Abstract
Dunes Creek, a small Lake Michigan coastal stream that drains sandy aquifers and wetlands of Indiana Dunes, has chronically elevated Escherichia coli levels along the bathing beach near its outfall. This study sought to understand the sources of E. coli in Dunes Creek's central branch. A systematic survey of random and fixed sampling points of water and sediment was conducted over 3 years. E. coli concentrations in Dunes Creek and beach water were significantly correlated. Weekly monitoring at 14 stations during 1999 and 2000 indicated chronic loading of E. coli throughout the stream. Significant correlations between E. coli numbers in stream water and stream sediment, submerged sediment and margin, and margin and 1 m from shore were found. Median E. coli counts were highest in stream sediments, followed by bank sediments, sediments along spring margins, stream water, and isolated pools; in forest soils, E. coli counts were more variable and relatively lower. Sediment moisture was significantly correlated with E. coli counts. Direct fecal input inadequately explains the widespread and consistent occurrence of E. coli in the Dunes Creek watershed; long-term survival or multiplication or both seem likely. The authors conclude that (i) E. coli is ubiquitous and persistent throughout the Dunes Creek basin, (ii) E. coli occurrence and distribution in riparian sediments help account for the continuous loading of the bacteria in Dunes Creek, and (iii) ditching of the stream, increased drainage, and subsequent loss of wetlands may account for the chronically high E. coli levels observed.
Fecal coliforms, particularly Escherichia coli, are considered a reliable indicator of recreational water quality (26). The U.S. Environmental Protection Agency recommends a maximum geometric mean of 126 CFU of E. coli/100 ml from five samples over a 30-day period for swimming waters; the single-sample criterion is set at 235 CFU/100 ml. Regulatory and management agencies generally assume that the source of E. coli is humans and warm-blooded animals. However, it has become increasingly clear that E. coli and other fecal bacterial indicators (e.g., enterococci) can persist and perhaps replicate in soil and water and on plants under certain tropical and even temperate conditions (2, 4, 10, 14, 17, 19, 24). Nonpoint sources generated within the watershed are generally ignored in most sanitary surveys because there is limited acceptance of the existence of significant background reservoirs of E. coli (15).
It has become clear that E. coli and enterococci are ubiquitous and can persist for long periods of time in tropical and subtropical soils and water (10, 11, 13, 14, 24). Circumstantial evidence supports replication of indicator bacteria under certain conditions (2, 6, 7, 12, 24). Aside from that by Whitman et al. (28), few comparable studies addressing the persistence of E. coli in temperate or boreal lake watersheds have been conducted, and even fewer studies address comparable riparian situations. Most studies of E. coli sources within streams focus on exogenous influences (e.g., sewage, feedlot operations, and wildlife) since the assumption is that the streams are intrinsically unpolluted.
Indiana Dunes State Park beach, which is located along the shore of southern Lake Michigan, is a popular recreational area that attracts thousands of visitors annually. High levels of E. coli and consequent swimming advisories have been a chronic problem at the park. Concerns over these closures have prompted local managers and regulatory officials to seek the sources of E. coli. The immediate source of this bacterium is undoubtedly Dunes Creek, which empties directly onto the park's only swimming beach. Dunes Creek is one of three perennial streams draining and connecting the Great Marsh system and Lake Michigan. The Great Marsh is a linear wetland depression formed about 6,300 years ago (25). It was formerly lacustrine but has now become a mosaic of marsh, pond, and swamp wetlands. Dunes Creek is the only stream system within the state park, but a minor portion of it also drains rural and suburban lands. Sister streams, Derby and Kintzele Ditches, have also been implicated in the pollution of the respective neighboring beaches. Earlier studies suggested similar E. coli longitudinal distributions, loadings, and seasonality in these streams (24a, 27). There is no direct evidence for point source contamination of these creeks, and previous studies on Derby Ditch and Dunes Creek suggested that bacterial loadings into these creeks were mostly derived from unidentified non-point source contamination and that there was no significant human fecal input (27). These earlier studies provided circumstantial evidence suggesting that the persistent and increased concentrations of E. coli in the creeks were perhaps due to runoff from soil adjacent to the creeks and/or resuspension of sediment-borne bacteria.
In this study, we characterize the persistence and distribution of E. coli within a small northern Indiana sandy stream. We explore the possibility that the ubiquitous occurrence of this bacterium within the Dunes Creek watershed serves as a continual source and reservoir, which compromises its use as a reliable indicator of human and animal waste. The specific objectives of this study were to (i) confirm that Dunes Creek significantly increases E. coli concentrations in the state park's beach water, (ii) describe the spatial distribution of E. coli in Dunes Creek and related watersheds, and (iii) determine whether riparian sediment and soil are potential sources of E. coli loading to the creek.
MATERIALS AND METHODS
Site description.
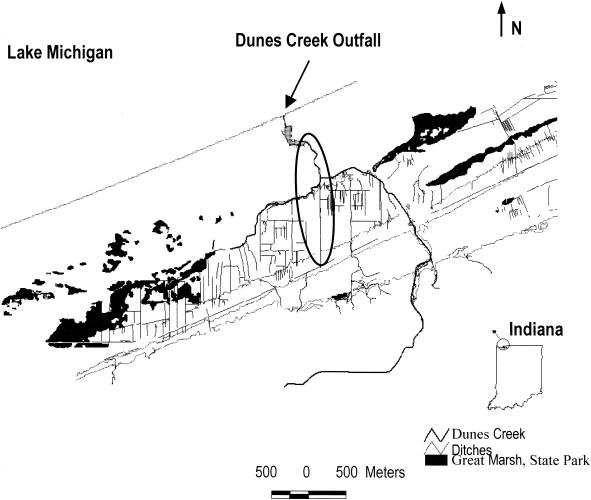
Most of the work occurred within the watershed of Dunes Creek's central branch (Fig. 1). The creek, which has a drainage area of 8.8 km2, is a natural stream that drains wetlands and seeps, but it has been ditched extensively to facilitate drainage. Over 90% of the watershed is natural area, with approximately one-third of this classified as aquatic or wetland habitat. The majority of the remaining land use is residential (6.5%) and agricultural (2.1%). Creek water is slightly brown (platinum-cobalt units, 142.4), circumneutral (pH 7.6), and moderately hard (CaCO3, 163.6 mg/liter) (Stewart et al., Ecological assessment of the three creeks draining the Great Marsh at Indiana Dunes National Lakeshore). The riparian zone around the sampling sites is dominated by white oak, although suburban development, agricultural lands, and train and highway corridors are located upstream. Study area soils are mostly sandy, with abundant deciduous litter and fine detritus. Riffles and margins are mostly clean to detritus-laden sands interspersed with slack areas and pools with silts grading to deeper sands. During the course of the riparian study, the stream depth ranged from 3 to 10 cm upstream and 10 to 20 cm downstream; stream width ranged from <1 m upstream to 2 to 3.5 m downstream. Eastern and western branches of the creek converge with the central branch at the park's campground, and then the creek flows freely for about 250 m before entering a culvert about 400 m long that delivers the waters to a swimming beach on Lake Michigan. Of the three main branches of Dunes Creek, the central branch is the shortest and least culturally developed, although historical maps suggest that it was created by ditching during the early 20th century.
FIG. 1.
Study site: Dunes Creek and its major branches. Roads are not shown for clarity. The encircled area is where most of the intensive sampling occurred. Thin lines indicate extensive ditching of the wetlands in the watershed.
Definition of sediment and soil samples.
In this study, various types of substrates collected from Dunes Creek and other similar watersheds were analyzed for E. coli. In this article, we distinguish two substrate types: (i) sediments, which are essentially sands with some fines that are poorly differentiated and structured material, mostly lying within the stream and along its banks (approximately 3 to 5 m from the middle of the creek), and (ii) soils, which in this case are mostly sandy (>95%), litter-covered, well-drained, and low-nutrient substrates that lie beyond the annual common flood zone.
Monitoring at beach outfall.
Between April and November 1999 and 2000, E. coli concentration was monitored weekly just east and west of the creek's outfall into Lake Michigan. Samples were analyzed for E. coli by the membrane filtration technique (1). Sampling and testing at beach locations were repeated whenever E. coli counts exceeded Environmental Protection Agency-recommended criteria for bathing waters. Samples were collected in knee-deep (45-cm) water in the morning, held at 4°C, and analyzed within 4 h of collection.
Weekly stream monitoring.
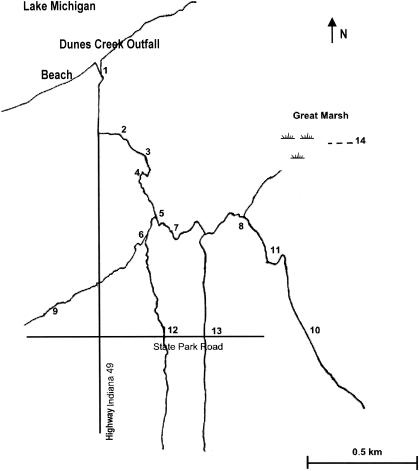
Weekly water samples were taken from 14 sites along Dunes Creek (Fig. 2) from June to November 1999 and from February to November 2000, for a total of 710 samples. The samples were analyzed for E. coli using the membrane filtration technique (1).
FIG. 2.
Sampling stations in the Dunes Creek watershed monitored for E. coli weekly during 1999 and 2000. Sampling occurred from June to November 1999 and February to November 2000. The area between stations 12 and 2 is where most of the intensive watershed surveys were conducted.
Lateral studies.
Five equally spaced transects were selected at a site approximately 750 m upstream from the Dunes Creek outfall to Lake Michigan, called the Nissaki 1 site. The selected location measured 20 (length) by 32 m (lateral distance that extended into the adjacent riparian forest). The initial transect was randomly selected, and the remaining four transects were equally spaced at 5-m intervals perpendicular to the stream and running southward. Sediment (0, 1, 2, and 4 m from stream margin) and soil (8, 16, and 32 m from stream margin) samples were collected at each transect. Within 8 cm of each sampling point, five subsamples (about 4 ml each) were taken with a sterile, liquid-medicine dispenser. The subsamples were mixed thoroughly, and a portion of the pooled sample was used later for further analysis. Special care was taken to avoid collection of overlying stream water when submerged sediments were collected. Large litter and twigs were carefully removed before forested soils were sampled. Sampling points were carefully inspected for animal droppings, and suspect areas were avoided. Sampling depth did not exceed 6 cm. Three water samples, one each between transects 1 and 2, 2 and 3, and 4 and 5, were collected. All samples were held at 4°C and analyzed within 4 h of sampling for E. coli by using the IDEXX (Westbrook, Maine) Colilert-18 system, which is based on a defined substrate technology (8, 9). E. coli was elutriated from sediments and soils as detailed below. Air, sediment, and water temperatures were determined in the field with a digital thermometer (data not shown).
Watershed E. coli distribution studies.
Fifteen randomly selected transects along the 800-m central tributary were selected with the ArcView GIS program (version 3.2; Environmental Systems Research Institute Inc., Redlands, Calif.). These points were located in the field with a geographic positioning system (GPS III Plus; Garmin International, Olathe, Kans.). The sample collection techniques were generally similar to the approach used in the lateral studies except for the sampling within each transect. Earlier lateral studies had demonstrated that soils beyond 4 m from the stream margin had unpredictable E. coli counts and distribution. Within each transect, water was collected from midstream; sediment samples were collected from midstream (0 m) and 0.25, 1, and 4 m landward from the stream margin. Samples were kept at 4°C and analyzed for E. coli within 6 h of collection.
Groundwater and reference streams.
The following source waters and associated sediments of Dunes Creek were surveyed: (i) seep water and sediments mostly above and below the park's campground and lateral ditch of the Nissaki 1 site; (ii) artesian spring, marginal sediment, and streamlet in the Beverly Shores area between Beverly Drive and U.S. Route 20; (iii) artesian springs, surrounding sediments, and associated seeps along a secondary branch of Dunes Creek; (iv) artesian well and associated sediments in Warren Woods State Park (Sawyer, Mich.), some 100 m from the margin and high above the floodplain of the St. Joseph River; (v) spring water about 500 m from the St. Joseph River; and (vi) submerged sediment, water, and marginal sands along Painterville Creek, a small coastal stream within Warren Dunes State Park that was very similar to Dunes Creek in land use and limnology.
Laboratory analysis.
Water, sediment, and soil samples were analyzed for E. coli by the Colilert-18 method. Preliminary elutriation efficiency experiments on related sediments using a variety of sonication, shaking, and vortexing approaches yielded the following optimized method. For sediment and soil samples, bacteria were elutriated by vortexing, for 2 min, 5 g of fresh substrate and 35 ml of sterile distilled water in a 50-ml centrifuge tube. After standing for two minutes, the suspension was serially diluted; 100-ml volumes of the appropriate dilutions were aseptically transferred into distribution trays (IDEXX; Quanti-Tray/2000), sealed, and incubated at 35°C for 18 h. After 18 h, wells that fluoresced upon exposure to long-wavelength UV light were counted to determine a most probable number (MPN) per 100 ml of water. To determine moisture content of soil and sediment samples, about 10 g of fresh sample was placed in a 100°C dry oven for 24 h and the weight differential was recorded. Percent moisture content was calculated by the following formula: (weight of fresh sample − weight of dry sample)/weight of dry sample × 100. Counts from soil and sediment samples were expressed per gram of dry weight of the sample. All sets of analyses for E. coli determinations included suitable blanks and a standard E. coli culture (ATCC 25922) for quality control purposes.
Our general protocol for E. coli identification in this study included the following steps. (i) Growth (broth) from wells that fluoresced was initially swabbed onto membrane (medium) thermotolerant E. coli (mTEC) agar, plates were incubated at 44.5°C for 22 h, and yellow or yellow-brown colonies that developed on the membrane were confirmed to be E. coli by the substrate test (urease activity). (ii) Well-isolated colonies from the mTEC agar plates (at least two per plate) were further streaked for primary isolation on MacConkey agar, followed by a secondary isolation on the same medium. (iii) At least two colonies from each MacConkey plate were again confirmed for their β-glucuronidase activity, which is a positive test for E. coli, by growing them on nutrient agar with methylumbelliferyl-β-glucuronide, and subsequently confirmed isolates were stored in tryptic soy broth for later use. (iv) Finally, the isolates that were presumed to be E. coli were speciated by using the BBL crystal identification scheme (Becton Dickinson Microbiology Systems, Sparks, Md.). For quality control and quality assurance, a standard isolate (E. coli ATCC 25922) was included in the identification protocol.
Data analyses.
Statistical analyses were performed using SPSS, version 10.01. Statistical procedures were performed on log10-transformed data to improve normality and equality of variance in large data sets. Nonparametric tests were used where a data set was too small to confirm or normalize. The statistical significance level was set at a P value of ≤0.05 unless otherwise stated.
RESULTS
E. coli counts at Dunes Creek outlet.
Water samples analyzed during the 1999 and 2000 monitoring seasons clearly demonstrated that E. coli concentrations in Dunes Creek were significantly correlated with the park's beach water (P < 0.0001, r = 0.520, n = 139). Dunes Creek empties directly onto the state park's only swimming beach, indicating that the creek directly impacts bathing water quality.
Weekly stream monitoring.
Over the course of the 2-year monitoring study, only stream samples from sites 8 and 14 were significantly lower than those from other sites sampled for E. coli concentrations (Table 1). Site 14 is located within the Great Marsh, and site 8 is 200 m downgradient from that site and part of the headwaters of the creek (Fig. 2). In general, E. coli counts were lower upstream. The unpaired t test shows that the mean E. coli count downstream (sites 1 to 6), 921 ± 62 CFU/100 ml, was significantly higher than that upstream (sites 7 to 13), 486 ± 49 CFU/100 ml (P ≤ 0.001, df = 660).
TABLE 1.
Mean separation (by station) of log-transformed E. coli counts by Duncan analysisa
| Station | Mean separationb (log CFU/100 ml) | nc |
|---|---|---|
| 14 | 2.14* | 38 |
| 8 | 2.39† | 36 |
| 9 | 2.61‡ | 32 |
| 13 | 2.74‡,§ | 53 |
| 12 | 2.75‡,§ | 53 |
| 11 | 2.88‡,§ | 52 |
| 10 | 2.86§ | 59 |
| 5 | 2.96§ | 52 |
| 6 | 2.91§ | 52 |
| 7 | 3.02§ | 52 |
| 3 | 2.92§ | 52 |
| 2 | 2.93§ | 52 |
| 1 | 3.02§ | 58 |
| 4 | 2.96§ | 59 |
Weekly water samples were taken from 14 sites along Dunes Creek from June to November 1999 and from February to November 2000 (see Fig. 2 for locations of stations). One-way analysis of variance showed that the stations were significantly different in mean E. coli density (F13, 686 = 13.8, P = 0.001).
Values with different symbols are significantly different (P ≤ 0.05).
n, number of samples.
E. coli concentrations tended to be higher during warmer months, peaking in late summer. During cooler months, however, density generally remained above 1.5 log10 units/100 ml (e.g., February mean = 114 ± 30 CFU/100 ml), indicating that the creek had a perennial source of E. coli. These data further demonstrated that the variations in E. coli numbers were substantially high both within and between years (data not shown). Both nontransformed and log-transformed data were normally distributed (P = 0.235 and 0.067, respectively). Data behaved far more normally when transformed according to normal cumulative proportions probability plots of these data. The unpaired t test shows that there was no significant difference between 1999 and 2000 data when only common months were considered (P = 0.751, df = 519).
Lateral riparian E. coli distribution.
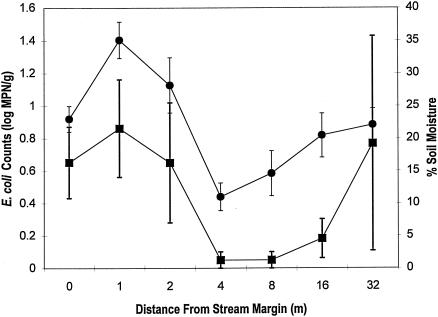
No significant difference between mean E. coli counts in sediment and soil samples collected at different distances (0, 1, 2, 4, 8, 16, and 32 m) from the stream margin was detected (F6, 28 = 1.187, P = 0.342) (Fig. 3). Variances within the first few meters were remarkably similar, and outliers were more common within forest soils. Pearson correlation analysis suggested that substrate moisture was correlated with E. coli counts (P = 0.003, r = 0.49, n = 35).
FIG. 3.
Mean E. coli counts (▪) and percent soil moisture (•) (±1 standard error) in samples collected along five equally spaced transects within the central branch of Dunes Creek.
Watershed E. coli distribution.
The median concentration of E. coli in water was 1,089 MPN/100 ml, whereas sediment samples collected at midstream (0 m) and 0.25, 1, and 4 m landward from the margin contained 71, 28, 21, and 13 MPN/g, respectively. E. coli counts in submerged (0 m) and margin sediments (0.25 m) were significantly correlated (P = 0.034, r = 0.55), as were counts between sediments collected at the margin and 1 m inland (P = 0.019, r = 0.60). A correlation between E. coli densities in stream water and submerged sediments was suggested, but these densities were not significantly correlated by using the statistical a priori α criteria originally set (P = 0.064, r = 0.49, n = 15).
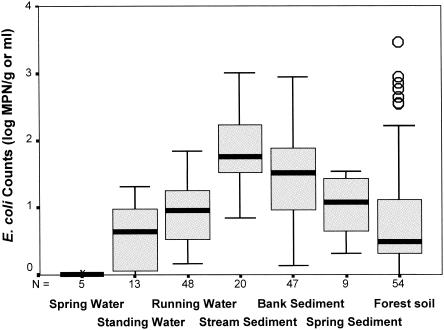
To examine the occurrence of E. coli within Dunes Creek and related stream watersheds but away from flooding and upstream bacterial input, we sampled springs and seeps along associated wetted sediments. In a loose sense, these samples acted as upstream controls relative to lower basin study sites. Mean E. coli counts were <0.1 (range, 0 to 0.1 CFU/ml), 0, and 0.8 CFU/ml (range, 0 to 2 CFU/ml) in artesian spring waters of Beverly Shores, Ind., Wilson Springs, Ind., and Warren Woods, Mich., respectively. Interestingly, spring water in Warren Woods was only 8°C, despite normal summer air temperatures. Wetted sediments contiguous to the springs were notably higher in counts, with mean E. coli counts of 22 MPN/g (range, 4 to 53 MPN/g) for Beverly Shores and 4 MPN/g (range, 1 to 8 MPN/g) for Warren Woods. The concentration of E. coli was even higher around sediments wetted by creek seeps, with a mean concentration within Dunes Creek of 36 MPN/g (range, 1 to 119 MPN/g) and a mean concentration within Painterville Creek (Warren Dunes, Mich.) of 84 MPN/g (range, 68 to 101 MPN/g).
When milliliters of water and grams of dry weight of sediment are assumed equal for hypothetical comparison, the median E. coli count was highest in stream sediments, followed by, in order of decreasing magnitude, bank sediments, spring sediments, stream water, and isolated pools (Fig. 4). In forest soils, E. coli counts were low but high outliers were common.
FIG. 4.
Mean E. coli densities in pooled samples of Dunes Creek and Warren Woods and Warren Dunes watersheds. Black bars, medians; gray rectangles, 25th and 75th percentiles; bars, range, excluding outliers; open circles, outliers.
E. coli identification and confirmation.
A total of 405 isolates were collected over a period of 6 months. Of the 405 presumptive isolates, 182 were used for positive identification by the BBL crystal identification scheme; 168 (92%) isolates were typed as E. coli, while the remaining 14 (8%) were not identified due to one or several ambiguous test reactions (data not shown).
DISCUSSION
This study was designed to examine the previously indicated nonpoint sources as potential contributors of E. coli in Dunes Creek (27). The results presented here were generally consistent with previous findings, further emphasizing that E. coli is common within the stream basin, especially in submerged, margin, and wetted bank sediments, with numbers rapidly decreasing landward beyond the banks. The results of this study clearly indicated that sediments and soils in the Dunes Creek watershed harbored E. coli, and the persistently elevated counts in the stream are perhaps due to the washing of the sediment-borne organisms into the water.
The present findings are particularly significant because, while there are a limited number of studies that address persistence and possible growth of E. coli in tropical areas (5, 7, 24), few comparable temperate or boreal lake or stream watersheds have been studied. Further, most studies of E. coli sources within streams, lakes, and rivers generally focus on influences such as sewage discharge, waste from feedlot operations, and wildlife (20) since the assumption is that these bodies of water are intrinsically unpolluted. Extensive studies conducted in Dunes Creek and related watersheds over the last 8 to 10 years have shown that the elevated E. coli counts in the creek are due to non-point source contamination rather than any sewage input (27, 29). The present study renders support to the previous observations.
Although E. coli counts in the riparian forest soils were highly variable (for instance, often less than 1 to several thousand organisms per g of dry weight), it is well known that, in natural environments, microbial distribution and abundance are not homogenous but patchy (3, 18, 30). While factors affecting the distribution of microbes in terrestrial habitats are not well understood, it is suggested that certain physical characteristics, such as soil type, particle size and distribution, and aggregate stability, affect microbial numbers and their biomass within and among soils (21). The high patchiness of E. coli population density in this study, particularly in the forested soils, makes population estimation in such environments difficult. Nonetheless, the ubiquity of E. coli along the banks and forested soils clearly suggests that increased E. coli loading in Dunes Creek water is mostly attributable to contributions from nonpoint (possibly nonfecal) sources held and subsequently released by soil and sediment erosion. These results are consistent with those found by Fujioka et al. (10), Hardina and Fujioka (14), and Solo-Gabriele et al. (24). Further, these findings may help account for many unresolved bacterial source identification and sanitary surveys for streams with easily transported basin sediments.
Springs and small pools in the watershed were essentially isolated from the creek, and seeps sampled were very rarely exposed to creek water through flooding. In almost every case, sediments surrounding issuing ground water were rich in E. coli, while the ground water itself had negligible quantities of E. coli. Preliminary samplings suggested that E. coli was highest in relatively clean moist sands and much lower in litter-laden sandy soils. These observations, coupled with the common occurrence of E. coli throughout the watersheds of the streams sampled, support the idea that E. coli is ubiquitous in riparian sediments and, perhaps, naturally occurring in the southern Lake Michigan coastal area. While the observations presented here support the idea that riparian E. coli is independent of fecal contamination, more research is needed to substantiate and understand this ecological phenomenon.
Many streams in the Midwest carry substantial quantities of E. coli relative to currently accepted U.S. guidelines for swimmable waters (22, 23). The tendency to relate E. coli occurrences to these guidelines is not practical because most of these streams, ditches, and creeks are not used for swimming-related activities although secondary contact is common. Regulators and sanitarians most often look for human-derived sources of fecal indicators when seeking the source of contamination on their swimming beaches. This approach can lead to frustration in conducting sanitary surveys because point sources may be elusive when E. coli is ubiquitous within a particular drainage. The integrated nature of streams and associated drainage areas is more complex; separating factors, such as ground water, land use, runoff, riparian soils, microflora, nutrients, fauna, vegetation, and associated ecosystem processes, that are closely tied can be difficult.
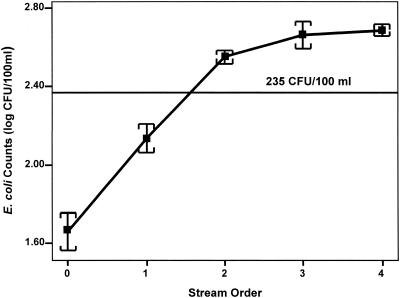
Even though this study and previous Dunes Creek studies (27, 29) have investigated nonpoint sources and their impact on E. coli levels in the Dunes Creek watershed, other complicating contributions have received little attention; one important factor is human impact, specifically, extensive ecosystem manipulation through ditching of the Great Marsh system. Over the years, the Great Marsh has been extensively ditched to drain the wetlands and accommodate urban and municipal development. Draining the wetlands, which in turn feed the creeks that eventually empty into Lake Michigan, has significantly modified these creeks. Dunes Creek, which used to be a small, natural stream, now has extensive drainage connections as a result of the massive ditching of wetlands (see Fig. 1). This has led to an unnatural increase in the complexity of the creek and has altered stream order. Stream order (e.g., 1°, 2°, 3°, etc.) describes the hierarchy of streams based on the number of additional confluences (16). The relationship between sediment loading and ionic and nonionic contents and stream order is well established, with these constituents generally increasing in lower reaches (higher orders) of a stream complex. In summary, extensive ditching of the wetlands has increased the stream order of Dunes Creek and altered drainage patterns, which have presumably increased potential inputs to and subsequent E. coli loadings in the creek (Fig. 5).
FIG. 5.
Mean (+1 standard error) E. coli concentrations plotted against stream order (as defined by Leopold et al. [16]) from weekly sampling of Dunes Creek during 1999 to 2000 at 14 fixed stations.
All streams share the tendency of organic material, salts, and microbial load to increase downstream. Past disturbance can cause environmental perturbations and exaggerations of these fluvial processes, resulting in artificially high E. coli loading introduced by exposed riparian sediments and drained wetlands. Such is the case for the extensive ditching that took place within the Dunes Creek watershed. Over time, natural fluvial and palustrine processes will recover, reducing the total, average, and peak loading of E. coli in stream water. Unfortunately, passive remediation may not be timely enough for local managers; consequently, faster remedial actions, such as wetland restoration and deditching, may have to be attempted appropriately.
In summary, Dunes Creek adversely influences the water quality of Indiana Dunes State Park beach. E. coli within the Dunes Creek watershed is abundant and ubiquitous. E. coli (i) occurs primarily in wetted sediments, (ii) occurs sporadically in forest soils, (iii) is plentiful around nearly every seep and spring examined, (iv) is consistently found in standing and running ditch water, (v) is relatively high throughout the year, and (vi) is relatively low in wetland waters and unditched drainage. Similar distribution patterns were found in other stream systems in Indiana (Derby Ditch) and Michigan (Warren Dunes and Warren Woods). E. coli decreases rapidly with distance from the streambed and is less variable in wetted sediments and water than forest soil. The relationship between E. coli concentration and stream order suggests that excessive ditching and, consequently, non-point source input via sediment transport are responsible for elevated E. coli density in the watershed. Further studies should not only examine if E. coli is widespread in nature but also seek understanding of its adaptations, the associated ecology, and the environmental implications of persistence.
Acknowledgments
M. Byappanahalli and R. Whitman contributed equally to this work.
We thank T. Coleman and D. Wickersham, Indiana Department of Natural Resources, for their continued support in Dunes Creek research projects. Special thanks to M. Dicky for collecting 1999-2000 monitoring samples. We thank M. Becker Nevers and N. Pavlovic, USGS, J. Marburger, National Park Service, and D. Wilcox, USGS, for providing valuable comments.
This work was supported by the Indiana Department of Natural Resources.
Footnotes
This article is contribution 1248 of the USGS Great Lakes Science Center.
REFERENCES
- 1.American Public Health Association. 1998. Standard methods for the examination of water and wastewater, 20th ed. American Public Health Association, Washington, D.C.
- 2.Anderson, S. A., S. J. Turner, and G. D. Lewis. 1997. Enterococci in the New Zealand environment: implications for water quality monitoring. Water Sci. Technol. 35:325-331. [Google Scholar]
- 3.Atlas, A. M., and R. Bartha. 1998. Microbial ecology: fundamentals and applications, 4th ed. Benjamin/Cummings Publishing Company, Inc., Menlo Park, Calif.
- 4.Bermudez, M., and T. C. Hazen. 1988. Phenotypic and genotypic comparison of Escherichia coli from pristine tropical waters. Appl. Environ. Microbiol. 54:979-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byappanahalli, M. N. 2000. Assessing the persistence and multiplication of fecal indicator bacteria in Hawaii's soil environment. Ph.D. thesis. University of Hawaii at Manoa, Honolulu.
- 6.Byappanahalli, M. N., and R. S. Fujioka. 1998. Evidence that tropical soil environment can support the growth of Escherichia coli. Water Sci. Technol. 38:171-174. [Google Scholar]
- 7.Desmarais, T. R., H. M. Solo-Gabriele, and C. J. Palmer. 2002. Influence of soil on fecal indicator organisms in a tidally influenced subtropical environment. Appl. Environ. Microbiol. 68:1165-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edberg, S. C., M. J. Allen, and D. B. Smith. 1991. Defined substrate technology method for rapid and specific simultaneous enumeration of total coliforms and Escherichia coli from water: collaborative study. J. Assoc. Off. Anal. Chem. 74:526-529. [PubMed] [Google Scholar]
- 9.Edberg, S. C., M. J. Allen, D. B. Smith, and N. J. Kriz. 1990. Enumeration of coliforms and Escherichia coli from source water by the defined substrate technology. Appl. Environ. Microbiol. 56:366-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujioka, R., C. Sian-Denton, M. Borja, J. Castro, and K. Morphew. 1999. Soil: the environmental source of Escherichia coli and enterococci in Guam's streams. J. Appl. Microbiol. Symp. Suppl. 85:83S-89S. [DOI] [PubMed]
- 11.Fujioka, R. S. 1983. Stream water quality assessment on fecal coliform and fecal streptococcus analysis. Technical memorandum report no. 70. University of Hawaii at Manoa, Honolulu.
- 12.Fujioka, R. S., and M. N. Byappanahalli. 2001. Microbial ecology controls the establishment of fecal bacteria in tropical soil environment, p. 273-283. In K. H. T. Matsuo, S. Takizawa, and H. Satoh (ed.), Advances in water and wastewater treatment technology: molecular technology, nutrient removal, sludge reduction and environmental health. Elsevier, Amsterdam, The Netherlands.
- 13.Fujioka, R. S., K. Tenno, and S. Kansako. 1988. Naturally occurring fecal coliforms and fecal streptococci in Hawaii's freshwater streams. Toxic. Assess. 3:613-630. [Google Scholar]
- 14.Hardina, C. M., and R. S. Fujioka. 1991. Soil: the environmental source of Escherichia coli and enterococci in Hawaii's streams. Environ. Toxicol. Water Qual. 6:185-195. [Google Scholar]
- 15.Leclerc, H., D. A. A. Mossel, S. C. Edberg, and C. B. Struijk. 2001. Advances in the bacteriology of the coliform group: their suitability as markers of microbial water quality. Annu. Rev. Microbiol. 55:201-234. [DOI] [PubMed] [Google Scholar]
- 16.Leopold, L. B., M. G. Wolman, and J. P. Miller. 1964. Fluvial processes in geomorphology. W. H. Freeman and Company, San Francisco, Calif.
- 17.Muller, T., A. Ulrich, E. M. Ott, and M. Muller. 2001. Identification of plant-associated enterococci. J. Appl. Microbiol. 91:268-278. [DOI] [PubMed] [Google Scholar]
- 18.Nunan, N., K. Ritz, D. Crabb, K. Harris, K. Wu, J. W. Crawford, and I. M. Young. 2001. Quantification of the in situ distribution of soil bacteria by large-scale imaging sections of undisturbed soil. FEMS Microbiol. Ecol. 37:67-77. [Google Scholar]
- 19.Ott, E. M., T. Muller, M. Muller, C. M. A. P. Franz, A. Ulrich, and M. Gabel. 2001. Population dynamics and antagonistic potential of enterococci colonizing the phyllosphere of grasses. J. Appl. Microbiol. 91:54-66. [DOI] [PubMed] [Google Scholar]
- 20.Patrick, R., F. Douglass, D. M. Palavage, and P. M. Stewart. 1992. Surface water quality: have the laws been successful? Princeton University Press, Princeton, N.J.
- 21.Richaume, A., C. Steinberg, L. Jocteur-Monrozier, and G. Faurie. 1993. Differences between direct and indirect enumeration of soil bacteria: the influence of soil structure and cell location. Soil Biol. Biochem. 25:641-643. [Google Scholar]
- 22.Silcox, C. A., B. A. Robinson, and T. C. Willoughby. 2001. Concentrations of Escherichia coli in the Kankakee and lower Wabash River watersheds in Indiana, June-September 1999. Water Resources Investigations Report 00-4018. U.S. Department of the Interior and Indiana Department of Environmental Management, Indianapolis, Ind.
- 23.Silcox, C. A., D. C. Voelker, and T. C. Willoughby. 2000. Concentrations of Escherichia coli in streams in the upper Wabash River watershed in Indiana, June-September 1998. Water Resources Investigations Report 00-4021. U.S. Department of the Interior and Indiana Department of Environmental Management, Indianapolis, Ind.
- 24.Solo-Gabriele, H., M. A. Wolfert, T. R. Desmarais, and C. J. Palmer. 2000. Sources of Escherichia coli in a coastal subtropical environment. Appl. Environ. Microbiol. 66:230-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24a.Stewart, P. M., J. T. Butcher, and M. E. Becker. 1997. Ecological assessment of the three creeks draining the Great Marsh at Indiana Dunes National Lakeshore. Report to the Indiana Dunes National Lakeshore. U. S. Geological Survey, Porter, Ind.
- 25.Thompson, T. A. 1994. History and architecture of wetland development in the Indiana Dunes. Proc. Indiana Acad. Sci. 103:167-176. [Google Scholar]
- 26.United States Environmental Protection Agency. 1986. Ambient water quality criteria for bacteria EPA-440/5-84-002. U.S. Environmental Protection Agency, Washington, D.C.
- 27.Whitman, R. L., A. V. Gochee, W. A. Dustman, and K. J. Kennedy. 1995. Use of coliform bacteria in assessing human sewage contamination. Nat. Areas J. 15:227-233. [Google Scholar]
- 28.Whitman, R. L., T. G. Horvath, M. L. Goodrich, M. B. Nevers, M. J. Wolcott, and S. K. Haack. 2001. Characterization of E. coli levels at 63rd Street Beach. Report to the City of Chicago, Department of the Environment and the Chicago Park District U.S. Geological Survey, Porter, Ind.
- 29.Whitman, R. L., M. B. Nevers, and P. J. Gerovac. 1999. Interaction of ambient conditions and fecal coliform bacteria in southern Lake Michigan waters: monitoring program implications. Nat. Areas J. 19:166-171. [Google Scholar]
- 30.Wollum, I. A. G., and D. K. Cassel. 1984. Spatial variability in Rhizobium japonicum in two North Carolina (USA) soils. Soil Sci. Soc. Am. J. 48:1082-1086. [Google Scholar]