Abstract
Streptococcus suis serotype 2 is a major pathogen found in the upper respiratory tract of swine. In this study, isolates of this bacterial species were tested for the production of bacteriocin-like inhibitory substances (BLIS). Of the 38 strains tested, four inhibited the growth of other S. suis isolates according to a deferred-antagonism plate assay. Interestingly, three of the strains were originally isolated from healthy carrier pigs and were considered nonvirulent. Three isolates (94-623, 90-1330, and AAH4) that produced BLIS in liquid broth were selected for further characterization. None of the inhibitory activities was related to the production of either organic acids or hydrogen peroxide. The BLIS produced by these strains were heat stable and proteinase K, pronase, and elastase sensitive but were trypsin and chymotrypsin resistant. They were stable at pH 2 and 12 and had molecular masses in the range of 14 to 30 kDa. Maximum production was observed during the mid-log phase. Following a curing procedure with novobiocin, only 90-1330 lost the ability to produce BLIS, suggesting that the BLIS might be plasmid encoded. Analysis of the inhibitory spectra revealed that the BLIS-producing strains also inhibited the growth of Actinobacillus minor, Actinobacillus porcinus, Enterococcus durans, Micrococcus luteus, Streptococcus agalactiae, Streptococcus dysgalactiae subsp. dysgalactiae, Streptococcus equi subsp. zooepidemicus, and S. dysgalactiae subsp. equisimilis. This study reports for the first time the ability of the swine pathogen S. suis serotype 2 to produce BLIS with the characteristics of classic bacteriocins. Further studies are required to investigate the possibility of using bacteriocin-producing strains to prevent swine infections caused by virulent strains of S. suis serotype 2.
A number of chemical substances, including bacteriocins, are secreted by cells during the various stages of bacterial growth. Bacteriocins have the ability to inhibit closely related and sometimes more distantly related strains of bacteria and thus play a major role in the natural defense systems of several bacterial species (12). Bacteriocins produced by indigenous bacteria may be critical for the maintenance of normal microflora and host health by preventing invasion by exogenous pathogens (3). Since a number of bacterial species, including Streptococcus suis, Haemophilus parasuis, Rothia nasimurium, Staphylococcus aureus, Arcanobacterium pyogenes, and Actinomyces hyovaginalis, are found in the upper respiratory tract (URT) of pigs (2), it is possible that the URT may be a favorable environment for the secretion of competition factors like bacteriocins.
The predominant streptococcal species in the URT of swine, more particularly the tonsils and nasal cavities, is S. suis (2). There are presently 35 serotypes of S. suis (serotypes 1 to 34 and serotype 1/2) recognized on the basis of capsular antigens, although serotype 2 is the strain most frequently associated with infections in pigs (8, 24), especially meningitis (7, 8, 24). Other pathologies, such as arthritis, pneumonia, endocarditis, and septicemia leading to sudden death, have also been reported (7, 8, 24). S. suis infections are largely controlled by using prophylactic and therapeutic antibiotics, including penicillin (8). However, a recent increase in antibiotic-resistant S. suis isolates combined with the growing public concern over the use of prophylactic antimicrobials in farming indicates that alternative strategies for controlling S. suis infections are required (1, 4, 13, 15, 26). Until now, most vaccines developed to protect swine against S. suis infections have used inactivated bacteria and results have been inconsistent (8). In this study, we propose that bacterial interference or bacteriocins may be a promising new avenue for the prevention and treatment of S. suis infections. Since there are no scientific data on antagonistic interactions involving S. suis, we investigated the production of bacteriocin-like inhibitory substances (BLIS) by S. suis serotype 2 isolates.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The bacterial isolates used in this study are listed in Table 1 and were kindly provided by M. Gottschalk from the Groupe de Recherche sur les Maladies Infectieuses du Porc at Université de Montréal (Montreal, Quebec, Canada). Micrococcus luteus ATCC 272 was obtained from M. Lavoie (Université Laval, Quebec City, Quebec, Canada). Bacteria were routinely grown aerobically at 37°C in Todd-Hewitt broth (THB; Becton Dickinson, Cockeysville, Md.) or on THB agar (THA) plates. LA broth medium was used to investigate the kinetics of BLIS production and to obtain BLIS-containing supernatants. LA broth contains 1% glucose, 2% peptone (Proteose Peptone no. 3; Difco, Detroit, Mich.), 0.3% K2HPO4, 0.2% KH2PO4, 0.01% MgSO4 · 7H2O, 0.002% MnSO4 · 6H2O, and 0.5% NaCl (9). BLIS production in THB, Trypticase soy broth (Becton Dickinson) supplemented with 3% yeast extract, and M17 broth (Difco) supplemented with 0.5% glucose was also evaluated.
TABLE 1.
Bacterial strains used in this study
| Strain | Origin | Geographic origin |
|---|---|---|
| A. minor AMX 2B | Healthy carrier pig | Quebec, Canada |
| A. porcinus 96-0088-3F | Healthy carrier pig | Quebec, Canada |
| E. durans DM | NAc | NA |
| E. faecalis ATCC 19433 | NA | NA |
| E. faecalis ATCC 29212 | Urine | NA |
| E. hirae ATCC 8043 | NA | NA |
| H. parasuis 99-9048-B | Diseased pig | Quebec, Canada |
| M. luteus ATCC 272 | NA | NA |
| P. multocida 01-14-97-2 | Diseased pig | Quebec, Canada |
| S. hyicus ATCC 11249 | Pig with epidermitis | NA |
| S. agalactiae ATCC 13813 | NA | NA |
| S. dysgalactiae subsp. dysgalactiae ATCC 27957 | Infected bovine | NA |
| S. dysgalactiae subsp. dysgalactiae ATCC 9926 | NA | NA |
| S. equi subsp. zooepidemicus ATCC 6580 | Horse with strangles | United States |
| S. equi subsp. zooepidemicus ATCC 43079 | Bovine mastitis | England |
| S. dysgalactiae subsp. equisimilis ATCC 9542 | NA | NA |
| S. bovis ATCC 9809 | NA | NA |
| S. bovis B457C | Diseased pig, kidney | NA |
| S. suis S428 serotype 1 | Diseased pig | Europe |
| S. suis 2651 serotype 1/2 | Diseased pig | Europe |
| S. suis 31533 serotype 2 | Diseased pig | France |
| S. suis 89-999 serotype 2 | Diseased pig | Quebec, Canada |
| S. suis 94-623 serotype 2 | Healthy carrier pig | France |
| S. suis 90-1330 serotype 2 | Healthy carrier pig | Canada |
| S. suis 94-3037 serotype 2 | Human; meningitis | Quebec, Canada |
| S. suis S735 serotype 2a | Diseased pig | The Netherlands |
| S. suis mutant 2A serotype 2b | S-735 | Quebec, Canada |
| S. suis Reims serotype 2 | Human | France |
| S. suis 166 serotype 2 | Diseased pig | France |
| S. suis 24 serotype 2 | Diseased pig | France |
| S. suis 65 serotype 2 | Healthy carrier pig | Europe |
| S. suis B268a serotype 2 | Healthy carrier pig | Quebec, Canada |
| S. suis T15 serotype 2 | Healthy carrier pig | Europe |
| S. suis TD10 serotype 2 | Healthy carrier pig | United Kingdom |
| S. suis AAH4 serotype 2 | Diseased pig | United States |
| S. suis SS 93 serotype 2 | Diseased pig | France |
| S. suis D 282 serotype 2 | Diseased pig | The Netherlands |
| S. suis JL 590 serotype 2 | Diseased pig | Mexico |
| S. suis 17 serotype 2 | NA | The Netherlands |
| S. suis 89-5046 serotype 2 | Diseased pig | Quebec, Canada |
| S. suis 4/3 HI serotype 2 | Healthy carrier pig | Quebec, Canada |
| S. suis LM 90-559 serotype 2 | Diseased pig | Canada |
| S. suis EA 0891/90 serotype 2 | Diseased pig | Canada |
| S. suis 95-8242 serotype 2 | Diseased pig | Quebec, Canada |
| S. suis 89-4223 serotype 2 | Healthy carrier pig | Quebec, Canada |
| S. suis 98-3473-5 serotype 2 | Pig; clinical strain | Quebec, Canada |
| S. suis 98-B575 serotype 2 | Pig; clinical strain | Quebec, Canada |
| S. suis 90-2741-7 serotype 2 | Diseased pig | Quebec, Canada |
| S. suis 770 353 serotype 2 | Human; meningitis | The Netherlands |
| S. suis 770 297 serotype 2 | Human; meningitis | The Netherlands |
| S. suis 96-52466 serotype 2 | Human; arthritis | France |
| S. suis 98-3634 serotype 2 | Human; endocarditis | Quebec, Canada |
| S. suis 4961 serotype 3 | Diseased pig | Europe |
| S. suis Amy12C serotype 5 | Pig; clinical strain | Quebec, Canada |
| S. suis 4B serotype 5 | Pig; clinical strain | Quebec, Canada |
ATCC 43765 S. suis serotype 2 reference strain.
S. suis S735 mutant that does not express capsular polysaccharide.
NA, not available.
Deferred-antagonism plate assay.
The bacterial isolates listed in Table 1 were tested for antagonistic interactions on THA plates. The producing bacteria were inoculated along a straight line through the center of THA plates by using sterile cotton-tipped swabs dipped in overnight broth cultures. After a 24-h incubation at 37°C to allow bacterial growth and BLIS production, the indicator strains were inoculated perpendicular to the producing bacteria with sterile cotton-tipped swabs dipped in overnight broth cultures. The plate contents were incubated for a further 24 h at 37°C before they were examined for the presence of inhibition zones at the intersections of the streaks.
Kinetics of BLIS production.
The kinetics of BLIS production by S. suis 94-623 was determined in LA broth. Tubes of LA broth were inoculated with a 10% (vol/vol) inoculum of an 18-h culture and were incubated at 37°C. Samples were taken every 20 min (15 min during the exponential growth phase). The optical densities at 660 nm (OD660) were monitored, and the BLIS titers of the culture supernatants were determined by using a plate diffusion assay. The culture supernatants were adjusted to pH 7.0, filter sterilized, and serially diluted in LA broth containing 1% (vol/vol) Tween 20. Fifty-microliter samples of the dilutions were placed in glass penicylinders (8 by 8 mm; Bellco Glass Inc., Vineland, N.J.) on THA plates that had been inoculated with a standardized quantity of indicator strain S. suis 24 (100 μl of a bacterial suspension at an OD660 of 0.4). After a 24-h incubation at 37°C, the BLIS titer was expressed in arbitrary units (AU) defined as the reciprocal of the highest dilution for which the growth of the indicator strain was inhibited.
Mode of action.
The mode of action of the BLIS was investigated as follows: the BLIS-producing strains (S. suis 94-623, 90-1330, and AAH4) were grown in LA broth for 48 h. The culture supernatants were collected by centrifugation (10,000 × g for 15 min), adjusted to pH 7.0, and filter sterilized. One milliliter of each culture supernatant was added to 10 ml of an early-log-phase culture of S. suis S735 in THB. The OD660 was then measured at various times for up to 24 h. One milliliter of culture supernatant from the non-BLIS-producing S. suis S735 strain was used as a negative control.
Plasmid curing.
Plasmid curing of the four BLIS-producing S. suis isolates was attempted by using the procedure described by Shehane and Sizemore (23). Tubes of THB medium containing 1.0, 4.0, 8.0, 10.0, or 20.0 μg of novobiocin/ml (Sigma Chemical Co., St. Louis, Mo.) were inoculated with overnight S. suis cultures and were incubated for 48 h at 37°C prior to spreading serial dilutions on THA plates. After an overnight incubation, 100 bacterial colonies from each isolate were selected and tested for BLIS production as follows: bacterial colonies grown on THA plates were covered with 7 ml of soft THA (0.75% [wt/vol] agar) containing 0.75 ml of an overnight culture of the indicator strain S. suis 24 (adjusted to an OD660 of 0.2). The plate contents were incubated for a further 24 h and were then examined for inhibition zones.
Characterization of BLIS.
The susceptibility of the BLIS produced by strains 94-623, 90-1330, and AAH4 to enzymatic, pH, and temperature treatments was determined by using the plate diffusion assay described above. The proteolytic enzymes used were trypsin (type I; Sigma) α-chymotrypsin (type II; Sigma), elastase (type II-A; Sigma), pronase (Boehringer Mannheim, Laval, Quebec, Canada), and proteinase K (Boehringer Mannheim), each at a final concentration of 0.5 mg/ml. The effect of catalase (Sigma) at a final concentration of 1,000 U/ml was also investigated. Aliquots of culture supernatants from BLIS-producing S. suis strains grown in LA broth medium were treated for 2 h at 37°C with each enzyme individually. They were then boiled for 5 min to inactivate the enzymes. The pHs of the culture supernatants were adjusted to 2 or 12 by using 1 N HCl or 1 N NaOH, respectively, to evaluate the sensitivity of the BLIS to extreme pHs. The supernatants were incubated at room temperature for 2 h, the pH was then readjusted to 7.0, and the BLIS titers were determined. To evaluate temperature stability, the supernatants were incubated at 100°C for 15 min or 121°C for 20 min and were rapidly chilled on ice and the BLIS titers were determined. Lastly, the culture supernatants of BLIS-producing S. suis strains grown in LA broth were dialyzed by using tubing with a 12- to 14-kDa molecular mass cutoff. Following dialysis for 18 h at 4°C, samples were taken and tested for the presence of BLIS with the plate diffusion assay. BLIS-containing supernatants were also subjected to ultrafiltration by using membranes with 10- and 30-kDa molecular mass cutoffs. The ultrafiltrates were tested for the presence of BLIS with the plate diffusion assay. To exclude the possibility that growth inhibition could be related to a low pH resulting from acid production, the effect on the inhibition zones of adding 1% CaCO3 to THA plates was tested. The indicator strain used throughout the characterization process was S. suis 24.
RESULTS
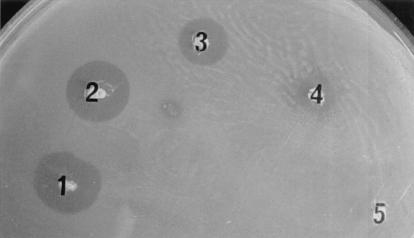
Thirty-eight strains of S. suis serotype 2 were tested for BLIS production with a deferred-antagonism plate assay. Four strains (94-623, 90-1330, AAH4, and 65) produced inhibitory activity against other S. suis strains (serotypes 1, 1/2, 2, 3, and 5) (Table 2). Strains 94-623, 90-1330, and AAH4 produced BLIS that were active against all S. suis isolates except themselves, while strain 65 inhibited the growth of all S. suis isolates except itself. Figure 1 shows the growth-inhibitory zones of the indicator strain S. suis 24 caused by the four BLIS-producing isolates. Strains 94-623, 90-1330, and 65 were originally isolated from healthy carrier pigs (from Canada and Europe), while AAH4 was isolated from a diseased pig (from the United States). BLIS production in LA broth by these four isolates was tested by using a plate diffusion assay. The supernatants of three of the isolates (94-623, 90-1330, and AAH4) contained BLIS activity, which was further characterized.
TABLE 2.
Susceptibility of S. suis strains to the four BLIS-producing S. suis strains as determined by a deferred-antagonism plate assay
| Indicator strain | Susceptibility to BLIS-producing strain:
|
|||
|---|---|---|---|---|
| 94-623 | 90-1330 | AAH4 | 65 | |
| 94-623 | −a | − | − | + |
| 90-1330 | − | − | − | + |
| AAH4 | − | − | − | + |
| 65 | +b | + | + | − |
| T15 | + | + | + | + |
| TD10 | + | + | + | + |
| S428 | + | + | + | + |
| 2651 | + | + | + | + |
| 31533 | + | + | + | + |
| 89-999 | + | + | + | + |
| 94-3037 | + | + | + | + |
| S735 | + | + | + | + |
| Mutant 2A | + | + | + | + |
| Reims | + | + | + | + |
| 166 | + | + | + | + |
| 24 | + | + | + | + |
| B268a | + | + | + | + |
| SS 93 | + | + | + | + |
| D 282 | + | + | + | + |
| JL 590 | + | + | + | + |
| 17 | + | + | + | + |
| 89-5046 | + | + | + | + |
| 4/3 HI | + | + | + | + |
| LM 90-559 | + | + | + | + |
| EA 0891/90 | + | + | + | + |
| 95-8242 | + | + | + | + |
| 89-4223 | + | + | + | + |
| 98-3473-5 | + | + | + | + |
| 98-B575 | + | + | + | + |
| 90-2741-7 | + | + | + | + |
| 770 353 | + | + | + | + |
| 770 297 | + | + | + | + |
| 96-52466 | + | + | + | + |
| 98-3634 | + | + | + | + |
| 4961 | + | + | + | + |
| Amy12C | + | + | + | + |
| 4B | + | + | + | + |
−, not inhibited.
+, inhibited.
FIG. 1.
BLIS production by S. suis 94-623 (1), S. suis 90-1330 (2), S. suis AAH4 (3), S. suis 65 (4), and S. suis 31-533 (5) (negative control). Fresh overnight cultures were spotted on THA plates and were incubated overnight. Plates were then overlaid with THA soft agar that had been inoculated with the indicator strain used (S. suis 24), and their contents were further incubated overnight.
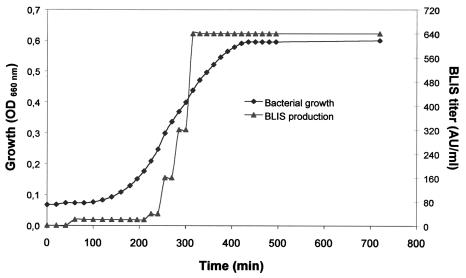
The kinetics of BLIS production in LA broth by S. suis 94-623 is presented in Fig. 2. Anti-S. suis 24 BLIS activity reached a maximum (640 AU/ml) in the culture supernatant after a 5.5-h incubation period, which corresponded to the mid-log growth phase. The activity appeared relatively stable, since the titer did not decrease when the culture was incubated for up to 24 h. On the other hand, the BLIS titer in THB, supplemented with 3% yeast extract and M17 supplemented with 0.5% glucose reached only a maximum of 20 AU/ml, despite comparable cell densities in all four media.
FIG. 2.
Kinetics of BLIS production during growth of S. suis 94-623. The growth of S. suis 94-623 was evaluated by measuring the OD660, and the BLIS titer against S. suis 24 was expressed as AU per milliliter as described in Materials and Methods.
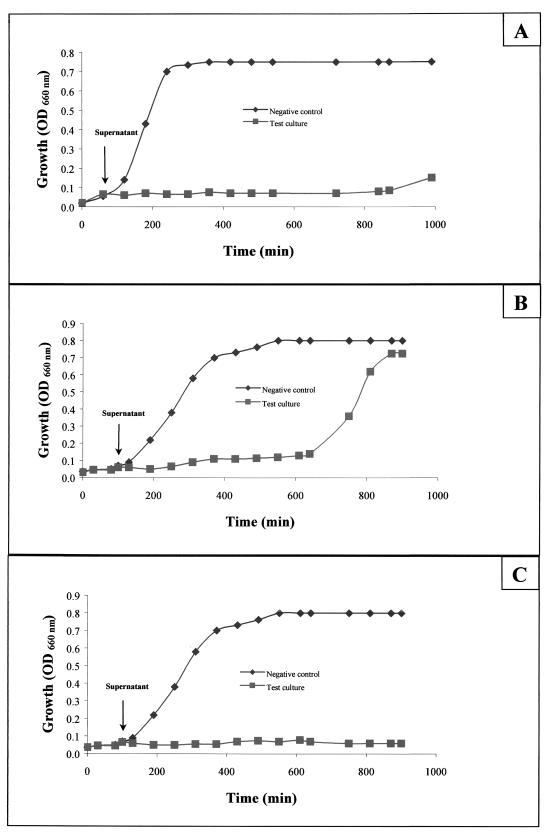
The modes of action of the BLIS produced by 94-623, 90-1330, and AAH4 are presented in Fig. 3. The growth of indicator strain S735 stopped immediately after the addition of the BLIS-containing supernatants. The BLIS produced by AAH4 appeared to be the most efficient in inhibiting bacterial growth. However, after a 24-h incubation period, the OD660 of all three test cultures was comparable to that of the control culture, suggesting that the BLIS were bacteriostatic.
FIG. 3.
Mode of action of the BLIS produced by S. suis 90-1330 (A), 94-623 (B), and AAH4 (C) on the growth of S. suis S735. The BLIS-containing supernatant (test culture) or the supernatant from a nonproducing strain (negative control) was added in the early exponential growth phase.
Following a curing procedure with novobiocin, 100 colonies of each BLIS-producing strain were tested to determine whether they retained their ability to produce BLIS. For 90-1330, all colonies lost their ability to produce BLIS when 10 μg of novobiocin/ml was added to the media, whereas only 73% of the colonies lost their BLIS activity when 4 μg of novobiocin/ml was used. All the colonies from 94-623, AAH4, and 65 retained their ability to produce BLIS in the presence of 10 μg of novobiocin/ml.
The four BLIS-producing strains were tested for their capacity to inhibit the growth of a number of gram-positive and gram-negative bacteria, most of which were isolated from swine (Table 3). Actinobacillus minor AMX 2B, Actinobacillus porcinus 96-0088-3F, Micrococcus luteus ATCC 272, Streptococcus equi subsp. zooepidemicus ATCC 6580, Streptococcus dysgalactiae subsp. dysgalactiae ATCC 27957, Streptococcus dysgalactiae subsp. equisimilis ATCC 9542, Streptococcus agalactiae ATCC 13813, and Enterococcus durans DM were inhibited by all four BLIS-producing strains, while the growth of Streptococcus bovis ATCC 9809, Pasteurella multocida 01-14-97-2, Haemophilus parasuis 99-9048-B, S. equi subsp. zooepidemicus ATCC 43079, Enterococcus faecalis ATCC 19433, E. faecalis ATCC 29212, Enterococcus hirae ATCC 8043, and Staphylococcus hyicus ATCC 11249 was unaffected. In addition, strain 65, but not strains 94-623, 90-1330, and AAH4, inhibited E. hirae ATCC 8043, S. dysgalactiae subsp. dysgalactiae ATCC 9926, and S. bovis B457C.
TABLE 3.
Properties of the BLIS produced by S. suis serotype 2
| Property or strain tested | Inhibitory activity or resistance shown by producing strain:
|
|||
|---|---|---|---|---|
| 94-623 | 90-1330 | AAH4 | 65 | |
| Production in | ||||
| Todd-Hewitt agar plates | +a | + | + | + |
| Todd-Hewitt agar plates + CaCO3 | + | + | + | + |
| Todd-Hewitt broth | + | + | + | −b |
| LA broth | + | + | + | − |
| Sensitivity to | ||||
| 100°C, 15 min | Rc | R | R | NDe |
| 121°C, 20 min | Sd | S | S | ND |
| pH 2.0 | R | R | R | ND |
| pH 12.0 | R | R | R | ND |
| Trypsin | R | R | R | ND |
| Chymotrypsin | R | R | R | ND |
| Elastase | S | S | S | ND |
| Proteinase K | S | S | S | ND |
| Pronase | S | S | S | ND |
| Dialysis (12-14 kDa)f | + | + | + | ND |
| Ultrafiltration (30 kDa)g | + | + | + | ND |
| Activity spectrum for bacteria | ||||
| Gram-positive | ||||
| E. durans DM | + | + | + | + |
| E. faecalis ATCC 19433 | − | − | − | − |
| E. faecalis ATCC 29212 | − | − | − | − |
| E. hirae ATCC 8043 | − | − | − | − |
| M. luteus ATCC 272 | + | + | + | + |
| S. hyicus ATCC 11249 | − | − | − | − |
| S. bovis ATCC 9809 | − | − | − | − |
| S. bovis B457C | − | − | − | + |
| S. agalactiae ATCC 13813 | + | + | + | + |
| S. dysgalactiae subsp. dysgalactiae ATCC 27957 | + | + | + | + |
| S. dysgalactiae subsp. dysgalactiae ATCC 9926 | − | − | − | + |
| S. equi subsp. zooepidemicus ATCC 6580 | + | + | + | + |
| S. equi subsp. zooepidemicus ATCC 43079 | − | − | − | − |
| S. dysgalactiae subsp. equisimilis ATCC 9542 | + | + | + | + |
| Gram-negative | ||||
| A. minor AMX 2B | + | + | + | + |
| A. porcinus 96-0088-3F | + | + | + | + |
| H. parasuis 99-9048-B | − | − | − | − |
| P. multocida 01-14-97-2 | − | − | − | − |
+, inhibitory activity.
−, no inhibitory activity.
R, resistant.
S, sensitive.
ND, not determined.
BLIS activity in dialysis tubing.
BLIS activity in ultrafiltrates.
Table 3 lists the effects of various treatments on anti-S. suis 24 activity. BLIS from 94-623, 90-1330, and AAH4 retained their activity after heating at 100°C for 15 min but were inactivated following treatment at 121°C for 20 min. All three BLIS were resistant to pH 2 and 12 and trypsin and chymotrypsin treatments. However, they were completely inactivated by pronase, proteinase K, and elastase. The BLIS did not pass through a dialysis membrane with a 12- to 14-kDa molecular mass cutoff but were detected in the ultrafiltrate following ultrafiltration through a membrane with a 30-kDa molecular mass cutoff. The above properties could not be determined for the BLIS produced by S. suis 65, since it could not be recovered from the culture supernatant.
DISCUSSION
Positive and negative interactions are known to modulate microbial ecosystems. Because of the ecological diversity and the high bacterial density of the URT in pigs, bacteria must compete for the same ecological niches. Bacteriocins or BLIS may play an important role in this competitive process. The aim of our study was to highlight the presence of antagonistic interactions between various S. suis serotype 2 isolates and to determine the properties of the BLIS that they produced. Only 4 of 38 S. suis serotype 2 isolates produced BLIS that were active against other S. suis isolates and other swine pathogens. Since the BLIS produced by strains 94-623, 90-1330, AAH4, and 65 inhibited only one of two S. dysgalactiae and S. equi subsp. zooepidemicus strains, it is possible that the BLIS produced by these two strains and by S. suis are closely related. Indeed, S. dysgalactiae ATCC 9926 and S. equi subsp. zooepidemicus ATCC 43079 produced BLIS that were active against several S. suis serotype 2 strains (data not shown). The fact that different BLIS can have similar immunity proteins could explain these results. For instance, the immunity proteins of curvacin A and acidocin A share 50% similarity, even though the bacteriocins share only 34% sequence homology (10).
S. suis 65 consistently produced BLIS on THA plates but never in broth media. We tried to extract the BLIS from THA plates, but we obtained inconsistent results. Similar observations have been reported for bacteriocins produced by other streptococci (11, 21). The hydrophobic nature of some BLIS and bacteriocins, which can attach to the bacterial cell surface, may explain their absence in the culture supernatant. BLIS produced by 94-623, 90-1330 and AAH4 were heat stable, were unaffected by extreme pHs, and did not pass through a membrane with a 12- to 14-kDa cutoff. However, the BLIS could pass through an ultrafilter with a 30-kDa cutoff, indicating that they had molecular masses between 12 to 14 and 30 kDa. Although bacteriocins with molecular masses in the 30- to 50-kDa range have been previously reported (25, 27), it is possible that the BLIS produced by S. suis are low- molecular-weight molecules but form protein aggregates because they are highly hydrophobic. The fact that the BLIS were inactivated by proteinase K, pronase, and elastase indicated that they are proteins. Considering their properties, the BLIS produced by S. suis 94-623, 90-1330, and AAH4 may be classic bacteriocins, although studies on purified BLIS would be required to confirm their true nature. They had similar inhibitory spectra and did not interfere with the growth of the other producing strains, suggesting that they may be similar molecules. Bacteriocins produced by lactic acid bacteria are known to possess a specific mechanism that provides self-protection against the toxicity of their own bacteriocins (5). Each bacteriocin is associated with an immunity protein, and they are generally produced concomitantly. These immunity proteins may provide cross-immunity against bacteria that produce related bacteriocins (12). Amino acid sequencing of the BLIS produced by 94-623, 90-1330, and AAH4 is required to determine whether they are different molecules.
The extended lag times observed following the addition of the BLIS-containing supernatants to the S735 cultures and the fact that the OD660 after the 24-h incubation periods was similar to that of the control culture indicated that the BLIS produced by S. suis 94-623, 90-1330, and AAH4 were bacteriostatic. S. suis 94-623 produced BLIS constitutively in broth media, and maximum activity was reached at the mid-log phase. This is in agreement with previous reports on bacteriocin production by various gram-positive bacteria (6, 9, 17, 18, 20). Bacteriocin production was influenced by the culture medium used and was most pronounced in LA broth medium, which had the highest concentration of glucose. Previous studies with Lactobacillus plantarum and Pediococcus damnosus also showed that bacteriocin production is dependent on glucose concentration and a decrease in pH (14, 19).
Following a curing procedure, only 90-1330 lost the capacity to produce BLIS, suggesting that this BLIS may be encoded by a plasmid. Conversely, the fact that BLIS production by 94-623, AAH4, and 65 was not affected by this curing procedure suggested that these BLIS were not encoded by a plasmid. Analysis of the plasmid profiles of 90-1330 and the cured strains by agarose gel electrophoresis revealed no differences in plasmids under 10 kb (data not shown). Pulsed-field gel electrophoresis analysis, which allows large plasmids to be detected, is currently in progress to identify the nature of the plasmid coding for the BLIS produced by 90-1330. Bacteriocins encoded by large plasmids have been previously reported (16, 18).
As S. suis 94-623, 90-1330, and 65 were isolated from healthy carrier pigs and produce BLIS that were against many virulent S. suis serotype 2 strains as well as a number of other swine pathogens, they may be of great interest for use as probiotic strains. The possibility of preventing S. suis serotype 2 infections by a precocious colonization of piglets with bacteriocin-producing nonvirulent S. suis strains deserves serious consideration. Bacteriocin treatments have already been proposed to control infectious diseases because they appear to be inexpensive, effective, and nontoxic to animals and humans (3, 22).
Acknowledgments
We are grateful to Marcelo Gottschalk and Marc Lavoie for generously providing the strains used in this study.
The financial assistance of the Canadian Research Network on Bacterial Pathogens of Swine and La Fédération des Producteurs de Porcs du Québec is duly acknowledged.
REFERENCES
- 1.Aarestrup, F. M., S. R. Rasmussen, K. Artursson, and N. E. Jensen. 1998. Trends in the resistance to antimicrobial agents of Streptococcus suis isolates from Denmark and Sweden. Vet. Microbiol. 63:71-80. [DOI] [PubMed] [Google Scholar]
- 2.Baele, M., K. Chiers, L. A. Devriese, H. E. Smith, H. J. Wisselink, M. Vaneechoutte, and F. Haesebrouck. 2001. The gram-positive tonsillar and nasal flora of piglets before and after weaning. J. Appl. Microbiol. 91:997-1003. [DOI] [PubMed] [Google Scholar]
- 3.Brook, I. 1999. Bacterial interference. Crit. Rev. Microbiol. 25:155-172. [DOI] [PubMed] [Google Scholar]
- 4.Cantin, M., J. Harel, R. Higgins, and M. Gottschalk. 1992. Antimicrobial resistance patterns and plasmid profiles of Streptococcus suis isolates. J. Vet. Diagn. Investig. 4:170-174. [DOI] [PubMed] [Google Scholar]
- 5.Cintas, L. M., M. P. Casaus, C. Herranz, I. F. Nes, and P. E. Hernandez. 2001. Review: bacteriocins of lactic acid bacteria. Food Sci. Tech. Int. 7:281-305. [Google Scholar]
- 6.Crupper, S. S., and J. J. Iandolo. 1996. Purification and partial characterization of a novel antibacterial agent (Bac1829) produced by Staphylococcus aureus KSI1829. Appl. Environ. Microbiol. 62:3171-3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gottschalk, M., and M. Segura. 2000. The pathogenesis of the meningitis caused by Streptococcus suis: the unresolved questions. Vet. Microbiol. 76:259-272. [DOI] [PubMed] [Google Scholar]
- 8.Higgins, R., and M. Gottschalk. 1999. Streptococcal diseases, p. 563-578. In B. E. Straw, S. D'Allaire, W. L. Mengelin, and D. J. Taylor (ed.), Diseases of swine, 8th ed. Iowa State University Press, Ames.
- 9.Ikeda, T., T. Iwanami, M. Hirasawa, C. Watanabe, J. R. McGhee, and T. Shiota. 1982. Purification and certain properties of a bacteriocin from Streptococcus mutans. Infect. Immun. 35:861-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ingolf, F. N., and H. Helge. 2000. Class II antimicrobial peptides from lactic acid bacteria. Biopolymers 55:50-61. [DOI] [PubMed] [Google Scholar]
- 11.Iverson, W. G., and N. F. Millis. 1976. Bacteriocins of Streptococcus bovis. Can. J. Microbiol. 22:1040-1047. [DOI] [PubMed] [Google Scholar]
- 12.Jack, R. W., J. R. Tagg, and B. Ray. 1995. Bacteriocins of gram-positive bacteria. Microbiol. Rev. 59:171-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kataoka, Y., T. Yoshida, and T. Sawada. 2000. A 10-year survey of antimicrobial susceptibility of Streptococcus suis isolates from swine in Japan. J. Vet. Med. Sci. 62:1053-1057. [DOI] [PubMed] [Google Scholar]
- 14.Leal-Sánchez, M. V., R. Jiménez-Diaz, A. Maldonado-Barragán, A. Garrido-Fernández, and J. L. Ruiz-Barba. 2002. Optimization of bacteriocin production by batch fermentation of Lactobacillus plantarum LPC010. Appl. Environ. Microbiol. 68:4465-4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martel, A., M. Baele, L. A. Devriese, H. Goossens, H. J. Wisselink, A. Decostere, and F. Haesebrouck. 2001. Prevalence and mechanism of resistance against macrolides and lincosamides in Streptococcus suis isolates. Vet. Microbiol. 83:287-297. [DOI] [PubMed] [Google Scholar]
- 16.Martinez-Cuesta, M. C., G. Buist, J. Kok, H. H. Hauge, J. Nissen-Meyer, C. Pelaez, and T. Requena. 2000. Biological and molecular characterization of a two-peptide lantibiotic produced by Lactobacillus lactis IFPL 105. J. Appl. Microbiol. 89:249-260. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura, T., N. Yamazaki, H. Taniguchi, and S. Fujimura. 1983. Production, purification and properties of a bacteriocin from Staphylococcus aureus isolated from saliva. Infect. Immun. 39:609-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Navaratna, M. A., H. G. Sahl, and J. R. Tagg. 1998. Two-component anti-Staphylococcus aureus lantibiotic activity produced by Staphylococcus aureus C55. Appl. Environ. Microbiol. 64:4803-4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nel, H. A., R. Bauer, E. J. Vandamme, and L. M. Dicks. 2001. Growth optimization of Pediococcus damnosus NCFB 1832 and the influence of pH and nutrients on the production of pediocin PFD-1. J. Appl. Microbiol. 91:1131-1138. [DOI] [PubMed] [Google Scholar]
- 20.Ocaña, V. S., A. A. Pesce de Ruiz Holgado, and M. E. Nader-Macías. 1999. Characterization of a bacteriocin-like substance produced by a vaginal Lactobacillus salivarius strain. Appl. Environ. Microbiol. 65:5631-5635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parrot, M., M. Charest, and M. C. Lavoie. 1989. Production of mutacin-like substances by Streptococcus mutans. Can. J. Microbiol. 35:366-372. [DOI] [PubMed] [Google Scholar]
- 22.Reid, G., J. Howard, and B. S. Gan. 2001. Can bacterial interference prevent infection? Trends Microbiol. 9:424-428. [DOI] [PubMed] [Google Scholar]
- 23.Shehane, S. D., and R. K. Sizemore. 2002. Isolation and preliminary characterization of bacteriocin produced by Vibrio vulnificus. J. Appl. Microbiol. 92:322-328. [DOI] [PubMed] [Google Scholar]
- 24.Staats, J. J., I. Feder, O. Okwumabua, and M. M. Chengappa. 1997. Streptococcus suis: past and present. Vet. Res. Commun. 21:381-407. [DOI] [PubMed] [Google Scholar]
- 25.Thompson, J. K., M. A. Collins, and W. D. Mercer. 1996. Characterization of a proteinaceous antimicrobial produced by Lactobacillus helveticus CNRZ450. J. Appl. Microbiol. 80:338-348. [DOI] [PubMed] [Google Scholar]
- 26.Turgeon, P. L., R. Higgins, M. Gottschalk, and M. Beaudoin. 1994. Antimicrobial susceptibility of Streptococcus suis isolates. Br. Vet. J. 50:263-269. [DOI] [PubMed] [Google Scholar]
- 27.Van de Guchte, M., S. D. Ehrlich, and E. Maguin. 2001. Production of growth-inhibiting factors by Lactobacillus delbrueckii. J. Appl. Microbiol. 91:147-153. [DOI] [PubMed] [Google Scholar]