Abstract
Background
Fully understanding the determinants and sequelae of fetal growth requires a continuous measure of birth weight adjusted for gestational age. Published United States reference data, however, provide estimates only of the median and lowest and highest 5th and 10th percentiles for birth weight at each gestational age. The purpose of our analysis was to create more continuous reference measures of birth weight for gestational age for use in epidemiologic analyses.
Methods
We used data from the most recent nationwide United States Natality datasets to generate multiple reference percentiles of birth weight at each completed week of gestation from 22 through 44 weeks. Gestational age was determined from last menstrual period. We analyzed data from 6,690,717 singleton infants with recorded birth weight and sex born to United States resident mothers in 1999 and 2000.
Results
Birth weight rose with greater gestational age, with increasing slopes during the third trimester and a leveling off beyond 40 weeks. Boys had higher birth weights than girls, later born children higher weights than firstborns, and infants born to non-Hispanic white mothers higher birth weights than those born to non-Hispanic black mothers. These results correspond well with previously published estimates reporting limited percentiles.
Conclusions
Our method provides comprehensive reference values of birth weight at 22 through 44 completed weeks of gestation, derived from broadly based nationwide data. Other approaches require assumptions of normality or of a functional relationship between gestational age and birth weight, which may not be appropriate. These data should prove useful for researchers investigating the predictors and outcomes of altered fetal growth.
Keywords: MeSH Headings: Birth weight, fetal weight, gestational age, premature birth, ultrasonography
Background
Birth weight is a composite of fetal growth and length of gestation, each of which has different contributors and different sequelae. Removing the contribution of gestational age to birth weight is a first step in understanding the determinants of fetal growth. Preterm birth is a prime predictor of neonatal complications, mortality, and developmental delay.[1,2] Also, birth weight may predict both short- and long-term adverse outcomes. For example, higher birth weight among term infants is associated with birth complications,[3] as well as reduced risk of cardiovascular disease and hypertension in later life, but an increased risk of obesity. [4-9]
In 1996, Alexander et al. published birth weight curves using a 1991 nationwide United States reference, later updated using 1994–1996 data.[10,11] However, these authors included weights only for the 5th, 10th, 50th, 90th, and 95th percentiles at each completed week of gestation. While such categorical divisions may serve for clinical or demographic use, many research studies have been hampered by the traditional classifications "small for gestational age" (SGA) and "large for gestational age" (LGA), historically defined as those infants below the 10th or above the 90th percentile at each gestational age.[12] These divisions are arbitrary, and such gross categorizations may mask differences in risk within or between populations. In addition, associations of fetal growth with later disease appear to span the entire birth weight spectrum, and are not limited to infants above or below a particular cut-point.[13]
Thus, precise determination of birth weight relative to gestational age is needed to understand both the determinants of intrauterine growth and the sequelae of altered growth. The purpose of our analysis was to create a more nearly continuous reference measure of birth weight for gestational age using a recent nationwide dataset.
Methods
We obtained data from the National Center for Health Statistics 1999 and 2000 Natality Data Sets on CD-ROM.[14] These public-use data files include information recorded on birth certificates from all 3,963,465 live births in 1999 and 4,063,823 live births in 2000 that occurred in the United States. We limited analysis to singletons born to United States resident mothers at 22 to 44 completed weeks gestational age (n = 7,609,221). We included only births in which the gestational age was determined from a recorded last menstrual period, and for which plurality, birth weight and baby's sex were recorded (n = 6,714,495). We next trimmed the data to exclude birth weights inconsistent with the gestational age, according to the criteria published by Alexander et al.[10] We thus retained information on 6,690,717 babies.
For each completed week of gestation, we divided the total number of births into 100 equally sized groups, each representing an increment of 1 percentile point. We then used a resistant nonlinear smoothing technique, 4325H,[15] twice, to account for the same birth weight value straddling several percentiles within each week of gestation, which might represent a bias towards reporting round numbers of birth weight values. This approach differs from that used by Alexander, et al.,[10] who smoothed across gestational age groups. We report the highest weight in each group, except in the heaviest group. We present tables for all individuals, as well as those stratified by the baby's sex and birth order.
For situations in which a normally distributed index of birth weight for gestational age may be desirable, we provide what we term a z-value, printed in Table 1 (see additional file, worksheet 'Table 1-z-values'). In many analyses, a 'z-score' for a subject is determined by subtracting the mean and dividing by the standard deviation from a standardization population. The z-scores from the sample are interpreted as if they arise from a normally distributed population. In our analysis, however, we use data from the entire population of annual births in the United States, not just a sample, obviating the need for sampling strategies or normality assumptions. In fact, the distribution of birth weights at each completed week of gestation was not normal in this complete dataset (Kolmogorov-Smirnov test p < 0.01 for all weeks). For example, for an infant born at 427 g at 24 weeks, assuming a normal distribution of birth weight would assign a z score of -1.28 (10th percentile), whereas using our method the same infant would be assigned a z value of -1.695 (5th percentile), a difference of 0.4 units. Therefore, in comparison with the reference group, this infant is lighter than it would appear using a normal distribution assumption.
In contrast to this usual practice, we first calculated actual percentile ranks, and then assigned z-values for each percentile. This amounts to a non-parametric transformation to a normal distribution. One can manipulate the z-values as if they were conventional z-scores. Thus, a research subject in the 5th percentile or with a z-value of -1.695 is lighter than 95% and heavier than 4% of the reference population at that gestational age. We provide the z-value corresponding to the middle of each percentile step – e.g. for the 5th percentile, which spans babies from >4% to 5%, we present the z-value corresponding to 4.5%. Babies with weights greater than the 99th percentile should be assigned a z-value of 2.576, corresponding to 99.5%.
We performed all analyses using SAS version 8.2 (SAS Institute, Cary NC), with the exception of the smoothing procedure, for which we used STATA version 7 (STATA Corporation, College Station TX)
Results
The number of infants at each gestational age ranged from a low of 2,497 at 22 weeks to a maximum of 1,677,211 at 39 weeks. There were 3,423,215 (51.2%) boys and 3,267,502 (48.8%) girls. A total of 2,755,841 (41.2%) mothers were primiparous. There were 3,979,490 (59.5%) infants born to non-Hispanic white mothers, and 954,021 (14.4%) born to non-Hispanic black mothers.
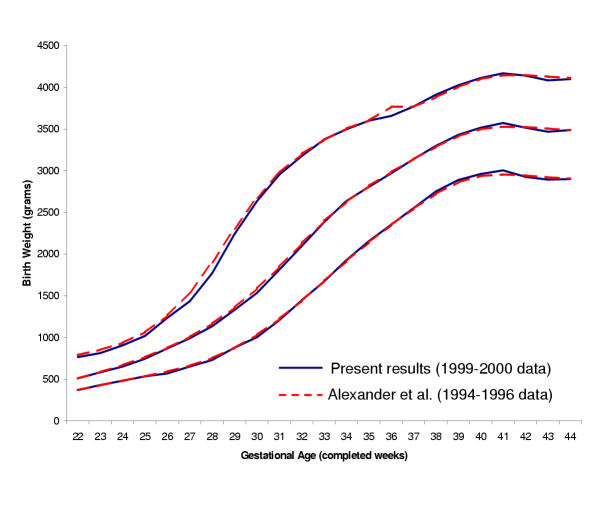
For all births, Table 2 shows the birth weight delineating the top of each percentile step from 1 through 99 percent, for each completed week of gestation (see additional file,worksheet 'Table 2-All'). The top row of the table reports the number of observations represented per percentile cell. For example, at 24 weeks of gestation, there were approximately 4200 reference births, or 42 births per cell; at 39 weeks of gestation, there were 16,772 per cell. In Figure 1, we show that the 10th, 50th, and 90th percentiles from this analysis of 1999 and 2000 births are virtually identical to those published by Alexander et al., based upon the combined 1994–1996 birth cohorts.[11] This figure displays the expected increasing slopes during the third trimester and a leveling off beyond 40 weeks previously seen in menstrually dated pregnancies.[10,16]
Figure 1.
Select reference percentiles for birth weight at each gestational age from 22 to 44 completed weeks for all singleton infants: 10th, 50th, and 90th percentiles from our analysis of 6,690,717 births in the 1999–2000 US Natality datasets, compared with the corresponding percentiles published by Alexander et al. (1999) from 1994–96 US Natality data.
The maximum variance in the estimated percentile cut point occurs at the 50th percentile within each gestation week cohort. For the 24 week cohort of all births, the point estimate of the 50th percentile is 652 grams, and the 95% confidence interval for the point estimate includes values between 648 and 655 grams. This includes the presented values for the 48th through 51st percentile, and z-values between -0.038 and 0.013. Thus the birth weight z-score should be treated as a covariate measured with error[17] though the error is very small even in this worst case. For more extreme percentiles at 24 weeks the confidence interval is smaller; for percentiles greater than 84 or less than 13, the interval excludes the values for both neighboring percentiles. Similarly, as the number of births per percentile increases, the confidence interval at 50% gets smaller. For weeks with more than 80 births per percentile (>28 weeks), the confidence interval even at 50% excludes the neighboring percentiles.
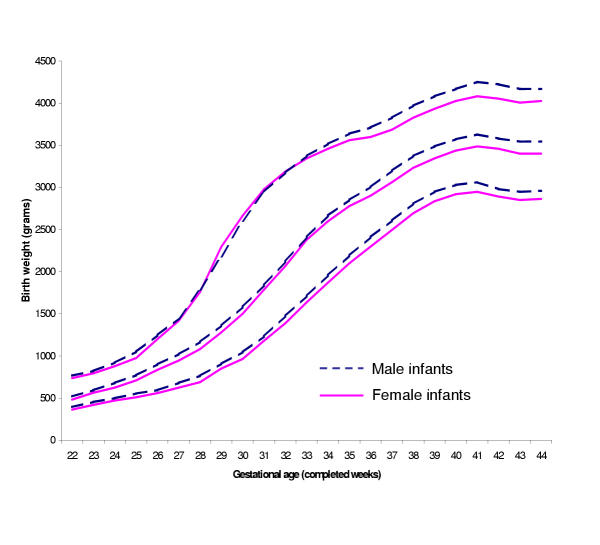
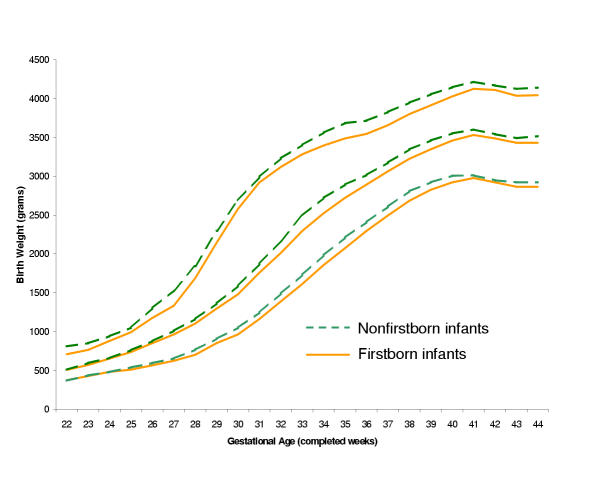
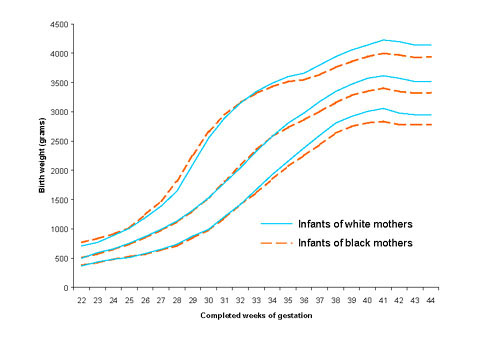
Tables 3 and 4 contain percentile limits for boys and girls (see additional file, worksheets 'Table 3-Males' and 'Table 4-Females'). As others have reported,[11,18] boys were generally heavier than girls at each gestational age (Figure 2). Tables 5 and 6 provide percentile values for firstborn and non-firstborn infants (see additional file, worksheets 'Table 5-Firstborn' and 'Table 6-Nonfirstborn'). Children born to parous mothers had higher birth weights at each gestational age, as anticipated (Figure 3).[18,19] Finally, Tables 7 and 8 present birth weight percentiles for infants born to non-Hispanic white and black mothers only (see additional file, worksheets 'Table 7-Whiteonly' and 'Table 8-Blackonly'). From approximately 33 gestational weeks onwards, babies born to non-Hispanic white mothers are heavier than those born to black mothers (Figure 4), as has been seen previously.[11] Table 9 summarizes the contents of Tables 2-8.
Figure 2.
Select reference percentiles for birth weight at each gestational age from 22 to 44 completed weeks for male and female singleton infants: 10th, 50th, and 90th percentiles. Data from 3,423,215 male and 3,267,502 female infants in the 1999-2000 US Natality datasets.
Figure 3.
Select reference percentiles for birth weight at each gestational age from 22 to 44 completed weeks for firstborn and non-firstborn singleton infants: 10th, 50th, and 90th percentiles. Data from 2,755,841 firstborn and 3,917,426 non-firstborn infants in the 1999–2000 US Natality datasets.
Figure 4.
Select reference percentiles for birth weight at each gestational age from 22 to 44 completed weeks for infants born to non-Hispanic white and black mothers: 10th, 50th, and 90th percentiles. Data from 3,979,490 infants of white mothers and 954,021 infants of black mothers in the 1999–2000 US Natality datasets.
Table 9.
Contents of Tables 2-8, reporting multiple birth weight percentiles for singleton infants born at 22 through 44 completed weeks of gestation. All data are from the 6,690,717 singleton infants in the 1999–2000 US Natality Datasets eligible for inclusion. Investigators can electronically download the data incorporated in these tables as reference for analyses of the determinants or sequelae of fetal growth. Tables can be found in the attached in additional file named 'Tables 1-8.xls'.
| Table | Infants included | Number of births from which the percentiles are derived |
Name of worksheet within the Tables 1-8.xls file |
| Table 2 | All births | 6,690,717 | Table 2-All |
| Table 3 | Males | 3,423,215 | Table 3-Males |
| Table 4 | Females | 3,267,502 | Table 4-Females |
| Table 5 | Firstborn | 2,755,841 | Table 5-Firstborn |
| Table 6 | Non-firstborn | 3,917,426 | Table 6-Nonfirstborn |
| Table 7 | Non-Hispanic whites | 3,979,490 | Table 7-Whiteonly |
| Table 8 | Non-Hispanic blacks | 954,021 | Table 8-Blackonly |
Tables 2 through 8 are available for investigators to download electronically (see additional file). Investigators can then compare their data against these reference data using the statistical software package of their choice. In most cases, the reference data including all births (Table 2) should be used, and researchers wishing to account for infant sex, maternal race, or parity can do so using statistical adjustment with their own data. However, if such adjustment is not possible, then Tables 3-8 can be used.
We provide Table 10, which includes selected data from Tables 1, 2, 3, and 4, as an example for the use of our method. The percentile assigned to an infant is the smallest one with a value larger than the infant's birth weight. Thus an infant weighing 2500 grams born at 37 completed weeks of gestation would be assigned to the 9th percentile. This percentile, which includes infants from >8th to 9th percentile, corresponds to a z-value of -1.372. If the investigator preferred to use the sex specific tables, the same infant would be at the 7th percentile with a z-value of -1.514 if a boy, and at the 11th percentile with a z-value of -1.254 if a girl. In some cases the same birth weight straddles several percentiles for a given gestational age despite smoothing, particularly at the lowest gestational ages. This is an expected occurrence when a large number of birth weights have identical recorded values. In such cases, we recommend that users assign the mean of the z-values crossed by the single birth weight.
Table 10.
Birth weight percentiles from 6 – 23 for all births, and for boys and girls only, at 36 and 37 completed weeks of gestation. In the right half of the table we provide the z-values corresponding to the median of each percentile range. Data in these tables, provided for illustrative purposes, are a subset of data in Tables 1, 2, 3, and 4, published in the electronic pages of this Journal (see additional file 1, Tables 1-8.xls).
| Gestational age in completed weeks | |||||||||
| All Births | Boys Only | Girls Only | |||||||
| Percentile | 36 weeks | 37 weeks | 36 weeks | 37 weeks | 36 weeks | 37 weeks | Percentile range | Median of percentile range | z-value corresponding to median of percentile range |
| 6 | 2213 | 2429 | 2266 | 2486 | 2168 | 2375 | >5 to 6 | 5.5 | -1.598 |
| 7 | 2259 | 2465 | 2311 | 2523 | 2206 | 2409 | >6 to 7 | 6.5 | -1.514 |
| 8 | 2296 | 2496 | 2349 | 2552 | 2240 | 2439 | >7 to 8 | 7.5 | -1.440 |
| 9 | 2325 | 2524 | 2381 | 2580 | 2270 | 2467 | >8 to 9 | 8.5 | -1.372 |
| 10 | 2354 | 2552 | 2410 | 2609 | 2299 | 2495 | >9 to 10 | 9.5 | -1.311 |
| 11 | 2382 | 2580 | 2439 | 2637 | 2327 | 2524 | >10 to 11 | 10.5 | -1.254 |
| 12 | 2409 | 2609 | 2465 | 2666 | 2354 | 2551 | >11 to 12 | 11.5 | -1.200 |
| 13 | 2432 | 2634 | 2487 | 2692 | 2380 | 2574 | >12 to 13 | 12.5 | -1.150 |
| 14 | 2451 | 2654 | 2505 | 2713 | 2400 | 2593 | >13 to 14 | 13.5 | -1.103 |
| 15 | 2471 | 2672 | 2526 | 2731 | 2419 | 2611 | >14 to 15 | 14.5 | -1.058 |
| 16 | 2494 | 2692 | 2549 | 2751 | 2439 | 2631 | >15 to 16 | 15.5 | -1.015 |
| 17 | 2514 | 2711 | 2569 | 2770 | 2458 | 2649 | >16 to 17 | 16.5 | -0.974 |
| 18 | 2532 | 2728 | 2586 | 2786 | 2475 | 2667 | >17 to 18 | 17.5 | -0.935 |
| 19 | 2551 | 2743 | 2603 | 2800 | 2494 | 2685 | >18 to 19 | 18.5 | -0.896 |
| 20 | 2570 | 2758 | 2619 | 2816 | 2512 | 2700 | >19 to 20 | 19.5 | -0.860 |
| 21 | 2586 | 2773 | 2637 | 2832 | 2168 | 2375 | >20 to 21 | 20.5 | -0.824 |
| 22 | 2601 | 2789 | 2654 | 2847 | 2206 | 2409 | >21 to 22 | 21.5 | -0.789 |
| 23 | 2616 | 2804 | 2670 | 2863 | 2240 | 2439 | >22 to 23 | 22.5 | -0.755 |
Discussion
In this paper, we have used 1999 and 2000 United States nationwide Natality databases to generate multiple reference percentiles for birth weight at each completed week of gestation. Birth weight rose in a non-linear pattern as gestational age increased. In concordance with published data, at each gestational age birth weights were higher among boys than girls, and among non-firstborn infants than firstborns. Near term, infants born to non-Hispanic white mothers were larger than those born to non-Hispanic blacks. Our more detailed data correspond well with the limited percentiles published by Alexander et al. (Figure 1), which have been used as a reference standard within the United States.[10,11] Our data should prove useful to investigators working to understand both the determinants and the sequelae of fetal growth. The electronic publication of these data allows for dissemination and widespread use of such a detailed reference.
Previous studies have been hampered by the lack of a continuous measure of birth weight independent of gestational age. We first consider previous approaches for the use of fetal growth as an outcome. The interactions among fetal, maternal, and environmental factors that influence fetal growth remain poorly understood. Removing the contribution of gestational age to birth weight is a first step in understanding the roles of these factors in determining fetal growth.[20,21] Many researchers have attempted to remove the influence of gestational age when studying predictors of birth weight by using the categories small- (SGA), large- (LGA), and appropriate-for-gestational age (AGA). [22,23] However, this categorization reduces the power to detect small associations between fetal exposures and birth weight, unless there is a change in the relationship exactly at the arbitrary cutpoint. This is a particular problem when the size of the association is small in magnitude. Additionally, comparing SGA or LGA with AGA infants inhibits study of variation within the majority of babies that are AGA.
Others have used various methods to control for gestational age when investigating determinants of fetal growth. One procedure has been to use a "birth weight ratio." This measure is calculated by dividing an infant's birth weight by a reference median birth weight at the given gestational age.[16,24,25] However the reference median is chosen, this ratio will assume a linear relationship between birth weight and its influences across the range of birth weights, which may not be correct.
Another approach has been to include gestational age in a multiple regression equation along with other potential predictors of birth weight. This strategy has been used to generate a predicted weight, against which an individual baby's weight can be compared.[16,26] Like the birth weight ratio, regression analysis also typically assumes a linear relationship between birth weight and gestational age. This assumption may or may not be appropriate, but in any case is not required by our method, which is based on actual data.
In addition to examining determinants of fetal growth, adjusting birth weight for gestational age is also needed to understand the influence of fetal growth on later outcomes. While birth weight alone may predict risk for adult diseases,[8,27] most published data emanate from an era when few premature babies survived until adulthood. Future studies of the early life origins of adult disease will require disentangling the effects of length of gestation from fetal growth. A continuous measure is needed since many studies in this field suggest associations that span the entire range of birth weight, and are not limited to birth weight extremes.
For use of fetal growth as a predictor variable, previously published methods either assume a normal distribution of birth weights at each completed week of gestation,[20] or include both birth weight and gestational age in multivariable regression models. [28-30] However, these approaches are limited because they assume either no relationship or a linear relationship between birth weight and gestational age.
Our method provides comprehensive reference values from broadly based nationwide data without making parametric, functional, or other modeling assumptions. It is useful whether fetal growth is used as an outcome or as an exposure (predictor). These results are not intended to assign a percentile to an individual infant for clinical use. Rather, this approach should help researchers investigate the factors associated with infants born at, for example, the 20th as compared with the 40th, 60th, or 80th percentile, as well as the sequelae of such differences in fetal growth.
We have trimmed the dataset prior to analysis following the methods of Alexander et al.[10] This procedure primarily excludes infants with implausibly high birth weights at the younger gestational ages, likely because of inaccurate dating. Investigators might therefore wish to consider whether study subjects well beyond the 99.5th percentile at the youngest gestational ages have accurately recorded weights and gestational ages, prior to inclusion in any analyses. Additionally, some infants with inaccurate gestational ages may remain in the dataset despite trimming, which would tend to inflate values for percentiles above the median.
Several limitations should be considered. One is that relatively low numbers of births at the earlier gestational ages may make percentile estimates for these infants less stable. However, our major percentiles are quite consistent with the combined 1994–1996 data even at the earliest gestational ages (Figure 1).[10,11] We were not able to account for altitude, although few infants in the US are born at high altitude. In addition, infants born before term may have different growth patterns from those remaining in utero. These curves thus represent cross-sectional weights at birth rather than longitudinal fetal growth.
The Natality dataset calculates gestational age from last menstrual period. Some studies suggest that prenatal ultrasound may provide more accurate dates, even when the last menstrual period is recalled with apparent certainty.[31,32] Thus, some investigators advocate use of ultrasound to generate birth weight norms.[25] Nevertheless, the use of ultrasound reference data has several problems. Because prenatal ultrasounds are not universally performed, these reference datasets are generally regional.[26,33] Additionally, data are available only for the subset of women who receive early ultrasounds, and may not include women seeking prenatal care late in pregnancy, or those who choose not to have an ultrasound. Further, different institutions may use different methods to estimate gestational age for a fetus of a given size. Thus, the United States Natality data remain most representative of pregnancies throughout the United States, and appropriate for use as reference.
In addition to calculating reference percentiles for all newborns, we have presented percentiles stratified by infant sex, birth order, and maternal race. Other researchers have advocated further adjusting estimates for factors such as maternal height and weight, [16,26] and even for weights of prior infants born to the same mother.[19] We fear that an overly stratified reference may obscure important predictors of birth weight. While sex and birth order are immutable, the other factors likely serve as surrogates for a combination of maternal exposures such as stress, socioeconomic position, and nutrition, and thus are not permanently bound to differences in fetal growth for future populations.
In conclusion, we have presented a United States national reference standard for size at birth over a broad range of birth weight percentiles from 22 through 44 completed weeks of gestation. Advances in computer technology now permit manipulation of such large data sets as well as electronic publication of multiple reference percentiles for widespread use. These data should prove useful for researchers investigating the determinants and sequelae of altered fetal growth.
Competing Interests
None declared.
Authors' Contributions
EO participated in the study design, analysis of data, and was the primary author of the manuscript. KK participated in the study design, analysis of data, and revision of the manuscript. JRE participated in the study design and revision of the manuscript. MWG participated in the study design, analysis of data, and revision of the manuscript. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Supplementary Material
Acknowledgments
Acknowledgements
Funded in part by a grant from the NIH (HL68041), and by Harvard Medical School and the Harvard Pilgrim Health Care Foundation
Contributor Information
Emily Oken, Email: emily_oken@hphc.org.
Ken P Kleinman, Email: ken_kleinman@hphc.org.
Janet Rich-Edwards, Email: janet_rich-edwards@hphc.org.
Matthew W Gillman, Email: matthew_gillman@hphc.org.
References
- McCormick MC, et al. The health and developmental status of very low-birth-weight children at school age. JAMA. 1992;267:2204–8. doi: 10.1001/jama.267.16.2204. [DOI] [PubMed] [Google Scholar]
- McCormick MC. The contribution of low birth weight to infant mortality and childhood morbidity. N Engl J Med. 1985;312:82–90. doi: 10.1056/NEJM198501103120204. [DOI] [PubMed] [Google Scholar]
- Bennett BB. Shoulder dystocia: an obstetric emergency. Obstet Gynecol Clin North Am. 1999;26:445–58. doi: 10.1016/s0889-8545(05)70089-9. viii. [DOI] [PubMed] [Google Scholar]
- Curhan GC, et al. Birth weight and adult hypertension and obesity in women. Circulation. 1996;94:1310–5. doi: 10.1161/01.cir.94.6.1310. [DOI] [PubMed] [Google Scholar]
- Fall CH, et al. Fetal and infant growth and cardiovascular risk factors in women. BMJ. 1995;310:428–32. doi: 10.1136/bmj.310.6977.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips DI. Birth weight and the future development of diabetes. A review of the evidence. Diabetes Care. 1998;21:B150–5. [PubMed] [Google Scholar]
- Rich-Edwards JW, et al. Birth weight and risk of cardiovascular disease in a cohort of women followed up since 1976. BMJ. 1997;315:396–400. doi: 10.1136/bmj.315.7105.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich-Edwards JW, et al. Birthweight and the risk for type 2 diabetes mellitus in adult women. Ann Intern Med. 1999;130:278–84. doi: 10.7326/0003-4819-130-4_part_1-199902160-00005. [DOI] [PubMed] [Google Scholar]
- Oken E, Gillman MW. Fetal origins of obesity. Obes Res. 2003;11:496–506. doi: 10.1038/oby.2003.69. [DOI] [PubMed] [Google Scholar]
- Alexander GR, et al. A United States national reference for fetal growth. Obstet Gynecol. 1996;87:163–8. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- Alexander GR, Kogan MD, Himes JH. 1994–1996 U.S. singleton birth weight percentiles for gestational age by race, Hispanic origin, and gender. Matern Child Health J. 1999;3:225–31. doi: 10.1023/A:1022381506823. [DOI] [PubMed] [Google Scholar]
- Wilcox AJ. On the importance – and the unimportance – of birthweight. Int J Epidemiol. 2001;30:1233–41. doi: 10.1093/ije/30.6.1233. [DOI] [PubMed] [Google Scholar]
- Gillman MW. Epidemiologic challenges in studying the fetal origins of adult chronic disease. International Journal of Epidemiology. 2002;31:294–299. doi: 10.1093/ije/31.2.294. [DOI] [PubMed] [Google Scholar]
- National Vital Statistics System Birth Data. 2002. http://www.cdc.gov/nchs/births.htm
- Velleman PF. Definition and comparison of robust nonlinear data smoothing algorithms. J Am Stat Assn. 1980;75:609–615. [Google Scholar]
- Wilcox MA, et al. The individualised birthweight ratio: a more logical outcome measure of pregnancy than birthweight alone. Br J Obstet Gynaecol. 1993;100:342–7. doi: 10.1111/j.1471-0528.1993.tb12977.x. [DOI] [PubMed] [Google Scholar]
- Stefanski LA. The effects of measurement error on parameter estimation. Biometrika. 1985;72:583–92. [Google Scholar]
- Brenner WE, Edelman DA, Hendricks CH. A standard of fetal growth for the United States of America. Am J Obstet Gynecol. 1976;126:555–564. doi: 10.1016/0002-9378(76)90748-1. [DOI] [PubMed] [Google Scholar]
- Skjaerven R, Wilcox AJ, Russell D. Birthweight and perinatal mortality of second births conditional on weight of the first. Int J Epidemiol. 1988;17:830–8. doi: 10.1093/ije/17.4.830. [DOI] [PubMed] [Google Scholar]
- Wilcox AJ, Skjaerven R. Birth weight and perinatal mortality: the effect of gestational age. Am J Public Health. 1992;82:378–82. doi: 10.2105/ajph.82.3.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer MS. Intrauterine growth and gestational duration determinants. Pediatrics. 1987;80:502–11. [PubMed] [Google Scholar]
- Kjos SL, et al. A randomized controlled trial using glycemic plus fetal ultrasound parameters versus glycemic parameters to determine insulin therapy in gestational diabetes with fasting hyperglycemia. Diabetes Care. 2001;24:1904–10. doi: 10.2337/diacare.24.11.1904. [DOI] [PubMed] [Google Scholar]
- Doctor BA, et al. Perinatal correlates and neonatal outcomes of small for gestational age infants born at term gestation. Am J Obstet Gynecol. 2001;185:652–9. doi: 10.1067/mob.2001.116749. [DOI] [PubMed] [Google Scholar]
- Nolan CJ, et al. Maternal serum triglyceride, glucose tolerance, and neonatal birth weight ratio in pregnancy. Diabetes Care. 1995;18:1550–6. doi: 10.2337/diacare.18.12.1550. [DOI] [PubMed] [Google Scholar]
- Marsal K, et al. Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr. 1996;85:843–8. doi: 10.1111/j.1651-2227.1996.tb14164.x. [DOI] [PubMed] [Google Scholar]
- Gardosi J, et al. Customised antenatal growth charts. Lancet. 1992;339:283–7. doi: 10.1016/0140-6736(92)91342-6. [DOI] [PubMed] [Google Scholar]
- Barker D. Mothers, babies, and health in later life. Second. Edinburgh: Harcourt Brace and Company; 1998. [Google Scholar]
- Barker M, et al. Birth weight and body fat distribution in adolescent girls. Arch Dis Child. 1997;77:381–3. doi: 10.1136/adc.77.5.381. [DOI] [PubMed] [Google Scholar]
- Phillips DI, et al. Thinness at birth and insulin resistance in adult life. Diabetologia. 1994;37:150–4. doi: 10.1007/s001250050086. [DOI] [PubMed] [Google Scholar]
- Mi J, et al. Effects of infant birthweight and maternal body mass index in pregnancy on components of the insulin resistance syndrome in China. Ann Intern Med. 2000;132:253–60. doi: 10.7326/0003-4819-132-4-200002150-00002. [DOI] [PubMed] [Google Scholar]
- Mongelli M, Wilcox M, Gardosi J. Estimating the date of confinement: ultrasonographic biometry versus certain menstrual dates. Am J Obstet Gynecol. 1996;174:278–81. doi: 10.1016/s0002-9378(96)70408-8. [DOI] [PubMed] [Google Scholar]
- Gjessing HK, Skjaerven R, Wilcox AJ. Errors in gestational age: evidence of bleeding early in pregnancy. Am J Public Health. 1999;89:213–8. doi: 10.2105/ajph.89.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doubilet PM, et al. Improved birth weight table for neonates developed from gestations dated by early ultrasonography. J Ultrasound Med. 1997;16:241–9. doi: 10.7863/jum.1997.16.4.241. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.