Abstract
A variety of lactic acid bacteria were screened for their ability to produce folate intracellularly and/or extracellularly. Lactococcus lactis, Streptococcus thermophilus, and Leuconostoc spp. all produced folate, while most Lactobacillus spp., with the exception of Lactobacillus plantarum, were not able to produce folate. Folate production was further investigated in L. lactis as a model organism for metabolic engineering and in S. thermophilus for direct translation to (dairy) applications. For both these two lactic acid bacteria, an inverse relationship was observed between growth rate and folate production. When cultures were grown at inhibitory concentrations of antibiotics or salt or when the bacteria were subjected to low growth rates in chemostat cultures, folate levels in the cultures were increased relative to cell mass and (lactic) acid production. S. thermophilus excreted more folate than L. lactis, presumably as a result of differences in the number of glutamyl residues of the folate produced. In S. thermophilus 5,10-methenyl and 5-formyl tetrahydrofolate were detected as the major folate derivatives, both containing three glutamyl residues, while in L. lactis 5,10-methenyl and 10-formyl tetrahydrofolate were found, both with either four, five, or six glutamyl residues. Excretion of folate was stimulated at lower pH in S. thermophilus, but pH had no effect on folate excretion by L. lactis. Finally, several environmental parameters that influence folate production in these lactic acid bacteria were observed; high external pH increased folate production and the addition of p-aminobenzoic acid stimulated folate production, while high tyrosine concentrations led to decreased folate biosynthesis.
Folate is an essential component in the human diet. It is involved, as a cofactor, in many metabolic reactions, including the biosynthesis of the building blocks of DNA and RNA, the ribonucleotides. The daily recommended intake for an adult varies from 200 μg in Europe to 400 μg in the United States. For pregnant women, a double dose is recommended, since folate is known to prevent neural tube defects in newborns (39, 40). Recently, claims that high-folate diets protect against cardiovascular disease (6, 7) and even some forms of cancer (2) have been made. Several recent reports have indicated that folate deficiency is common in various population groups, including women of childbearing age (16, 24).
Folate is produced by various green plants and by some microorganisms. Vegetables and dairy products are the main source of folate for humans. Milk is a well-known source of folate. It contains between 20 and 50 μg of folate per liter and thus contributes significantly to the daily requirement of the average human. Some fermented milk products, especially yogurt, are reported to contain even larger amounts of folate (1). Up to 110 μg of folate per liter has been found in yogurt. This high level is a direct result of the production of additional folate by the lactic acid bacteria in the yogurt. Of the two lactic acid bacterial species in yogurt, Lactobacillus bulgaricus and Streptococcus thermophilus, only the latter is reported to produce folate (28). Recently, it has been reported that other lactic acid bacteria produce folate during milk fermentation (17).
Folate is synthesized from the precursors GTP, p-aminobenzoate (PABA), and glutamate. These three building blocks are modified in a number of enzymatic steps and used for the further production of various folate derivatives that are necessary for C1 metabolism, such as tetrahydrofolate, 5-formyl tetrahydrofolate, 5,10-methenyl tetrahydrofolate,10-formyl tetrahydrofolate, and 5,10-methylene tetrahydrofolate (12, 33).
In earlier work, we reported the effect of metabolic engineering of the folate biosynthesis pathway on folate production (36). In the present work, we study the influence of growth conditions and medium composition, especially the role of pH and PABA, on folate biosynthesis and folate distribution in lactic acid bacteria. We concentrate on Lactococcus lactis MG1363 (11) as the best-studied model lactic acid bacterium for metabolic engineering and starter bacterium for cheese, butter, and buttermilk production and on Streptococcus thermophilus NIZO strain B119 as a starter bacterium for the production of yogurt, probiotic dairy products, and several cheese varieties. Large differences in folate production for different strains and different growth conditions were observed.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
A total of 15 Lactococcus lactis strains, 2 Leuconostoc strains, 7 Lactobacillus strains, 3 Streptococcus thermophilus strains, and 1 Enterococcus strain were tested for their ability to produce folate. The strains are listed in Table 1. The NIZO strain number, the original code as found in the official strain collection, literature reference in case of previous publications, and the source of isolation are also given in Table 1. Lactococcus strains were cultivated in M17 medium (38) or chemically defined medium (CDM) (26) supplemented with 0.5% glucose at 30°C. Lactobacillus and Leuconostoc strains were cultivated in MRS medium (8) at 37 and 30°C, respectively. S. thermophilus strains were cultivated at 37°C in M17 medium. All strains were routinely stored in litmus milk with 0.1% yeast extract at −40°C.
TABLE 1.
Extra- and intracellular folate production before and after enzymatic deconjugation by different species and strains of lactic acid bacteriaa
| Lactic acid bacterial species | Growth conditionb | Origin or original strain codec | NIZO strain no. | Extracellu- lar folate (μg/liter) | Extracellu- lar deconj. folate (μg/liter) | Intracellu- lar folate (μg/liter) | Intracellu- lar deconj. folate (μg/liter) | Total deconj. folate
|
|
|---|---|---|---|---|---|---|---|---|---|
| μg/liter | μg/liter/ OD600 unit | ||||||||
| Lactococcus lactis subspecies | HP | B42 | 14 | 14 | 44 | 81 | 95 | 39 | |
| L. lactis subsp. cremoris | A | ||||||||
| L. lactis subsp. cremoris | A | E8 | B64 | 10 | 9 | 47 | 99 | 92 | 33 |
| L. lactis subsp. cremoris | A | SK110 | B697 | 41 | 46 | 40 | 69 | 116 | 50 |
| L. lactis subsp. lactis | A | MG1363 (13) | 10 | 8 | 37 | 87 | 94 | 31 | |
| L. lactis subsp. lactis | A | KB (23) | B628 | 9 | 6 | 39 | 84 | 90 | 25 |
| L. lactis subsp. lactis | AC | KB (23) | B628 | 15 | 11 | 165 | 245 | 256 | 29 |
| L. lactis subsp. lactis | AC | NZ 9000 (5a) | 13 | 9 | 38 | 106 | 116 | 43 | |
| L. lactis subsp. lactis | AC | NZ 9010 (ldh negative) (5a) | 20 | 22 | 184 | 269 | 291 | 39 | |
| L. lactis subsp. lactis | A | Radish | B26 | 20 | 26 | 25 | 65 | 91 | 32 |
| L. lactis subsp. lactis | A | Frozen peas | B27 | 12 | 8 | 27 | 61 | 69 | 25 |
| L. lactis subsp. lactis | A | Silage (3) | B1171 | 11 | 14 | 30 | 47 | 62 | 22 |
| L. lactis subsp. lactis | A | Silage (3) | B1172 | 11 | 10 | 29 | 53 | 63 | 23 |
| L. lactis subsp. lactis | A | Silage (3) | B1173 | 4 | 5 | 25 | 52 | 57 | 20 |
| L. lactis subsp. lactis biovar diacetylactis | A | C17 (32) | B621 | 17 | 21 | 40 | 77 | 98 | 37 |
| L. lactis subsp. lactis biovar diacetylactis | A | Ru4 (32) | B86 | 17 | 16 | 42 | 84 | 100 | 38 |
| L. lactis subsp. lactis biovar diacetylactis | A | ZK (32) | B87 | 19 | 14 | 34 | 65 | 79 | 35 |
| Enterococcus species | A | Termite gut | B28 | −8 | −18 | 1 | 0 | −18 | −8 |
| Streptococcus thermophilus | A | Yogurt | B103 | 6 | 25 | 3 | 4 | 29 | 13 |
| Streptococcus thermophilus | A | Yogurt | B108 | 25 | 23 | 83 | 179 | 202 | 51 |
| Streptococcus thermophilus | A | Spray dryer | B119 | 48 | 40 | 69 | 80 | 120 | 214 |
| Leuconostoc lactis | B | 60 (32) | B911 | 8 | 37 | 5 | 7 | 45 | 26 |
| Leuconostoc paramesenteroides | B | 7-1 (32) | B629 | 6 | 33 | 8 | 10 | 44 | 25 |
| Lactobacillus plantarum | B | WCFS-1 | 6 | 27 | 11 | 18 | 45 | 4 | |
| Lactobacillus helveticus | B | ATCC 10797 | B219 | −9 | −1 | 2 | 90 | 89 | 22 |
| Lactobacillus helveticus | B | Cheese | −9 | 3 | 0 | −1 | 2 | 1 | |
| Lactobacillus acidophilus | B | Ki (15) | B230 | −8 | 0 | 0 | 1 | 1 | 0 |
| Lactobacillus casei | B | Cheese | −9 | −45 | 1 | 32 | −13 | −3 | |
| Lactobacillus casei subsp. rhamnosus | B | ATCC 7469 | B236 | −10 | −98 | 1 | 34 | −63 | −7 |
| Lactobacillus delbrueckii subsp. bulgaricus | B | Rb from RR starter (9) | B194 | −9 | 12 | 2 | 41 | 54 | 31 |
| M17 medium | 10 | 69 | |||||||
| MRS medium | 9 | 108 | |||||||
Extracellular and intracellular folate concentrations are shown and were corrected for folate present in the medium. Positive values indicate that folate was produced. Negative values indicate that the folate present in the growth medium was consumed. Folate concentration was measured after enzymatic deconjugation of the polyglutamyl tail (deconj. folate). Standard deviations varied between 5 and 20%.
Growth conditions: A, grown on M17 medium; B, grown on MRS medium; C, grown aerobically.
Strain origin, original strain designation as in the official strain collection, literature reference in previous publications, and/or source of isolation.
To study the effects of cultivation conditions on folate production, L. lactis strain MG1363 was grown in CDM (26) without folate, PABA, and aromatic amino acids phenylalanine, tryptophan, and tyrosine. S. thermophilus strain B119 was grown in Difco folic acid casei medium (Becton Dickinson Microbiology Systems, Sparks, Md.). The growth medium was supplemented with erythromycin (0 to 0.1 μg/ml), chloramphenicol (0 to 2.5 μg/ml), NaCl (0 to 0.8% [wt/vol]), or hemin (10 μg/ml), as indicated in the figure legends and tables. Growth was determined by the increase in optical density at 600 nm (OD600). Aerobic growth conditions were obtained by growing 5-ml cell culture samples in 100-ml tubes with continuous shaking (210 rpm) in a water bath at 30°C (Gyrotory water bath, model G76; New Brunswick Scientific, Edison, N.J.).
Continuous fermentation.
For continuous cultivation, a 1-liter glass vessel (Applikon Dependable Instruments, Schiedam, The Netherlands), filled with 0.5 liter of growth medium, was used as described previously (32). Temperature was maintained at 30 or 37°C, and pH was controlled by titration with 5 N NaOH using the Biocontroller ADI 1020 (Applikon Dependable Instruments). Anaerobic conditions were maintained by sparging the headspace of the culture with N2 gas. To cultivate S. thermophilus NIZO strain B119, modified M17 medium was used with potassium phosphate (0.6 g/liter) replacing β-glycerophosphate and with 0.5% lactose as the growth-limiting substrate. For continuous cultivation of L. lactis MG1363, CDM without PABA, folate, and phenylalanine was used with 0.5% glucose as the growth-limiting substrate.
Analysis of intra- and extracellular folate concentrations.
Folate was quantified using a Lactobacillus casei microbiological assay (13). Cells and supernatant were recovered from a cell culture (5 ml) to measure both intra- and extracellular folate concentrations as described previously (36). The microbiological folate assay gives nearly equal responses to mono-, di-, and triglutamyl folate, while the response to longer-chain polyglutamyl folate (more than three glutamyl residues) decreases markedly in proportion to chain length (37). Consequently, total folate concentrations can be measured only after deconjugation of the polyglutamyl tails in samples containing folate derivatives with more than three glutamyl residues. The analysis of total folate concentration, including polyglutamyl folate, was done after enzymatic deconjugation of the folate samples for 4 h at 37°C and at pH 4.8 with human plasma (Sigma-Aldrich Chemie, Zwijndrecht, The Netherlands), as a source for γ-glutamyl hydrolase activity (36). Microbiological assay measurements were repeated, and the standard deviation varied between 0 and 20%. The microorganism used for detection, Lactobacillus casei subsp. rhamnosus strain ATCC 7469, was stored at −80°C in MRS medium supplemented with 15% glycerol. For use in the microbiological assay, Lactobacillus casei was pregrown in filter-sterilized folic acid casei medium supplemented with 0.3 μg of folate per liter; the culture was incubated for 18 h at 37°C. Cultures were cooled down on ice, and 40% cold, sterile glycerol was added. Aliquots (1.5 ml) of this mixture were stored in sterile tubes at −80°C until use for folate determination. The microbiological assay was performed in 96-well microtiter plates (well volume, 300 μl). The wells were filled by adding 150 μl of twofold-concentrated folic acid casei medium to 150 μl of an unknown or reference sample in 0.1 M potassium phosphate buffer (pH 6.3) containing 1% ascorbic acid. For reference samples, folic acid was dissolved in the same buffer at a concentration ranging from 0 to 0.25 μg/liter. Control wells were not inoculated to check for sterility of the procedure. Growth in the microtiter plates was determined by measuring the increase in absorbance at 580 nm using the microplate reader Emax (Molecular Devices Corporation, Sunnyvale, Calif.)
Folate measurement by HPLC.
For analysis of intracellular folate concentrations by high-performance liquid chromatography (HPLC), concentrated cell extracts were prepared as follows. L. lactis or S. thermophilus cells were recovered from a cell culture (50 ml) by centrifugation (12,000 × g, 10 min, 4°C) and washed with 20 ml of 50 mM H3PO4 (pH 2.3) containing 1% ascorbic acid. The cells were resuspended in 1 ml of the same buffer. Cell extract was prepared by the addition of 1 g of silica beads to the cell suspension, followed by disruption of the cells in an FP120 Fastprep cell disrupter (Savant Instruments Inc., Holbrook, N.Y.). The cell extract was heated at 100°C for 3 min to release folate from folate binding proteins and to precipitate proteins. Following centrifugation (two times at 12,000 × g, 3 min, 4°C), 100 μl of concentrated cell extract was injected into the chromatograph as soon as possible after extraction, although samples were stable over the working day.
Folate derivatives were purchased from Schircks (Jona, Switzerland). Small volumes of folate stock solutions were prepared at a concentration of 1 mg/ml and frozen. Working solutions were prepared by thawing microliter volumes and diluting to a concentration between 10 and 1,000 ng/ml as needed. The concentrated polyglutamyl folate samples were analyzed by mass spectrometry using a VG Quattro II mass spectrometer (Micromass UK Ltd., Manchester, United Kingdom).
The high-performance liquid chromatograph consisted of a Waters 600E pump (Waters Associates, Watford, United Kingdom), Waters 767 Plus autosampler injector, Shimadzu SPD-M10A photodiode array detector, and a Waters 470 fluorescence detector. Different mono- and polyglutamyl folate derivatives were discriminated by using a betasil phenyl column (250-mm-long column with an inner diameter of 3 mm) (bead diameter, 3 μm) (Keystone Scientific Inc., Bellefonte, Pa.) protected with a betasil phenyl guard column. Freshly prepared mobile phase consisting of 9% methanol and 1.5% formic acid, pH 3.0, was filtered through a 0.45-μm-pore-size Millipore filter (type durapore) and degassed. Chromatography was performed at 50°C using a flow rate of 0.5 ml/min, which produced a back pressure of 1,200 lb/in2. Detection was performed as follows. (i) Fluorimetric detection was achieved with an excitation wavelength of 310 nm and an emission wavelength of 352 nm. The optimal signal-to-noise ratio for sensitive detection was an attenuation of 64 and a gain value of 100 with a filter value of 4 s. (ii) Photodiode array detection data were collected at wavelengths between 220 and 500 nm at an optical resolution of 2 nm in order to discriminate fine structural details of the similar mono- and polyglutamyl folate spectra. Postanalysis routines were studied using Shimadzu Class VP 5.0 software.
RESULTS
Screening for folate production.
Several species and strains from the lactic acid bacterial genera Lactococcus, Lactobacillus, Streptococcus, and Leuconostoc were screened for intra- and extracellular folate production (Table 1). The highest folate level was detected in an aerobically grown L. lactis strain defective in lactate dehydrogenase (291 μg/liter). However, the highest folate production per biomass was found in S. thermophilus strain B119 (214 μg/liter/OD600 unit). In general, Lactobacillus strains consumed (small amounts of) folate and did not produce folate, with the exception of Lactobacillus plantarum. In most strains, e.g., L. lactis MG1363, folate production levels were higher after deconjugation of the polyglutamyl tail (see Materials and Methods), indicating the (intracellular) presence of polyglutamyl folates with more than three glutamyl residues. In contrast, in other strains, e.g., S. thermophilus B119, deconjugation of the folate samples had no effect on folate analysis, indicating the absence of polyglutamyl folates with more than three glutamyl residues.
The extent to which cells excreted the produced folate into the medium varied from strain to strain (Table 1). All L. lactis strains, except SK110, showed high intracellular accumulation of folate (approximately 90%). The other lactic acid bacteria, including S. thermophilus, showed extensive excretion of folate into the medium. For S. thermophilus strain B119, this excretion of folate was observed to be strongly dependent on the pH of the cultivation medium. Under controlled growth conditions in a chemostat at pH 5.5, the cytoplasmic retention of folate in S. thermophilus was relatively low (45%), resulting in increased excretion of folate into the medium. At higher pH (6.0 and 6.5), most folate was found inside the cells (Table 2). Interestingly, increased dilution rates, and consequently increased growth rates, also resulted in increased retention of folate by S. thermophilus (data not shown). At the higher pH values and higher growth rates, a smaller fraction of the total folate was retained inside the streptococcal cells than the 90% (or more) intracellular retention usually observed for L. lactis under all growth conditions. pH did not affect the intracellular and extracellular distribution of folate in L. lactis (Table 2).
TABLE 2.
Folate production by S. thermophilus strain B119 and L. MG1363 in continuous cultures at different pHsa
| pH |
S. thermophilusb
|
L. lactisb
|
||||||
|---|---|---|---|---|---|---|---|---|
| Extracellular folate (μg/liter) | Intracellular folate (μg/liter) | % Excreted | Total folate (μg/liter) | Extracellular folate (μg/liter) | Intracellular folate (μg/liter) | % Excreted | Total folate (μg/liter) | |
| 5.0 | NDb | ND | ND | ND | 3 ± 2 | 32 ± 5 | 8 | 37 |
| 5.5 | 77 ± 7 | 65 ± 15 | 54 | 142 | 4 ± 2 | 36 ± 3 | 9 | 44 |
| 6 | 94 ± 8 | 292 ± 30 | 24 | 386 | 6 ± 1 | 42 ± 9 | 11 | 54 |
| 6.5 | 103 ± 8 | 431 ± 30 | 19 | 534 | ND | ND | ND | ND |
| 7.0 | ND | ND | ND | ND | 16 ± 1 | 91 ± 9 | 8 | 107 |
| 7.5 | ND | ND | ND | ND | 15 ± 1 | 88 ± 1 | 8 | 102 |
S. thermophilus strain B119 was cultivated in modified M17 medium (potassium phosphate instead of β-glycerophosphate) with 0.5% lactose as the growth-limiting substrate, and L. lactis was grown in CDM (26) without phenylalanine, PABA, and folate, with 0.5% glucose as the growth-limiting substrate.
Extracellular, intracellular, and total folate concentrations produced and percent excreted by the species. ND, not determined.
Identification of polyglutamyl folates.
The total folate concentration and the intra- and extracellular distribution of folate in L. lactis and S. thermophilus was determined by a microbiological assay. HPLC was used to determine the different intracellular mono- and polyglutamyl folate derivatives in these two lactic acid bacteria. The retention times and spectral characteristics of specific folate calibrators were used to identify the different folate derivatives in the cells of S. thermophilus and L. lactis. S. thermophilus produces 5-formyl tetrahydrofolate and 5,10-methenyl tetrahydrofolate, both in the triglutamyl form. Figure 1 shows the chromatograms of several 5-formyl polyglutamyl tetrahydrofolate calibrators (at 280 nm) and 5,10-methenyl tetrahydrofolate (at 360 nm) and cell extracts of S. thermophilus. On the basis of the characteristic UV absorption maximum around 360 nm of 5,10-methenyl tetrahydrofolate (19), the components detected at 360 nm can be identified as 5,10-methenyl tetrahydrofolate with different lengths of the polyglutamate tail. The elution time of 5,10-methenyl tetrahydrofolate is 2 min less than the elution time of 5-formyl tetrahydrofolate. It can be assumed that 5,10-methenyl polyglutamyl folates also elute just before the corresponding 5-formyl polyglutamyl folates. Thus, the detected 5,10-methenyl tetrahydrofolates in S. thermophilus could be identified as 5,10-methenyl tetrahydrofolate with three glutamate residues. After enzymatic deconjugation, most of the 5,10-methenyl polyglutamyl folate was transformed into 5,10-methenyl mono- and diglutamyl folate.
FIG. 1.
Chromatograms of folate calibrators (A and C), cell extracts of S. thermophilus before (B and D) and after deconjugation (E) monitored by UV absorption at 280 nm (A and B) and 360 nm (C to E). Peaks of the following folate compounds are shown: 5-formyl tetrahydrofolate-Glu1 (S and R diastereoisomer) (peak 1), 5-formyl tetrahydrofolate-Glu2 (peak 2), 5-formyl tetrahydrofolate-Glu3 (peak 3), 5-formyl tetrahydrofolate-Glu4 (peak 4), 5-formyl tetrahydrofolate-Glu5 (peak 5), 5,10-methenyl tetrahydrofolate-Glu1 (peak 6), 5,10-methenyl tetrahydrofolate-Glu2 (peak 7), and 5,10-methenyl tetrahydrofolate-Glu3 (peak 8). Elution time is shown on the x axis, and UV absorption (in milli absorbance units [mAU]) is shown on the y axis.
In the cell extracts of L. lactis, 5,10-methenyl polyglutamyl folate could also be detected at 360 nm (in this case with four, five, or six glutamate residues) (data not shown). The fluorescence detection and UV absorption of the L. lactis samples also show the presence of polyglutamyl folates, with a UV absorption maximum of 262 nm (data not shown). On the basis of the UV absorption maximum of 262 nm, we assumed that the unknown polyglutamyl folates (also with four, five, or six glutamate residues) are 10-formyl polyglutamyl folates (M. D. Lucock, personal communication). No other folate derivatives, including 5-formyl polyglutamyl folate, could be identified.
Effects of cultivation conditions on folate production.
The effects of growth conditions and medium composition were analyzed further, especially the influence of pH, PABA, hemin, and growth rate. Under controlled growth conditions in a chemostat, the total amount of folate produced in both L. lactis and S. thermophilus increased more than threefold when the pH was increased from 5.5 to 7.5 (Table 2).
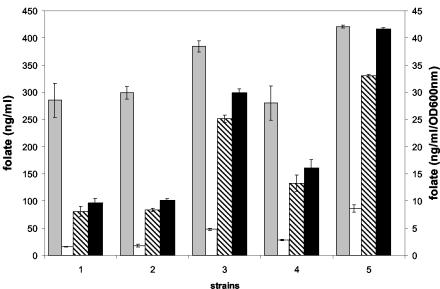
A further increase in folate production was observed in pH-controlled batch fermentations with excess glucose. The amount of biomass produced increased by more than threefold in pH-controlled batch fermentations over that in non-pH-controlled batch fermentations. The increase in folate production is even more than the increase in biomass and reaches levels up to 400 ng/ml (data not shown). Folate production per cell also increased in L. lactis strain KB, which is naturally defective in lactate dehydrogenase (LDH) activity, and an engineered LDH-negative strain (L. lactis NZ9010) (Table 1). To promote maximum cell growth, these strains were grown aerobically. Further increases in biomass and folate production per cell were observed in strains that were defective in LDH activity and that were grown under aerobic conditions in the presence of hemin (Fig. 2). Analysis of the fermentation characteristics of these cells showed that lactate production was decreased and that production of acetate and acetoin was increased (data not shown) compared to that of cells grown anaerobically.
FIG. 2.
Folate production and distribution in different L. lactis strains grown aerobically and anaerobically with and without hemin in M17 medium. Total folate production per OD600 unit (right y axis) (grey bars) and extracellular folate (white bars), intracellular folate (hatched bars), and total folate production (black bars) (left y axis) are shown. Bars: 1, wild-type strain grown anaerobically; 2, wild-type strain grown aerobically; 3, ldh mutant strain grown aerobically; 4, wild-type strain grown anaerobically with hemin; 5, ldh mutant strain grown aerobically with hemin.
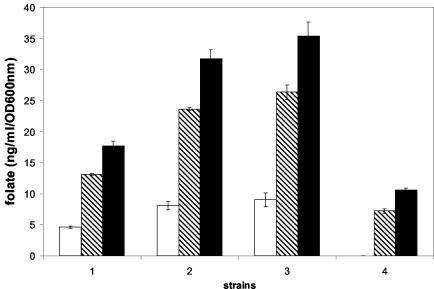
One of the folate precursors is PABA, which is synthesized via glycolysis in the pentose phosphate pathway and shikimate pathway. In L. lactis, folate production was shown to be dependent on the concentration of PABA in the medium. The addition of PABA at a concentration ranging from 1 to 100 μM to minimal medium lacking aromatic amino acids, purines, and folate resulted in a twofold increase of folate production (Fig. 3). Concentrations of PABA above 100 μM did not result in a further increase in folate. Folate production decreased twofold by the addition of tyrosine (1.2 mM) to the medium (Fig. 3). However, the negative effect of tyrosine on folate production could be compensated for by the addition of PABA (Fig. 3). The changes measured in the folate microbiological assay upon the addition of PABA or tyrosine were not a result of direct growth stimulation or inhibition of Lactobacillus casei (data not shown).
FIG. 3.
Influence of PABA and tyrosine on folate production of L. lactis grown in CDM. Extracellular folate (white bars), intracellular folate (hatched bars), and total folate production (black bars) are shown. Strains were grown under the following conditions: without PABA and without tyrosine (bars 1), with 100 μM PABA and 1.2 mM tyrosine (bars 2), with 100 μM PABA but without tyrosine (bars 3), and with 1.2 mM tyrosine but without PABA (bars 4).
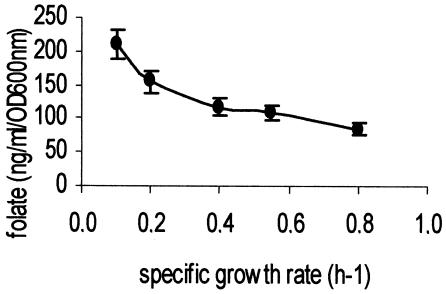
Folate production could be improved further in the two lactic acid bacteria by inhibiting growth with antibiotics or with increasing concentrations of NaCl. In L. lactis, the addition of growth-inhibitory concentrations of chloramphenicol (2 μg/ml) and erythromycin (0.1 μg/ml) lead to 40 and 215% increases in folate production, respectively. The addition of NaCl to the growth medium increased folate production in both L. lactis and S. thermophilus. In the latter bacterium, 0.8% (wt/vol) NaCl was the highest salt concentration allowing growth in a semisynthetic medium. Under these conditions, the specific growth rate was strongly reduced (90%) and a 10-fold increase in folate production was observed. The relationship between growth rate and folate production was further investigated in continuous cultures. S. thermophilus NIZO strain B119 was grown in modified M17 medium with lactose as the growth-limiting substrate. The growth rate was controlled by the flow rate of the incoming growth medium. At low dilution rates, the yield of folate in the bioreactor was increased (Fig. 4), although the specific folate production (in nanograms per milliliter per OD600 unit per hour) did decrease slightly with the lower dilution rates. A similar inverse relationship between growth rate and folate production was observed for L. lactis MG1363, with glucose as the growth-limiting substrate in a CDM (data not shown).
FIG. 4.
Total folate production by S. thermophilus NIZO strain B119 grown at different dilution rates (= growth rates) in continuous culture in modified M17 medium (see Materials and Methods).
DISCUSSION
Several species and strains from the lactic acid bacterial genera Lactococcus, Lactobacillus, Streptococcus, and Leuconostoc were screened for folate production. The lactic acid bacteria L. lactis MG1363 and S. thermophilus B119 were further analyzed for folate production under different growth conditions. L. lactis, S. thermophilus, and Leuconostoc spp. produced folate in the range of 5 to 291 μg/liter. Lactobacillus strains, with the exception of Lactobacillus plantarum, did not produce folate. In several strains, folate analysis performed after deconjugation resulted in detection of higher folate levels. This indicates that part of the folate is present as polyglutamyl folate with more than three glutamate residues.
All folate-producing strains showed partial excretion of folate into the external medium. In L. lactis, up to 90% of the total produced folate remained in the cell and was identified as 5,10-methenyl tetrahydrofolate and presumably 10-formyl tetrahydrofolate, both with four, five, or six glutamate residues. In S. thermophilus, much less of the total produced folate remained in the cell and was identified as 5-formyl tetrahydrofolate and 5,10-methenyl tetrahydrofolate, both with three glutamate residues. The difference in distribution can probably be explained by the different length of the polyglutamyl tail of the two microorganisms. One of the functions of the polyglutamyl tail is believed to be the retention of folate within the cell (22, 30). The longer polyglutamyl tail identified in L. lactis improves the retention of folate. It can be assumed that cell retention of folates is mainly a result of the negative charge of the carboxyl groups of (polyglutamyl) folate (pKa of 4.6). Hence, polyglutamyl folates with longer glutamyl tails will have a more negative charge than polyglutamyl folates with short glutamyl tails. Moreover, in S. thermophilus, the intra- and extracellular folate distribution was influenced by the pH. Cells that were grown at low pH had a larger extracellular folate fraction than cells that were cultured at a high pH. In both S. thermophilus and L. lactis cells, it has been observed that when the extracellular pH was decreased, the intracellular pH also decreased (31; J. Hugenholtz, M. Starrenburg, and T. Abee, Actes du Colloque LACTIC 97, p. 469, 1997). Consequently, at a lower intracellular pH, a higher concentration of the folates is protonated and electrically neutral, enhancing transport across the membrane. In L. lactis, no influence of pH on intra- and extracellular folate distribution was observed. We assume that the polyglutamyl folates with four, five, and six glutamate residues are not protonated at lower intracellular pH to the same extent as the polyglutamyl folates with three glutamate residues, as detected in S. thermophilus.
In continuous cultures, it was observed that the production of folate increased with increasing pH. This is in agreement with literature data that report on the high pH optima (between 7.3 and 9.3) of all the enzymes involved in folate biosynthesis analyzed in several (micro)organisms (4, 20, 21, 25, 27, 29, 34, 42). Therefore, we assume that the activity of folate-synthesizing enzymes is increased under conditions with a high external pH that lead to a more alkaline cytosol compared to conditions with low external pH (31; Hugenholtz et al., Actes du Colloque LACTIC 97). This hypothesis is supported by the observations that more folate per cell is produced in batch fermentations with controlled pH. Under such conditions, acidification of the medium is delayed, and consequently, growth of the microorganisms is prolonged and the intracellular pH may be maintained at a more optimum level for efficient folate biosynthesis. Slow acidification of the fermentation broth and increased folate production are also achieved during the growth of a L. lactis strain that is defective in lactate production because of the deletion of a LDH-encoding gene. The total folate levels were increased in this strain because of increased biomass and probably, more efficient folate biosynthesis at higher intracellular pH. The addition of hemin to the growth medium resulted in a further increase of folate produced per cell biomass. We assume that hemin stimulates the direct oxidation of NADH by oxygen. As a consequence, NADH levels decrease and pyruvate is consumed via alternative nonreducing pathways, resulting in decreased acidification (less lactate, more acetoin) (18). Recently, it was reported that during aerobic growth the addition of hemin extends the growth period (10) and that hemin may reconstitute proton extrusion (5). This may indicate that the proton gradient in aerobically grown cells in the presence of hemin is higher and that, consequently, a higher intracellular pH is maintained. As was already described for pH-controlled fermentations, folate biosynthesis is assumed to increase at higher intracellular pH.
Folate production was stimulated further when growth was inhibited. This was observed in batch cultures in the presence of growth-inhibiting concentrations of several antibiotics and in the presence of high salt concentrations. In continuous cultures, folate concentration in the reactor increased at lower growth rate (Fig. 4). However, the folate production per biomass per hour decreased at lower growth rates. The reason for increased folate production when growth was inhibited is not yet known. It could be that under conditions of low growth rate, GTP, one of the folate precursors, accumulates because of decreased DNA and RNA synthesis. Interestingly, in earlier work we reported that overproduction of GTP cyclohydrolase I (EC 3.5.4.16), the first enzyme involved in folate biosynthesis, leads to increased production of folates by L. lactis (36). Despite uncertainties about the mechanism, our results show that food fermentations aimed at increasing in situ folate levels could be best performed at low growth rates and a high pH.
Folate can be synthesized in L. lactis in the absence of PABA, indicating that L. lactis has the ability to synthesize PABA. However, the addition of PABA to the medium leads to an increase in folate production. This suggests that the synthesis of PABA is a rate-determining step in the production of folate by L. lactis. The biosynthesis pathway of PABA and aromatic amino acids may proceed via a common pathway to chorismate. Studies of the synthesis of aromatic amino acids in Corynebacterium glutamicum have shown that the shikimate pathway is under tight control of tyrosine (14). The addition of tyrosine to the medium of L. lactis resulted in a decrease in folate production. It can be assumed that in L. lactis also, tyrosine leads to a feedback inhibition of phospho-2-dehydro-3-deoxyheptonate aldolase and that folate production is indirectly affected by the biosynthesis of aromatic amino acids. We are currently working on the development of strains that are deregulated in the biosynthesis of aromatic amino acids as an approach to increase the in situ production of PABA and folate.
The observation that the level of folate produced is influenced by the specific lactic acid bacterium, growth conditions, and medium used could have a large impact on the manufacture of dairy products. For instance, by specifically selecting high-folate-producing strains as part of the starter culture, yogurt with elevated levels of folate could be produced (35, 41). Furthermore, it is expected that in combination with specific growth conditions and metabolic engineering approaches (36), the current contribution of yogurt of 10 to 20% to the average daily intake for folate could be substantially increased.
Acknowledgments
L.T. was supported by the Stichting Zuivel Voeding & Gezondheid (SZVG).
REFERENCES
- 1.Alm, L. 1980. Effect of fermentation on B-vitamin content of milk in Sweden. J. Dairy Sci. 65:353-359. [Google Scholar]
- 2.Ames, B. N. 1999. Micronutrient deficiencies cause DNA damage and cancer. Food Sci. Agric. Chem. 1:1-15. [Google Scholar]
- 3.Ayad, E. H. E., A. Verheul, C. de Jong, J. T. M. Wouters, and G. Smit. 1999. Flavour forming abilities and amino acid requirements of Lactococcus lactis strains isolated from artisanal and non-dairy origin. Int. Dairy J. 10:169-179. [Google Scholar]
- 4.Ballantine, S. P., F. Volpe, and C. J. Delves. 1994. The hydroxymethyldihydropterin pyrophosphokinase domain of the multifunctional folic acid synthesis Fas protein of Pneumocystis carinii expressed as an independent enzyme in Escherichia coli: refolding and characterization of the recombinant enzyme. Protein Expr. Purif. 5:371-378. [DOI] [PubMed] [Google Scholar]
- 5.Blank, L. M., B. J. Koebmann, O. Michelsen, L. K. Nielsen, and P. R. Jensen. 2001. Hemin reconstitutes proton extrusion in an H+-ATPase-negative mutant of Lactococcus lactis. J. Bacteriol. 183:6707-6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5a.Bongers, R. S., M. H. N. Hoefnagel, M. J. C. Starrenburg, M. A. J. Siemerink, J. G. A. Arends, J. Hugenholtz, and M. Kleerebezem.2003. IS981-mediated adaptive evolution recovers lactate production by ldhB transcription activation in a lactate dehydrogenase-deficient strain of Lactococcus lactis. J. Bacteriol. 185:4499-4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boushey, C. J., A. A. Beresford, G. S. Omenn, and A. G. Moltulsky. 1996. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. JAMA 274:1049-1057. [DOI] [PubMed] [Google Scholar]
- 7.Brattstrom, L. 1996. Vitamins as homocysteine-lowering agents. J. Nutr. 126:1276S-1280S. [DOI] [PubMed]
- 8.De Man, J. C., M. Rogosa, and M. E. Sharpe. 1960. Cultivation medium for lactobacilli. J. Appl. Bacteriol. 23:130-135. [Google Scholar]
- 9.Driessen, F. M., J. Ubbels, and J. Stadhouders. 1977. Continuous manufacture of yogurt: optimal conditions and kinetics of the prefermentation process. Biotechnol. Bioeng. 21:821-839. [Google Scholar]
- 10.Duwat, P., S. Sourice, B. Cesselin, G. Lamberet, K. Vido, P. Gaudu, Y. Le Loir, F. Violet, P. Loubiere, and A. Gruss. 2001. Respiration capacity of the fermenting bacterium Lactococcus lactis and its positive effects on growth and survival. J. Bacteriol. 183:4509-4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gasson, M. J. 1983. Plasmid complementation of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J. Bacteriol. 154:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green, J., B. P. Nichols, and R. G. Matthews. 1996. Folate biosynthesis, reduction, and polyglutamylation, p. 665-673. In F. C. Neidhardt, J. L. Ingraham, K. B. Low, B. Magasanik, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella typhimurium: cellular and molecular biology, 1st ed. ASM Press, Washington, D.C.
- 13.Horne, D. W., and D. Patterson. 1988. Lactobacillus casei microbiological assay of folic acid derivatives in 96-well microtiter plates. Clin. Chem. 34:2357-2359. [PubMed] [Google Scholar]
- 14.Ikeda, M., and R. Katsumata. 1992. Metabolic engineering to produce tyrosine or phenylalanine in a tryptophan-producing Corynebacterium glutamicum strain. Appl. Environ. Microbiol. 58:781-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klaver, F. A. M., F. Kingma, and A. C. Bolle. 1990. Growth relations between bifidobacteria and lactobacilli in milk. Voedingmiddelentechnologie 9:13-16. [Google Scholar]
- 16.Konings, E. J., H. H. Roomans, E. Dorant, R. A. Goldbohm, W. H. Saris, and P. A. van den Brandt. 2001. Folate intake of the Dutch population according to newly established liquid chromatography data for foods. Am. J. Clin. Nutr. 73:765-776. [DOI] [PubMed] [Google Scholar]
- 17.Lin, M. Y., and C. M. Young. 2000. Folate levels in cultures of lactic acid bacteria. Int. Dairy J. 10:409-414. [Google Scholar]
- 18.Lopez de Felipe, F., and J. Hugenholtz. 1999. Pyruvate flux distribution in NADH-oxidase-overproducing Lactococcus lactis strain as a function of culture conditions. FEMS Microbiol. Lett. 179:461-466. [DOI] [PubMed] [Google Scholar]
- 19.Lucock, M. D., M. Green, M. Priestnall, I. Daskalakis, M. I. Levene, and R. Hartley. 1995. Optimisation of chromatographic conditions for the determination of folates in foods and biological tissues for nutritional and clinical work. Food Chem. 55:329-338. [Google Scholar]
- 20.Mathis, J. B., and G. M. Brown. 1970. The biosynthesis of folic acid. XI. Purification and properties of dihydroneopterin aldolase. J. Biol. Chem. 245:3015-3025. [PubMed] [Google Scholar]
- 21.McGuire, J. J., P. Hsieh, J. K. Coward, and J. R. Bertino. 1980. Enzymatic synthesis of folylpolyglutamates. Characterization of the reaction and its products. J. Biol. Chem. 255:5776-5788. [PubMed] [Google Scholar]
- 22.McGuire, J. J., and J. R. Bertino. 1981. Enzymatic synthesis and function of folylpolyglutamates. Mol. Cell. Biochem. 38:19-48. [DOI] [PubMed] [Google Scholar]
- 23.McKay, L. L., and K. A. Baldwin. 1974. Altered metabolism of Streptococcus lactis C2 deficient in lactic dehydrogenase. J. Dairy Sci. 57:181-186. [DOI] [PubMed] [Google Scholar]
- 24.O'Brien, M. M., M. Kiely, K. E. Harrington, P. J. Robson, J. J. Strain, and A. Flynn. 2001. The efficacy and safety of nutritional supplement use in a representative sample of adults in the North/South Ireland Food Consumption Survey. Public Health Nutr. 4:1069-1079. [DOI] [PubMed] [Google Scholar]
- 25.Pongsamart, S., R. I. Ho, L. Corman, and W. O. Foye. 1984. Characterization and inhibition of dihydrofolate synthetase from Neisseria gonorrhoeae. Mol. Cell. Biochem. 59:165-171. [DOI] [PubMed] [Google Scholar]
- 26.Poolman, B., and W. N. Konings. 1988. Relation of growth of Streptococcus lactis and Streptococcus cremoris to amino acid transport. J. Bacteriol. 170:700-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rao, K. N. 2000. Purification and characterization of dihydrofolate reductase from Lactobacillus leichmannii. Indian J. Biochem. Biophys. 37:121-129. [PubMed] [Google Scholar]
- 28.Rao, D. R., A. V. Reddy, S. R. Pulusani, and P. E. Cornwell. 1984. Biosynthesis and utilization of folic acid and vitamin B12 by lactic cultures in skim milk. J. Dairy Sci. 67:1169-1174. [Google Scholar]
- 29.Richey, D. P., and G. M. Brown. 1969. The biosynthesis of folic acid. IX. Purification and properties of the enzymes required for the formation of dihydropteroic acid. J. Biol. Chem. 244:1582-1592. [PubMed] [Google Scholar]
- 30.Shane, B., and E. L. Stokstad. 1975. Transport and metabolism of folates by bacteria. J. Biol. Chem. 250:2243-2253. [PubMed] [Google Scholar]
- 31.Siegumfeldt, H., K. B. Rechinger, and M. Jakobsen. 2000. Dynamic changes of intracellular pH in individual lactic acid bacterium cells in response to a rapid drop in extracellular pH. Appl. Environ. Microbiol. 66:2330-2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Starrenburg, M. J. C., and J. Hugenholtz. 1991. Citrate fermentation by Lactococcus and Leuconostoc spp. Appl. Environ. Microbiol. 57:3535-3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stover, P., and V. Schirch. 1993. The metabolic role of leucovorin. Trends Biochem. Sci. 18:102-106. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki, Y., T. Yasui, and S. Abe. 1979. Occurrence of GTP cyclohydrolase I in Bacillus stearothermophilus. J. Biochem. (Tokyo) 86:1679-1685. [DOI] [PubMed] [Google Scholar]
- 35.Sybesma, W., M. Starrenburg, I. Mierau, M. Kleerebezem, W. M. De Vos, and J. Hugenholtz. 2002. Control of folate production in lactic acid bacteria by using metabolic engineering, p. 623-630. In S. Milstien, G. Kapatos, R. A. Levine, and B. Shane (ed.), Chemistry and biology of pteridines and folates. Kluwer Academic Publishers, Boston, Mass.
- 36.Sybesma, W., M. Starrenburg, M. Kleerebezem, I. Mierau, W. M. de Vos, and J. Hugenholtz. 2002. Increased production of folate by metabolic engineering of Lactococcus lactis. Appl. Environ. Microbiol. 69:3069-3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tamura, T., Y. S. Shin, M. A. Williams, and E. L. R. Stokstad. 1972. Lactobacillus casei response to pteroylpolyglutamates. Anal. Biochem. 49:517-521. [DOI] [PubMed] [Google Scholar]
- 38.Terzaghi, B. E., and W. E. Sandine. 1975. Improved medium for lactic streptococci and their bacteriophages. Appl. Microbiol. 29:807-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van der Put, N. M., H. W. van Straaten, F. J. Trijbels, and H. J. Blom. 2001. Folate, homocysteine and neural tube defects: an overview. Exp. Biol. Med. (Maywood) 226:243-270. [DOI] [PubMed] [Google Scholar]
- 40.Wald, N. 1991. Prevention of neural tube defects. Results of the Medical Research Council vitamin study. Lancet 338:131-137. [PubMed] [Google Scholar]
- 41.Wouters, J. T. M., E. H. E. Ayad, J. Hugenholtz, and G. Smit. 2002. Microbes from raw milk for fermented dairy products. Int. Dairy J. 12:91-109. [Google Scholar]
- 42.Yoo, J. C., J. M. Han, O. H. Ko, and H. J. Bang. 1998. Purification and characterization of GTP cyclohydrolase I from Streptomyces tubercidicus, a producer of tubercidin. Arch. Pharm. Res. 21:692-697. [DOI] [PubMed] [Google Scholar]