal growth and reproduction (9). PABS and other accessory bacteria are located in the body cavity of the insect host, in both cells and hemolymph (insect blood) (3, 13, 25). Gut tissue dissected from A. pisum is also positive for PABS as determined by a taxon-specific PCR assay (5).
The vertical transmission of accessory bacteria from mother to offspring via the ovaries has been described previously (2), and recent phylogenetic data suggest that these bacteria are also available for horizontal transmission (5, 25). The purposes of this study were (i) to quantify the incidence of vertical and horizontal transmission of PABS in A. pisum and (ii) to test the hypothesis that the incidence of transmission can account for the observed prevalence of PABS in natural populations of A. pisum.
MATERIALS AND METHODS
The aphids listed in Table 1 are cyclical parthenogens with multiple asexual generations in the summer months, followed by a single sexual generation that produces the overwintering eggs. They were maintained as parthenogenetic lines on Vicia faba cv. The Sutton at 18°C with a daily cycle consisting of 18 h of light and 6 h of darkness and were also raised on an aseptic chemically defined diet, formulation A (24) with 0.15 M amino acids and 0.5 M sucrose.
TABLE 1.
Aphids and their accessory bacteria
| Aphid clone | Sourcea | Accessory bacteriab |
|---|---|---|
| Acyrthosiphon pisum clones | ||
| UY2 | Pisum sativum, September 1993, University of York | PASS, PAR, PABS |
| LMB95/28 | Trifolium pratense, July 1995, University of York | PABS, Spiroplasma |
| JF98/24 | Vicia sp., June 1998, Silwood Park, Berkshire | PAUS |
| ACD01/04 | Trifolium pratense, July 2001, University of York | None |
| Aphis fabae HR 91/3 | Vicia faba, June 1991, Abingdon, Oxon | PAUS |
All locations are in the United Kingdom.
Accessory bacteria as determined by diagnostic PCR assays and by terminal restriction fragment length polymorphism and denaturing gradient gel electrophoresis analyses of 16S rRNA gene fragments (6).
The incidence of PABS in the samples was determined by a specific nested PCR assay (5). DNA was extracted with a DNeasy tissue kit (Qiagen, Crawley, United Kingdom) by following manufacturer's instructions. For the PCR assays we used the cycling conditions and primers described previously (5). For the first round, the general bacterial 16S ribosomal DNA primers used were 5′-GCT TAA CAC ATG CAA G-3′ and 5′-ACG GGC AGT GTG TAC AAG ACC-3′, corresponding to nucleotide positions 41 to 61 forward and 1405 to 1385 reverse in Escherichia coli; and for the second round, the specific forward primer was 5′-AGC GCA GTT TAC TGA GTT CA-3′. PABS was identified as a single band at ca. 1,330 bp after electrophoresis on 2% agarose gels, staining with ethidium bromide, and visualization under UV illumination. All gels included as molecular weight markers the 100-bp DNA ladder (Promega, Hemel Hempstead, United Kingdom), comprising 100- to 1,000-bp markers in 100- bp increments and one fragment at 1,500 bp; all PCR assays also included a negative control consisting of distilled water and a positive control consisting of aphid DNA template known to contain PABS.
The aphids used to test for vertical transmission of PABS were 20 parthenogenetic adults of lines UY2, LMB95/28, and JF98/24 and one live offspring of each aphid, collected as it was born onto a sterile DNA-free pipette tip; 20 sexual females (oviparae) of lines UY2 and LMB95/28; 10 sexual males of lines UY2 and LMB95/28; and 20 fertile sexual eggs of line LMB95/28. The sexual morphs were obtained by culturing the plant-reared aphids first for 3 days with 18 h of light and 6 h of darkness with a daily 1°C reduction in temperature to 13°C and then at 13°C with a daily 2-min reduction in photoperiod. The first sexual morphs were seen in culture at approximately day 50 (12.2 h of light and 11.8 h of darkness).
Three types of samples were used to explore the release of PABS from aphids. In the first type, five replicate groups of five aphids were allowed to feed on the undersides of plant leaves, and the honeydew deposited over 2 days onto a strip of tinfoil positioned below each leaf was collected in 25 μl of sterile distilled water for DNA analysis; collection from tinfoil below an aphid-free leaf was used as a negative control. The second type of samples comprised the fluid exudates from siphunculi of five aphids, produced when an aphid was agitated with the hairs of a paintbrush and collected in a microcapillary tube. Finally, groups of five aphids were fed on diet sachets for 7 days, and diet droplets were then aseptically removed from the sachets with a sterile microsyringe; droplets collected from sachets incubated without aphids were used as a negative control.
Two experimental designs were used to test for acquisition of PABS via feeding. In the first design bacteria were isolated from A. pisum line LMB95/28 by the method described by Harrison et al. (15) and were aseptically added to the diets at a density of approximately 105 cells ml−1. Aphids of A. pisum line JF98/24 and Aphis fabae line HR91/3 were independently fed bacterium-supplemented diets, and aphids fed bacterium-free diets were used as controls. After 7 days, 10 test and control aphids were scored for PABS; five aphids from each treatment were transferred to plants and raised for three parthenogenetic generations, and then 10 aphids were tested for PABS. In the second design, three replicate cultures of the PABS-negative A. pisum ACD01/04 and A. fabae HR91/3 lines were each cultured in isolation or with the PABS-positive A. pisum LMB95/28 line (i.e., a total of 24 cages) on V. faba for three parthenogenetic generations. The different aphid lines were readily distinguished by color (line ACD01/04 is green, LMB95/28 is pink, and HR91/3 is black). Five aphids of each clone from each cage (i.e., 80 samples) were tested for PABS at the start and end of the experiment.
Maximum and minimum transmission rates of PABS were calculated by assuming a binomial distribution (P = RN and P = 1 − RN, respectively, where P is the critical level of significance [0.05], R is the rate of transmission, and N is the sample size) (10). In the subsequent analysis we quantified the level of horizontal transmission required to maintain the frequency of PABS-positive aphids at 37%, as reported previously (5) for a natural population of A. pisum, for different levels of vertical transmission. The equation dI/dt = (βSI/N) − DI was used to estimate the transmission coefficient (β) required to maintain no change in the frequency of an infected host (i.e., dI/dt = 0), where I and S are the proportions of insects infected and not infected with PABS, respectively, N is the sample size (taken as 100 individuals), and D is the loss of infected hosts from the population through failed vertical transmission. All PABS-negative aphids were assumed to be susceptible (i.e., S = 1 − I). Then, β was used to calculate the force of transmission (λ) required to maintain no change in the frequency of PABS-positive aphids by using the equation λ = βI/N.
RESULTS
Vertical transmission of PABS.
Among the parthenogenetic aphids, every mother and offspring of A. pisum lines UY2 and LMB95/28 bore PABS, and every individual of line JF98/24 was PABS-free. All the oviparae (sexual females) and males of lines UY2 and LMB95/28 and all the fertile sexual eggs of line LMB95/28 tested were also positive for PABS. When the binomial distribution was applied, the minimum transmission rates were calculated to be 74% for each sample of male aphids (n = 10) and 86% for each sample of parthenogenetic aphids, oviparae, and eggs (n = 20). Combining the data for all samples across the data (for UY2, n = 50; for LMB95/28, n = 70), a minimum overall transmission rate of 98% was obtained.
Over the course of this study, the routine parthenogenetic cultures of A. pisum lines UY2, LMB95/28, and JF98/24 were monitored for the presence of PABS over an estimated 114, 297, and 111 parthenogenetic generations. Lines UY2 and LMB95/28 were stably PABS positive, and JF98/24 was PABS negative throughout this period.
Horizontal transmission of PABS.
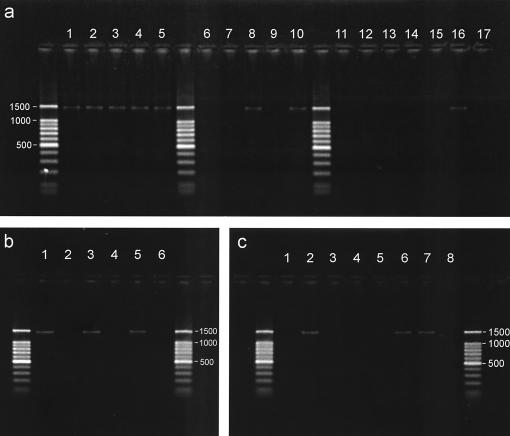
PABS was detected in the siphuncular fluid samples collected from all five aphids tested and in two (40%) of the five honeydew samples but in none of the diet sachets from which PABS-positive aphids had fed (Fig. 1a). The diet sachets probed by the aphids (but not the aphid-free sachets) did, however, become contaminated by bacteria other than PABS, as indicated by a product in the first round of the nested PCR assay, but the source and identity of the bacteria were not investigated further in this study. As determined by the binomial distribution, the minimal occurrence of PABS in siphuncular fluid was 55% (n = 5), and the maximum occurrence of release of PABS into the food substrate during feeding was 18% (n = 15).
FIG. 1.
Incidence of horizontal transmission of PABS. (a) Release of PABS from A. pisum line LMB95/28 via siphuncular fluid (lanes 1 to 5), honeydew (lanes 6 to 10), and feeding on diet sachets (lanes 11 to 15). Lane 16 contained a PABS-positive control, and lane 17 contained a negative control. (b) Acquisition of PABS by A. pisum line JF98/24 (lanes 1 and 2) and A. fabae line HR91/3 (lanes 3 and 4) from diet sachets that either bore PABS (lanes 1 and 3) or were microbiologically sterile (lanes 2 and 4). After feeding on the test diets, aphids were reared on plants for three generations prior to analysis. Lane 5 contained a PABS-positive control, and lane 6 contained a negative control. (c) PABS status of A. pisum lines ACD01/04 and LMB95/28 in coculture. ACD01/04 (lane 1) and LMB95/28 (lane 2) were examined at the start of the experiment; one of five replicate aphids of line ACD01/04 from each cage (lanes 3 to 5) and of LMB95/28 from one cage (lane 6) were examined at the end of the experiment. Lane 7 contained a PABS-positive control, and lane 8 contained a negative control.
When aphids of the PABS-negative lines A. pisum JF98/24 and A. fabae HR91/3 were fed on diets bearing bacteria from A. pisum clone LMB95/28, they were positive for PABS, and the third-generation descendants of these aphids maintained on plants were also PABS positive. The cultures of clones JF98/24 and HR91/3 derived from aphids fed bacterium-free diets were PABS negative (Fig. 1b).
In the final experiment, groups of the PABS-negative lines A. pisum ACD01/04 and A. fabae HR91/3 were cultured with the PABS-positive A. pisum line LMB95/28 on V. faba plants for three parthenogenetic generations. At the start and at the end of the experiment, all of the aphids of line LMB95/28 tested were PABS positive, and none of the aphids of lines ACD01/04 and HR91/3 bore PABS. Representative data for the ACD01/04 line are shown in Fig. 1c.
Modeled impact of transmission patterns on frequency of PABS in aphid populations.
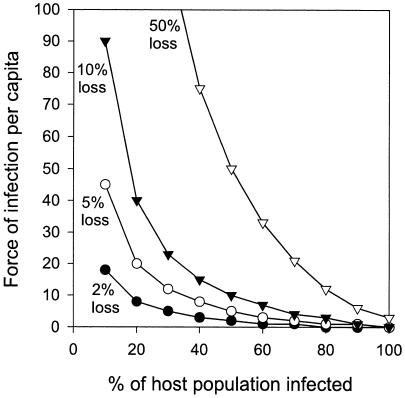
The deterministic model was used to calculate the per capita force of infection required to maintain a stable frequency of PABS-positive aphids (19). For a calculated maximum rate of failure of vertical transmission of 2% (see above) and a frequency of PABS-positive aphids in natural populations of 37% (5), the force of infection was 3% (Fig. 2). Figure 2 also shows that when the rate of failure of vertical transmission was elevated, the force of infection required to maintain a stable frequency of PABS in the aphid population increased dramatically at low PABS frequencies but that high PABS frequencies could be maintained by a low force of infection even with 50% loss via vertical transmission.
FIG. 2.
Force of infection in a frequency-dependent model required to maintain the proportion of PABS-positive aphids in a population with 2 to 50% loss through failure of vertical transmission.
DISCUSSION
The central question addressed in this study is whether the observed prevalence of PABS in natural aphid populations can be accounted for by the transmission patterns in the absence of selection either for or against aphids bearing PABS. Under these conditions, PABS can be described as a commensal that can exploit the aphid habitat without having an impact on aphid fitness.
For the observed high fidelity of vertical transmission (>98%) in A. pisum, for the model output shown in Fig. 2 low levels of horizontal transmission (e.g., 3%) were required to maintain the 37% prevalence of PABS reported previously (5), and even lower transmission levels were required for the higher prevalence of PABS in some A. pisum populations (7). The question, therefore, is whether a 3% force of infection is realistic for the aphid-PABS system. The estimated incidence of horizontal transmission of <18%, as obtained from coculturing aphid lines containing and lacking PABS, is compatible with the expectation of the model output (if the estimated values for horizontal transmission had been appreciably higher than 3%, the data would have been indicative of selection against PABS-positive aphids); and the experiments in which we examined the release and acquisition of PABS by the oral route suggested that PABS can be transmitted horizontally. Siphuncular fluid, which consistently bore PABS in our experiments, comprises modified hemolymph, and access of hemolymph-borne PABS to this material is not restricted by any anatomical barriers. The presence of PABS in honeydew and the stable vertical transmission via the insect ovaries of PABS acquired from ingested food together indicate that PABS can readily breach the aphid gut wall in both directions (i.e., to and from the gut lumen). PABS has previously been detected associated with dissected guts of A. pisum (6), and the site of transfer across the gut wall is an important issue for future research. Many other microbial taxa are known to be acquired by insects via the gut (18).
The low predicted rate of horizontal transmission between cocultured aphids (<18%), when combined with the estimated incidence of release (>55%) and assured acquisition of PABS, suggests that unidentified factors may restrict the incidence of horizontal transmission. Perhaps the populations of PABS released from aphids may have low infectivity or low viability, either at the time of release or because of inhospitable conditions on the plant surface (27). Also, aphids which have specialized mouth parts for feeding on plant sap may rarely ingest material from the plant surface. These issues may not be specific to PABS because an accessory bacterium known as PASS (R-type) also is not transmitted at a high frequency by coculture of PASS-positive and PASS-negative aphids (4).
In summary, our findings suggest that PABS can be maintained at the frequency observed in aphid populations by a combination of vertical transmission with high fidelity and occasional horizontal transmission. A priority for future research is to obtain more precise quantitative data for vertical and horizontal transmission rates by using increased sample sizes. Environmental factors, especially temperature and rearing plant, may affect horizontal transmission by influencing the density or characteristics of released PABS cells, the proximity between aphids, and aphid probing behavior, as well as the persistence and vertical transmission of the bacterial cells in the insect tissues. In addition, horizontal transmission of these bacterial through aborted attack by a parasitoid whose ovipositor is contaminated with bacteria from a previous aphid victim has been suggested (5, 25), but this has not been investigated experimentally.
The conclusions of this study are fully compatible with the finding that aphids from natural populations containing and lacking PABS did not differ significantly in terms of fitness under field conditions on the host plant V. faba (7). However, these conclusions apply strictly to aphids on V. faba in the absence of natural enemies under summer conditions in the United Kingdom The possibility that the prevalence of PABS-positive aphids under different conditions may be influenced by selective factors has been raised by several studies in which possession of accessory bacteria other than PABS has been correlated with plant affiliation and temperature (4, 20, 26). A priority for future research is to elucidate the contribution of transmission and selective factors to the observed prevalence of accessory bacteria in their animal hosts across different biotic and abiotic regimens.
Acknowledgments
We thank Julia Ferrari (Imperial College at Silwood Park) for providing aphid line JF98/24.
Grant GST/02/1842 from the Natural Environment Research Council, United Kingdom, and a MAFF studentship to A.C.D. provided financial support.
REFERENCES
- 1.Boman, H. G. 2000. Innate immunity and the normal microflora. Immunol. Rev. 173:5-16. [DOI] [PubMed] [Google Scholar]
- 2.Buchner, P. 1966. Endosymbiosis of animals with plant microorganisms. John Wiley & Sons, New York, N.Y.
- 3.Chen, D.-Q., and A. H. Purcell. 1997. Occurrence and transmission of facultative endosymbionts in aphids. Curr. Microbiol. 34:220-225. [DOI] [PubMed] [Google Scholar]
- 4.Chen, D.-Q., C. B. Montllor, and A. H. Purcell. 2000. Fitness effects of two facultative endosymbiotic bacteria on the pea aphid, Acyrthosiphon pisum, and the blue alfalfa aphid, A. kondoi. Entomol. Exp. Appl. 95:315-323. [Google Scholar]
- 5.Darby, A. C., L. M. Birkle, S. L. Turner, and A. E. Douglas. 2001. An aphid-borne bacterium allied to the secondary symbionts of whitefly. FEMS Microbiol. Ecol. 36:43-50. [DOI] [PubMed] [Google Scholar]
- 6.Darby, A. C. 2002. The microbiota of the pea aphid, Acyrthosiphon pisum. Ph. D. thesis. University of York, York, United Kingdom.
- 7.Darby, A. C., C. R. Tosh, K. F. A. Walters, and A. E. Douglas. 2003.. The significance of a facultative bacterium to natural populations of the pea aphid Acyrthosiphon pisum. Ecol. Entomol. 28:145-150. [Google Scholar]
- 8.Douglas, A. E. 1994. Symbiotic interactions. Oxford University Press, Oxford, United Kingdom.
- 9.Douglas, A. E., A. C. Darby, L. M. Birkle, and K. F. A. Walters. 2003.. The ecological significance of symbiotic microorganisms in animals—perspectives from the microbiota of aphids, p. 306-325. In R. M. Hails, J. Berringer, and H. C. J. Godfray (ed.), Genes in the environment. Blackwell Scientific Publishers, Oxford, United Kingdom.
- 10.Ebbert, M. A. 1991. The interaction phenotype in the Drosophila willistoni-Spiroplasma symbiosis. Evolution 45:971-988. [DOI] [PubMed] [Google Scholar]
- 11.Ewald, P. W. 1994. Evolution of infectious disease. Oxford University Press, New York, N.Y.
- 12.Falk, P. G., L. V. Hooper, T. Midtvedt, and J. I. Gordon. 1998. Creating and maintaining the gastrointestinal ecosystem: what we know and need to know about gnotobiology. Microbiol. Mol. Biol. Rev. 62:1157-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukatsu, T., N. Nikoh, R. Kawai, and R. Koga. 2000. The secondary endosymbiotic bacterium of the pea aphid Acyrthosiphon pisum (Insecta: Homoptera). Appl. Environ. Microbiol. 66:2748-2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hafner, M. S., and R. D. M. Page. 1995. Molecular phylogenies and host-parasite cospeciation—gophers and lice as a model system. Philos. Trans. R. Soc. London B 349:77-83. [DOI] [PubMed] [Google Scholar]
- 15.Harrison, C. P., A. E. Douglas, and A. F. G. Dixon. l989. A rapid method to isolate symbiotic bacteria from aphids. J. Invertebr. Pathol. 53:427-428.
- 16.Harrison, P. L., and C. C. Wallace. 1990. Reproduction, dispersal and recruitment of scleractinian corals, p. 133-207. In Z. Dubinsky (ed.), Coral reefs. Elsevier, Amsterdam, The Netherlands.
- 17.Hooper, L. V., and J. I. Gordon. 2001. Commensal host-bacterial relationships in the gut. Science 292:1115-1118. [DOI] [PubMed] [Google Scholar]
- 18.Kaslow, D. C., and S. Welburn. 1996. Insect-transmitted pathogens in the insect midgut, p. 434-463. In M. J. Lehane and P. F. Billingsley (ed.), Biology of the insect midgut. Chapman & Hall, London, United Kingdom.
- 19.McCallum, H., N. Barlow, and J. Hone. 2001. How should pathogen transmission be modelled? Trends Ecol. E. 16:295-300. [DOI] [PubMed] [Google Scholar]
- 20.Montllor, C. B., A. Maxmen, and A. H. Purcell. 2002. Facultative bacterial endosymbionts benefit pea aphids (Acyrthosiphon pisum) under heat stress. Ecol. Entomol. 27:189-195. [Google Scholar]
- 21.Moran, N., and P. Baumann. 1994. Phylogenetics of cytoplasmically inherited microorganisms of arthropods. Trends Ecol. E. 9:15-20. [DOI] [PubMed] [Google Scholar]
- 22.Nyholm, S. V., E. V. Stabb, E. G. Ruby, and M. J. McFall-Ngai. 2000. Establishment of an animal-bacterial association: recruiting symbiotic vibrios from the environment. Proc. Natl. Acad. Sci. USA 97:10231-10235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paterson, A. M., and J. Banks. 2001. Analytical approaches to measuring cospeciation of hosts and parasites: through a glass darkly. J. Parasitol. 31:1012-1022. [DOI] [PubMed] [Google Scholar]
- 24.Prosser, W. A., and A. E. Douglas. 1992. A test of the hypotheses that nitrogen is upgraded and recycled in an aphid (Acyrthosiphon pisum) symbiosis. J. Insect Physiol. 38:93-99. [Google Scholar]
- 25.Sandström, J. P., J. A. Russell, J. P. White, and N. A. Moran. 2001. Independent origins and horizontal transfer of bacterial symbionts of aphids. Mol. Ecol. 10:217-228. [DOI] [PubMed] [Google Scholar]
- 26.Tsuchida, T., R. Koga, H. Shibao, T. Matsumoto, and T. Fukatsu. 2002. Diversity and geographic distribution of secondary endosymbiotic bacteria in natural populations on the pea aphid, Acyrthosiphon pisum. Mol. Ecol. 11:2123-2135. [DOI] [PubMed] [Google Scholar]
- 27.Yang, C. H., D. E. Crowley, J. Borneman, and N. T. Keen. 2001. Microbial phyllosphere populations are more complex than previously realized. Proc. Natl. Acad. Sci. USA 98:3889-3894. [DOI] [PMC free article] [PubMed] [Google Scholar]