Abstract
Recent findings indicate that fishes from lakes in partially burned catchments contain greater mercury (Hg) concentrations than fishes from reference catchments. Increased methyl Hg (MeHg) concentrations in fishes can result in serious health problems for consumers. Here we show that a forest fire caused a 5-fold increase in whole-body Hg accumulation by rainbow trout (Oncorhynchus mykiss) and smaller Hg increases in muscle of several fish species in a mountain lake. The enhanced Hg accumulation was caused primarily by increased nutrient concentrations in the lake, which enhanced productivity and restructured the food web through increased piscivory and consumption of Mysis. This restructuring resulted in increases to the trophic positions and Hg concentrations of fishes. Forest fire also caused a large short-term release of total Hg (THg) and MeHg to streams and the lake. This release initiated a small pulse of MeHg in invertebrates that contributed to enhanced Hg accumulation by fishes. Climate change and prescribed burning to compensate for past fire suppression are predicted to increase future forest fire occurrence in North America, and increased Hg accumulation by fishes may be an unexpected consequence.
Keywords: productivity, stable isotopes, methyl mercury, food chain length, climate change
Concentrations of neurotoxic methyl mercury (MeHg) (1) in fishes are a complex function of MeHg and inorganic mercury inputs to waterbodies and in-lake processes such as MeHg production, biomagnification, and bioaccumulation. For example, inputs of mercury (Hg) to a waterbody can be influenced by atmospheric deposition (2), geology (3), soil and vegetation type (4), and large precipitation events (5). Increases in water temperature, dissolved organic carbon (DOC), and sulfate (SO42−) and declines in pH can increase in-lake Hg methylation (6). Increased lake productivity can reduce Hg biomagnification (7, 8) [by means of biomass (9, 10) and growth dilution (11)], causing fishes in nutrient-enriched lakes to have lower concentrations of Hg than fishes from reference lakes (7), although food chains are the same length. Conversely, increased Hg bioaccumulation may occur because of enhanced Hg inputs to lakes (1) or increases in food chain length (nitrogen stable isotope ratio; δ15N) (12, 13). As a result, MeHg concentrations in fishes from adjacent lakes that are not subjected to point-source pollution can range from very low to those that exceed guidelines to protect the health of human and wildlife consumers (14).
Forest fire potentially modifies complex interactions that control MeHg accumulation by fishes. For instance, forest fire alters soil and vegetation within burned catchments, which can affect nutrient transport (15), and postfire impacts on water chemistry (i.e., nutrients, DOC, SO42−, and pH) and water temperature have been documented (15). Fire-mobilized nutrients (usually N and sometimes P) (16) can enhance lake productivity (17). Forest fire also has been shown to cause small increases in δ15N of aquatic organisms (17–19), which some attributed to increased inputs of inorganic nitrogen from the catchment (18, 19), not food web alterations. Few have studied the effects of forest fire on the biogeochemical cycling of Hg. Fires are known to release Hg from forested catchments to the atmosphere, where long-distance transport occurs (20, 21). Elevated Hg concentrations in sediment (22) and fishes (18) have been identified in waterbodies located in catchments partially burned by forest fire, but mechanisms causing enhanced Hg accumulation by fishes have not been previously identified.
Mature forest ecosystems contain pools of nutrients and Hg equivalent to many years of deposition, particularly when subjected to fire suppression. In North America, forest fire has been suppressed for >100 years; however, prescribed burning has recently become a popular counteractive management option (23). Prescribed burning (17, 23) and climate change are expected to increase forest fire occurrence (24, 25). Our study demonstrates that forest fire and climate change are linked in previously unanticipated ways to factors that control MeHg concentrations in fishes.
Results and Discussion
An intensive study of Moab Lake [1,240 meters above sea level, area = 21.4 hectares (ha), mean depth = 8.6 m, undeveloped catchment] and proximate streams in Jasper National Park (Alberta, Canada) was conducted when a 1,120-ha forest fire burned 72% of Moab Lake's 342-ha catchment between July 12 and August 31, 2000.
Nutrient concentrations increased postfire when compared with prefire Moab Lake data from the 1970s (26) and 1990s. Nitrate + nitrite increased 9-fold from ≤2.0 μg/liter, total dissolved nitrogen increased 2-fold from 89 μg/liter, total phosphorus increased 4-fold from 5.0 μg/liter, and total dissolved phosphorus increased 5-fold from 1.0 μg/liter. All nutrient concentrations continued to increase in 2001 and may persist for several years (16, 17).
Nutrient enrichment caused increases in measures of lake productivity. Phytoplankton chlorophyll a was 1.5-fold greater (from 0.9 μg/liter) postfire. Zooplankton density was 1.6- to 3.0-fold higher in 2000 and 2001 than in the 1990s (13.2 crustaceans per liter). Conductivity, a surrogate for productivity (27), doubled from 113 μS/cm after the fire. Fire-associated nutrient loading can stimulate aquatic primary productivity (16, 17) and cause increased abundance of zooplankton (16) and likely benthic invertebrates (28). Postfire fish growth rates were 12–30% greater than in the 1970s (26). Enhanced lake productivity increases fish growth rates (27, 29), which may lead to earlier age of piscivory in lake trout (Salvelinus namaycush) (27, 29).
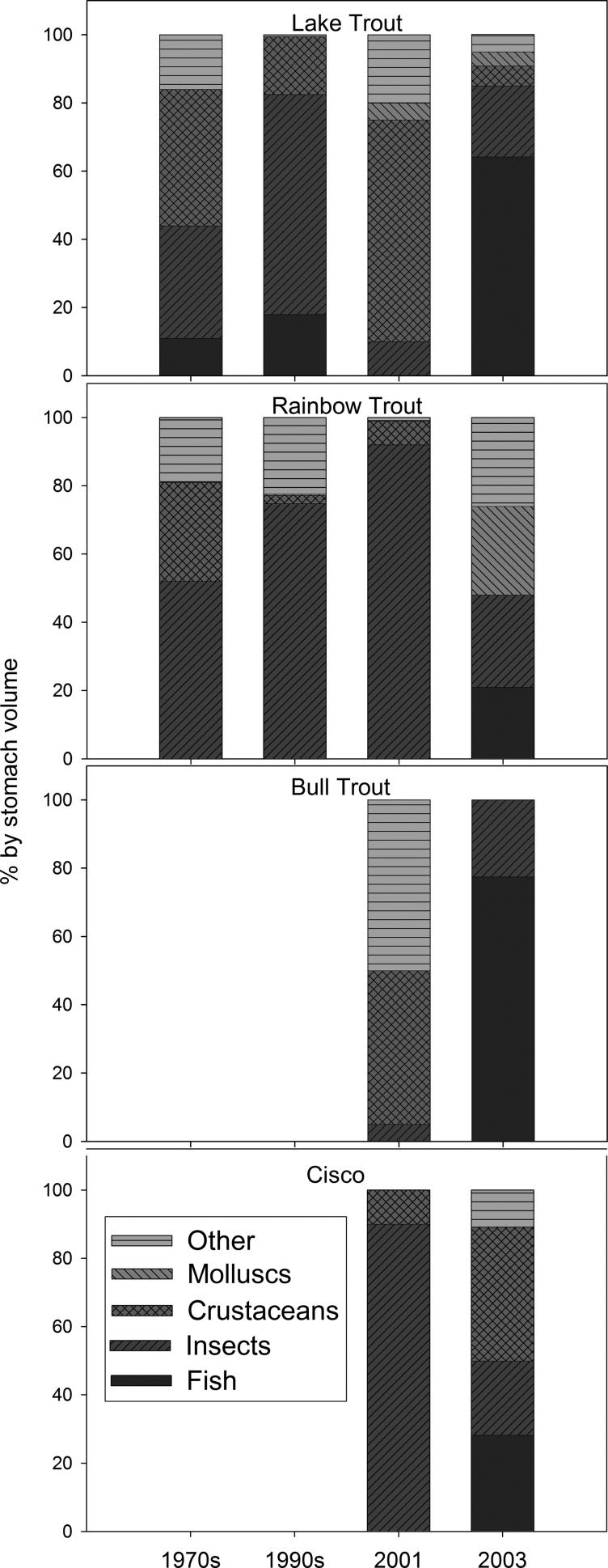
Increased fish growth in Moab Lake after the fire can be explained by dietary alterations. Before the fire, all fish species primarily consumed invertebrates (Fig. 1). Fishes switched from feeding on Hyalella (a detritivore) before the fire to Mysis (a zooplanktivore) in 2001 after the fire. In 2003, rainbow trout (Oncorhynchus mykiss), lake trout, bull trout (Salvelinus confluentus), and cisco (Coregonus artedi) consumed young rainbow trout, likely because of fire-related increases in rainbow trout recruitment from enhanced lake productivity and/or increased water temperatures in spawning streams. Forest fire can increase stream water temperatures by consuming forest canopy and riparian vegetation, resulting in a loss of shading (30). Strong year classes of rainbow trout in mountain lakes are related to increased summer temperatures, probably because warm water causes egg development and hatching times to decrease (31). However, a degree-day analysis indicated that enhanced recruitment was not because of year-to-year summer temperature variability, and recruitment was not unusual in other nearby lakes. Lake trout also preyed on cisco postfire in 2003. These dietary shifts altered the stable isotope composition of fishes.
Fig. 1.
Stomach contents (main taxa as percentage of stomach volume) of lake trout, rainbow trout, bull trout, and cisco from Moab Lake in 1979 (17), 1993, 2001, and 2003 (n = 1–26). In lake trout stomachs, both rainbow trout (1993/2003) and cisco (2003) were identified; in rainbow trout, bull trout, and cisco stomachs, the species of prey fish was rainbow trout/unidentifiable. Crustaceans included Daphnia (which were only present in cisco), Amphipoda, and Mysis; insects included Odonata, Trichoptera, Diptera, Ephemeroptera, and Hemiptera; molluscs included Pelecypoda and Gastropoda; and other included terrestrial items such as adult insects, ants, spiders, semiaquatic mammals, and unidentifiable matter.
The nitrogen stable isotope ratio (δ15N) is commonly used as a continuous measure of trophic position (32, 33). Baseline δ15N, measured in long-lived sphaeriid clams, increased from 0.15‰ before the fire to 2.58‰ in 2001. This increase was probably due to consumption of more autochthonous food sources (17) (indicated by a decline in sphaeriid clam δ13C from −22.2 to −29.8 from 1997 to 2001), and/or a positive shift in δ15N in runoff from burned areas. Others have shown that organisms from fire-impacted waterbodies were significantly enriched in δ15N when compared with reference waterbodies (17, 18).
We observed significant increases in the baseline-adjusted δ15N (BAδ15N) of lake trout (1994 x̄ = 7.25, 2001 x̄ = 9.13, 2003 x̄ = 12.6; Tukey test, P ≤ 0.05), rainbow trout (1994 x̄ = 4.22, 2001 x̄ = 5.59, 2003 x̄ = 9.47; Tukey test, P ≤ 0.05), and bull trout (2001 x̄ = 6.85, 2003 x̄ = 8.68; Mann–Whitney U test, P ≤ 0.05) within Moab Lake (Fig. 2A). Increases in BAδ15N reflect increased postfire consumption of Mysis and enhanced piscivory by all fish species (Fig. 1). Increases in BAδ15N of fishes is representative of a longer food chain. Productivity has been hypothesized to explain food chain length variability (34); however, little empirical supporting evidence exists and has generally been collected through multilake studies over a productivity gradient (34). In our study, a fire-related increase in lake productivity led to a longer food chain, which supports the productivity hypothesis. The length of a food chain is important because it affects the structure of aquatic communities and concentrations of contaminants in biota (34, 35).
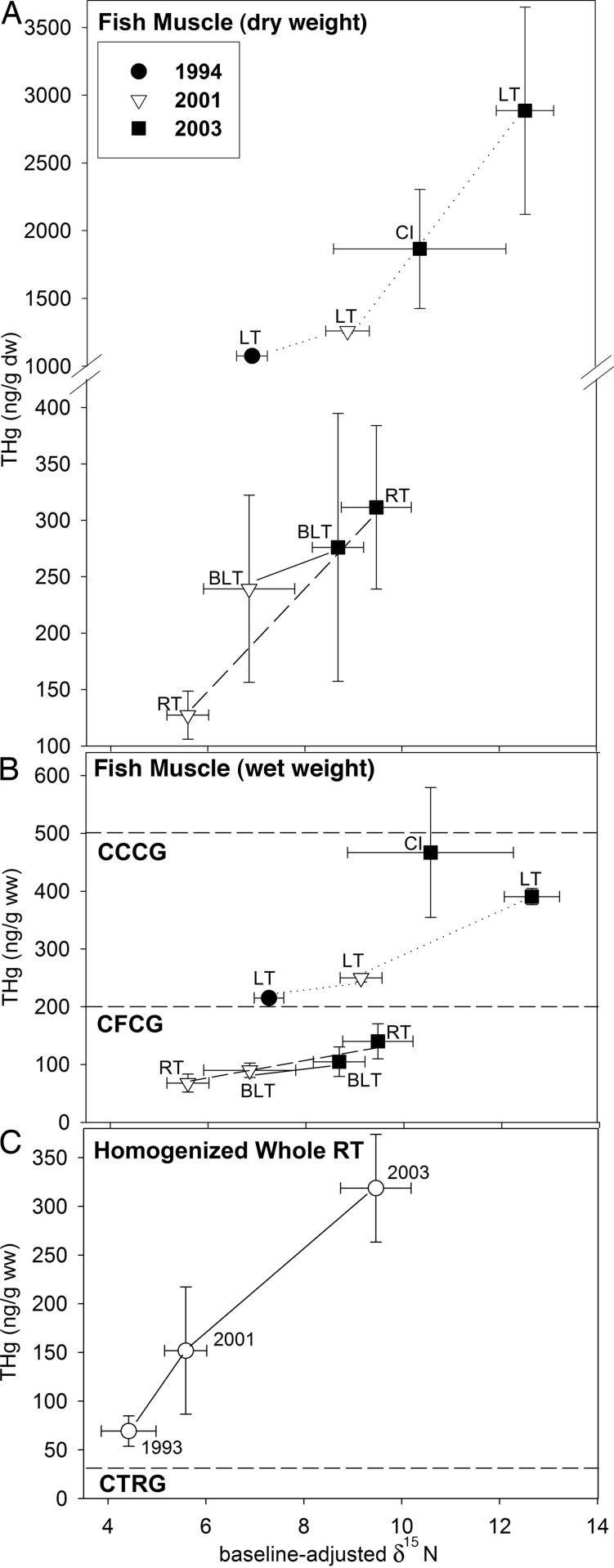
Fig. 2.
Mean THg in fish muscle vs. baseline-adjusted δ15N for fishes from Moab Lake in 1994, 2001, 2003. (A) THg concentration (dry weight). (B) THg concentration (wet weight). CCCG, Canadian Commercial Consumption Guideline; CFCG, Canadian Frequent Consumption Guideline (human health protection). (C) Mean THg for homogenized whole rainbow trout (RT) from Moab Lake vs. baseline-adjusted δ15N in 1993, 2001, and 2003. ww, wet weight; CRTG, Canadian Tissue Residue Guideline (wildlife health protection); LT, lake trout; BLT, bull trout; CI, cisco. Error bars show standard error. We assumed that fish THg concentrations were MeHg concentrations because >85% of Hg in fish is MeHg (6).
MeHg concentrations of organisms from Moab Lake were correlated with BAδ15N (r = 0.900; Fig. 2 A and B) as expected (12, 13). Hg in rainbow trout muscle increased significantly after fire [2001, 68.0 ng/g wet weight (ww); 2003, 140 ng/g dry weight (dw); Mann–Whitney U test, P ≤ 0.05]. By 2003, rainbow trout muscle Hg concentration and BAδ15N approached those of more piscivorous bull trout (Fig. 2A). A significant increase in the Hg concentration of lake trout muscle was also observed (1994, 215 ng/g ww; 2001, 250 ng/g ww; 2003, 391 ng/g ww; Tukey test, P ≤ 0.05). Enhanced piscivory caused increased Hg concentrations in lake trout (36). Prefire bull trout were not available for Hg analyses, but muscle Hg concentrations increased 1.2-fold from 2001 (90.1 ng/g ww) to 2003 (105 ng/g ww). The increase was not statistically significant (Mann–Whitney U test, P ≥ 0.05). In 2003, cisco (not normally a piscivorous species) began to eat fishes (Fig. 1), which could further increase Hg accumulation by lake trout that consume them. Young rainbow trout were present in stomachs of lake trout prefire and several species postfire, indicating they are preferred prey. Whole-body Hg concentrations of rainbow trout were 5-fold greater in 2003 than in 1994 (1994, 69.21 ng/g ww; 2001, 151.82 ng/g ww; 2003, 318.6 ng/g ww; Tukey test, P ≤ 0.05) (Fig. 2C).
Regressions of MeHg concentration and BAδ15N (invertebrates and lake trout only) were highly significant for 1994, 2001, and 2003 (P = 0.0001). BAδ15N explained 70.8% of the variation in Hg in 1994, 84.8% in 2001, and 88.5% in 2003. The slope of the regression line for 1994 was not significantly different from 2001 (t = −0.882, v = 27, P = 0.001) or other nearby lakes (t = −0.839, v = 41, P = 0.001), indicating similar Hg bioaccumulation rates. The slope of the regression line for 2003 was significantly steeper than for 1994 (t = −3.779, v = 24, P = 0.001), 2001 (t = −3.013, v = 29, P = 0.01), and other study lakes (t = −3.779, v = 38, P = 0.001), suggesting enhanced Hg bioaccumulation in 2003. When lake trout MeHg concentrations from regression lines are compared among years at a BAδ15N of 7 (common to regression lines for 1994, 2001, and 2003), ≈88% of the MeHg increase can be attributed to restructuring of the food web.
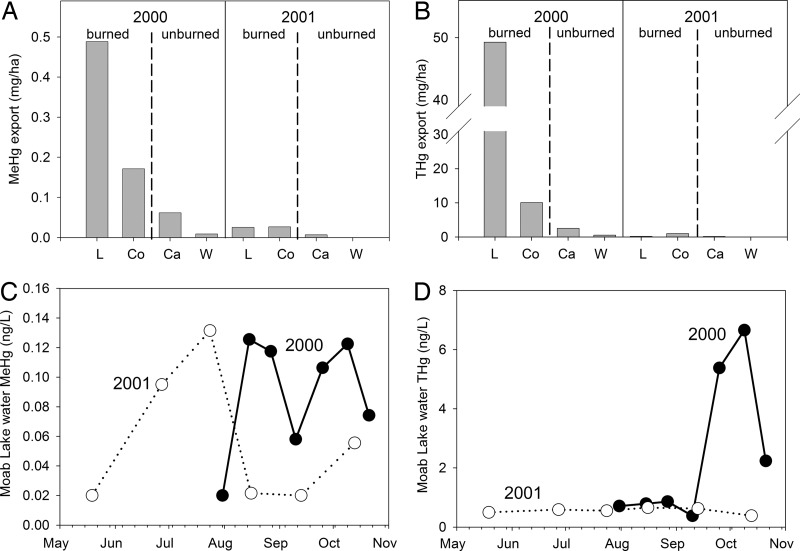
The first major postfire runoff event in September 2000 mobilized a large short-term pulse of MeHg and total Hg (THg) (Fig. 3A and B). This pulse of Hg was unexpected because fire-related volatilization of Hg to the atmosphere removes substantial amounts of Hg from soils (37) and vegetation (20), and decreased concentrations of Hg in soil have been identified even 50 years postfire (38). However, increased THg and MeHg concentrations in sediments close to an inflow draining a burned area of a New Mexico reservoir's catchment were attributed to sediment methylation of THg bound to organic matter and MeHg in runoff (22), but Hg inputs were not measured. Two streams near Moab Lake, in burned catchments, had peak MeHg concentrations of 0.14 and 0.13 ng/liter and THg concentrations of 102.4 and 21.4 ng/liter. In a high-elevation catchment in Rocky Mountain National Park (CO), stream MeHg levels peaked at 0.048 ng/liter just after snowmelt and were generally at or below their detection limits (<0.040 ng/liter) for the rest of the season (39). THg concentrations in the same streams ranged from 0.8 to 13.5 ng/liter. Concentrations of both MeHg (≤0.040 ng/liter) and THg (≈1.0 ng/liter) were lowest during September and October (39). Near Moab Lake, MeHg and THg exports to water were greater from burned catchments (0.33 mg of MeHg per ha, 29.7 mg of THg per ha) than unburned catchments (0.04 mg of MeHg per ha, 1.52 mg of THg per ha) in 2000. The exports from burned catchments were greater than the total annual export from high-elevation catchments in Colorado (0.13 mg of MeHg per ha and 6.5–23.4 mg of THg per ha) (39), although our export estimates were only for a 43-day period in September and early October. MeHg and THg exports decreased in both burned and unburned catchments close to Moab Lake from 2000 to 2001 (Fig. 3 A and B). Fire may release previously soil-bound MeHg for export in burned catchments. Increased Hg exports from burned and unburned catchments in 2000 may be indicative of local fire-related Hg deposition resulting from smoke and ash. Ash contains substantially lower MeHg and THg concentrations than unburned soil and vegetation because of volatilization (vegetation, 97% loss of THg and 94% loss of MeHg; soil, 79% loss of THg and 82% loss of MeHg) (40) and much greater concentrations of THg than MeHg (40). Soil fertilization has been shown to stimulate MeHg production in forest soils (38, 41). In this study, unburned catchments and unburned areas of burned catchments were likely subjected to nutrient deposition from the fire. This nutrient deposition could enhance methylation of both in situ and newly deposited fire-related THg, which would increase MeHg export from burned and unburned catchments. Thus, both burned and unburned (to a lesser extent) catchments may suffer deleterious effects from fire-related increases in MeHg and THg export.
Fig. 3.
MeHg and THg export from burned and unburned catchments near Moab Lake, and MeHg and THg concentrations in Moab Lake surface waters. (A and B) Export of MeHg (A) and THg (B) from two burned catchments, Lake Creek (L; 29.3% burned) and Corral Creek (Co; 68.4% burned), and two unburned catchments, Cavell Creek (Ca) and Wabasso Creek (W). (C and D) Seasonal concentrations of MeHg (C) and THg (D) in unfiltered water from Moab Lake in 2000 and 2001.
Export of MeHg and THg from the burned watershed caused a short-term increase in MeHg and THg concentrations in Moab Lake water during the autumn of 2000 (Fig. 3 C and D). The mean MeHg and THg concentrations in Moab Lake between August and mid-October 2000 were 0.10 and 2.72 ng/liter, respectively. Peak concentrations were 0.13 ng/liter MeHg and 6.66 ng/liter THg. August to mid-October mean concentrations declined to 0.03 ng/liter MeHg and 0.55 ng/liter THg in 2001. The 2000 mean concentrations were also higher than mean concentrations in 36 other lakes in the Canadian Rockies (August to September 2000: x̄ 0.06, range = 0.02–0.15 ng/liter MeHg, x̄ = 0.68, range = 0.36–2.24 ng/liter THg, May to July 2001 x̄ = 0.05, range = 0.02–0.15 ng/liter MeHg, x̄ = 0.49, range = 0.21–2.88 ng/liter THg), and in 90 high-altitude lakes located in 8 Rocky Mountain National Parks in the U.S. (September 1999 x̄ = 0.05, range = 0.01–0.73 ng/liter MeHg and x̄ = 1.07, range = 0.27–14.09 ng/liter THg) (42). Although direct evidence exists of increased MeHg and THg inputs to Moab Lake after fire, the possibility that in-lake methylation was stimulated by fire cannot be ruled out. The first pulse of MeHg in 2000 (Fig. 3C) coincided with fire-related changes in water chemistry (e.g., SO4 concentration was 8.5 mg/liter before the fire, 32.71 mg/liter at the time of the first MeHg pulse in 2000, and remained ≈16–18 μg/liter to the end of 2001). The second MeHg pulse in 2000 occurred at the same time as a large rain event (Fig. 3C). A small, short-lived peak in MeHg in invertebrates from Moab Lake (20–60 ng/g dw) coincided with the second fire-related peak in water MeHg concentration in September 2000. The overall increase in mass of MeHg and THg in Moab Lake water between July and mid-October was greater in 2000 (0.23 g of MeHg, 13 g of THg) than in 2001 (0.07 g of MeHg, 0.6 g of THg).
Although the baseline-adjusted rates of MeHg bioaccumulation in 1994 and 2001 were not significantly different, the elevation of the regression line (invertebrates and lake trout only) for 2001 was significantly higher than for 1994 (t = −2.199, df = 28, P = 0.05). When lake trout MeHg concentrations from regression lines are compared among years at a BAδ15N of 7, ≈12% of the MeHg increase is due to fire-enhanced Hg inputs to Moab Lake and/or increased in-lake methylation. However, if the pulse of Hg from the burned catchment occurred in summer rather than in autumn, Hg bioaccumulation could increase more than observed at Moab Lake because warmer water temperatures, greater nutrient concentrations, and increased DOC and SO42− could enhance production and uptake of fire-related MeHg.
Conclusion
We conclude that forest fire caused increased Hg accumulation by fishes in a partially burned catchment by means of two mechanisms. Food web restructuring was more important than increased Hg inputs and MeHg production at Moab Lake. We hypothesize that fire characteristics (i.e., fire severity, proportion of catchment burned, and timing and intensity of runoff) influence limiting nutrient and contaminant release from burned catchments, altering the relative importance of the two Hg accumulation mechanisms. This hypothesis provides an explanation for the differing results from studies conducted in severely and/or fully burned catchments (19, 43, 44). The postfire changes in Hg cycling observed in this study are likely not unique to Moab Lake. Forest fires can cause nutrient increases in both fluvial and lacustrine systems that persist for several years (16) in partially burned catchments. We recently observed elevated THg concentrations in streams in other burned catchments in the Canadian Rocky Mountains, indicating increased postfire Hg inputs (E.N.K., D.W.S., U. Silins, M. Wagner, and J. Graydon, unpublished data). In some lakes, forest fires could cause MeHg concentrations in fishes to exceed guidelines that protect the health of fish-eating birds and mammals, including humans (18). Hg in fishes often remains high for many years after Hg inputs have ceased (6). In North America, Hg contamination is already the most frequent reason for fish consumption advisories (45). If climate change and prescribed burning increase forest fires in the future in North America (17, 23–25), and the average annual area of forest burned continues to increase in Asia, Europe, the Caribbean, Oceania, South America, and some parts of Africa as it did from 1990 to 2000 (49), fish Hg contamination could become more widespread.
Methods
Field Sampling.
Moab Lake and creeks were sampled biweekly in 2000 and monthly in 2001. Lake sampling included measuring water temperature and collecting water samples for THg, MeHg [using ultraclean sampling protocol (47)], and water chemistry. A 140-μm mesh net was hauled vertically to capture zooplankton. Animals were preserved in formalin for enumeration and frozen for Hg analyses. Fishes were gillnetted in the 1970s, 1994, 2001, and 2003. Specimens were identified, measured, weighed, and frozen. Benthic macroinvertebrates were collected by using sweep nets and weighted mesh colonization traps. Invertebrates were sorted, rinsed, and frozen. At creeks, temperature, depth, and width were recorded. Flow was measured by using a Price AA current meter (Scientific Instruments, Inc., Milwaukee, WI). Water samples for Hg analyses were collected in Teflon bottles and processed the same as lake samples. THg water samples were acidified with concentrated trace metal-grade HCl equivalent to 0.2% of the sample volume. MeHg water samples were frozen. Unfiltered water for ammonia, nitrate + nitrite, and total phosphorus was kept cool until analysis. Water was filtered for SO42−, DOC, total dissolved nitrogen, and total dissolved phosphorus. Whatman (Maidstone, England) GF/F filters for chlorophyll a and water for nitrogen analyses were frozen.
Laboratory Methods.
Water analyses were completed at the University of Alberta Limnology Services Unit (www.biology.ualberta.ca/facilities/limnology). Invertebrates were identified and enumerated. Fishes were aged by using otoliths. Growth rates were determined from length–age regressions. Fish stomach contents were identified. Invertebrate and fish muscle samples were freeze-dried for 48 h. Whole fish were homogenized by using a stainless steel grinder. Fish samples were weighed before and after freeze-drying. Freeze-dried samples were powdered by using an acid-washed glass mortar and pestle. Hg analyses were conducted at the University of Alberta Low-Level Mercury Analytical Laboratory (www.biology.ualberta.ca/facilities/mercury).
THg in unfiltered water was determined by BrCl oxidation, SnCl2 reduction, purge and trap, and cold vapor atomic fluorescence spectrometry (CVAFS) (modified; refs. 48 and 49). The analytical detection limit was 0.05 ng/liter. For MeHg in unfiltered water, samples were distilled and ethylated, followed by gas chromatography separation with CVAFS (49, 50). The detection limit was 0.02 ng/liter. Fishes and freeze-dried invertebrates were digested with 7:3 HNO3:H2SO4 in closed Teflon bombs and analyzed for THg the same as water samples. Detection limits were 0.1–0.3 ng/g. For MeHg, zooplankton and macroinvertebrates were digested in a KOH–methanol solution, ethylated, and separated by gas chromatography with CVAFS. Detection limits were 0.1–0.3 ng/g dry weight. All Hg analyses included spike recoveries, duplicates, blanks, and National Research Council (Canada) certified reference materials (DORM-2), which were prepared alongside the original samples as matrix-matched biomaterial.
Freeze-dried invertebrates and fishes were analyzed for stable isotopes at the University of Ottawa G. G. Hatch Isotope Laboratories. Invertebrate were treated with HCl to dissolve shells and/or surficial carbonates, oven-dried at 60°C, and reground with a mortar and pestle. Samples were weighed in tin capsules (≈1–2 mg for invertebrates and ≈0.3–0.5 mg for fish) and combusted in an automated elemental analyzer (CE Elantech Inc., Lakewood, NJ) CE-1110 coupled to a Finnigan Mat DeltaPLUS Isotope Ratio mass spectrometer; (Thermo Scientific, Waltham, MA) with a Conflow III interface. Water was removed by magnesium perchlorate trap. Helium was the carrier gas. Stable isotopes are expressed in delta notation (δ):
where R = 15N/14N or 13C/12C (32). A normalized calibration curve based on NBS-22 and IAEACH-6 for carbon and IAEA-N-1 and IAEA-N-2 for nitrogen was used to calculate δ15N and δ13C. Standards were from the National Institute of Standards and Technology (NIST). An internal laboratory standard (caffeine) was run every 10th sample to correct for drift in values. Precision was found to be 0.2‰ for nitrogen and 0.3‰ for carbon (based on analyses of 20 replicates). Comparable methods were used for prefire samples at the Environment Canada Laboratory in Saskatoon (SK, Canada).
Hg Export Calculation Methods.
THg and MeHg export from catchments were calculated from THg and MeHg concentration, streamflow data from September 1 to October 13, 2000 and 2001, and catchment area. Dates were chosen based on available 2000 streamflow data. Hg concentration and streamflow values between sampling dates were interpolated from the sampling dates before and after.
δ15N Baseline-Adjustment Methods.
Nutrient inputs may cause baseline δ15N variability, which must be removed by baseline-adjustment before among-year comparison. Sphaeriid clams (collected in 1997, 2000, and 2001) were used as baseline organisms, and their δ15N was subtracted from invertebrate and fish δ15N.
Linear Regression Methods.
Invertebrate MeHg and baseline-corrected trophic position were extrapolated for 1994 and 2003. Data from 2000 before the postfire runoff event were used for 1994 (with 1997 sphaeriid clams as baseline), and data from fall 2001 were used for 2003. Only lake trout were included with invertebrates in regression analyses because muscle Hg data were available for 1994, 2001, and 2003. Linear regression models were created for 1994, 2001, 2003, and other proximate lakes. Some data varied slightly from a normal distribution, but transformation did not normalize the data. Other assumptions of linear regression were met. Regression is robust for slight violations of some assumptions (51); therefore, untransformed data were used. Slopes and elevations of regression lines were compared by using t tests (51). A BAδ15N of 7 (common to regression lines for 1994, 2001, and 2003) was chosen, and the difference between the lake trout Hg concentration in 2001 and 2003 was attributed to food web restructuring, whereas the difference between the lake trout Hg concentration in 1994 and 2001 was attributed to increased catchment inputs and in-lake methylation.
Acknowledgments
We thank B. Parker, M. Bowman, C. Lemmon, L. Cheperdak, M. Van Dorn, H. Swanson, and Parks Canada wardens for their help in the field and/or laboratory; D. Kelly, J. Kirk, M. Puchniak, and S. Wanigaratne of the University of Alberta Low-Level Mercury Analytical Laboratory for their technical assistance; and M. Demers, K. Hobson and L. Wassenaar for stable isotope analyses. This work was supported by the Alberta Science and Research Authority (ASRA), a Discovery Grant from the National Science and Engineering Research Council (NSERC) (to D.W.S.), an NSERC PGS-A Scholarship (to E.N.K.), Circumpolar/Boreal Alberta Research (C/BAR), Challenge Grants in Biodiversity, Science Horizons, and Parks Canada.
Abbreviations
- δ15N
nitrogen stable isotope ratio
- BAδ15N
baseline-adjusted δ15N
- THg
total mercury
- MeHg
methyl mercury
- DOC
dissolved organic carbon.
Footnotes
The authors declare no conflict of interest.
References
- 1.Fitzgerald WF, Clarkson TW. Environ Health Perspect. 1991;96:159–166. doi: 10.1289/ehp.9196159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swain EB, Engstrom DR, Brigham ME, Henning TA, Brezonik PL. Science. 1992;22:784–787. doi: 10.1126/science.257.5071.784. [DOI] [PubMed] [Google Scholar]
- 3.Vaidya OC, Howell GD. Water Air Soil Pollution. 2002;134:165–188. [Google Scholar]
- 4.Shanley JB, Kamman NC, Clair TA, Chalmers A. Ecotoxicology. 2005;14:125–134. doi: 10.1007/s10646-004-6264-z. [DOI] [PubMed] [Google Scholar]
- 5.Lawson NM, Mason RP. Water Res. 2001;35:4039–4052. doi: 10.1016/s0043-1354(01)00140-3. [DOI] [PubMed] [Google Scholar]
- 6.Ullrich SM, Tanton TW, Abdrahitova SA. Crit Rev Environ Sci Technol. 2001;31:241–293. [Google Scholar]
- 7.Essington TE, Houser JN. Trans Am Fish Soc. 2003;132:57–68. [Google Scholar]
- 8.Kidd KA, Paterson MJ, Hesslein RH, Muir DCG, Hecky RE. Can J Fish Aquat Sci. 1999;56:2193–2202. [Google Scholar]
- 9.Pickhardt PC, Folt CL, Chen CY, Klaue B, Blum JD. Sci Total Environ. 2005;339:89–101. doi: 10.1016/j.scitotenv.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 10.Chen CY, Folt CL. Environ Sci Technol. 2005;39:115–121. [PubMed] [Google Scholar]
- 11.Simoneau M, Lucotte M, Garceau S, Laliberte D. Environ Res. 2005;98:73–82. doi: 10.1016/j.envres.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Cabana G, Rasmussen JB. Nature. 1994;372:255–257. [Google Scholar]
- 13.Kidd KA, Hesslein RH, Fudge RJP, Hallard KA. Water Air Soil Pollution. 1995;80:1011–1015. [Google Scholar]
- 14.Allan RJ. Water Sci Technol. 1999;39:173–177. [Google Scholar]
- 15.Gresswell RE. Trans Am Fish Soc. 1999;128:193–221. [Google Scholar]
- 16.Carignan R, Steedman RJ. Can J Fish Aquat Sci. 2000;57(Suppl 2):1–4. [Google Scholar]
- 17.Spencer CN, Gabel KO, Hauer FR. Forest Ecol Manag. 2003;178:141–153. [Google Scholar]
- 18.Garcia E, Carignan R. Environ Toxicol Chem. 2005;24:685–693. doi: 10.1897/04-065r.1. [DOI] [PubMed] [Google Scholar]
- 19.Allen EW, Prepas EE, Gabos S, Strachan WMJ, Zhang WP. Can J Fish Aquat Sci. 2005;62:1963–1977. [Google Scholar]
- 20.Friedli HR, Radke LF, Lu JY, Banic CM, Leaitch WR, MacPherson JI. Atmos Environ. 2003;37:253–267. [Google Scholar]
- 21.Sigler JM, Lee X, Munger W. Environ Sci Technol. 2003;37:4343–4347. doi: 10.1021/es026401r. [DOI] [PubMed] [Google Scholar]
- 22.Caldwell CA, Canavan CM, Bloom NS. Sci Total Environ. 2000;260:125–133. doi: 10.1016/s0048-9697(00)00554-4. [DOI] [PubMed] [Google Scholar]
- 23.Weber MG, Stocks BJ. Ambio. 1998;27:545–550. [Google Scholar]
- 24.Flannigan MD, Logan KA, Stocks BJ, Wotton BM, Amiro BD, Todd JB. In: Forest Fire Research & Wildland Fire Safety. Viegas X, editor. Rotterdam: Millpress; 2002. pp. 1–5. [Google Scholar]
- 25.Flannigan MD, Stocks BJ, Wotton BM. Sci Total Environ. 2000;262:221–229. doi: 10.1016/s0048-9697(00)00524-6. [DOI] [PubMed] [Google Scholar]
- 26.Donald DB, DeHenau A-M. Limnological Studies in Jasper National Park, Part Eight A Limnological Survey and Management Study of 23 Lakes Near the Icefields Parkway. Edmonton: Canadian Wildlife Service; 1981. pp. 152–161. [Google Scholar]
- 27.Trippel EA, Beamish FWH. Can J Fish Aquat Sci. 1989;46:1531–1538. [Google Scholar]
- 28.Clarke KD, Knoechel R, Ryan PM. Can J Fish Aquat Sci. 1997;54:89–95. [Google Scholar]
- 29.Donald DB, Anderson RS. Trans Am Fish Soc. 1982;111:675–680. [Google Scholar]
- 30.Ice GG, Neary DG, Adams PW. J Forest. 2004;102:16–20. [Google Scholar]
- 31.Donald DB, Alger DJ. Can J Fish Aquat Sci. 1986;43:1733–1741. [Google Scholar]
- 32.Peterson BJ, Fry B. Annu Rev Ecol Syst. 1987;18:293–320. [Google Scholar]
- 33.Post DM. Ecology. 2002;83:703–718. [Google Scholar]
- 34.Post DM, Pace ML, Hairston NG. Nature. 2000;405:1047–1049. doi: 10.1038/35016565. [DOI] [PubMed] [Google Scholar]
- 35.Kidd KA, Schindler DW, Muir DCG, Lockhart WL, Hesslein RH. Science. 1995;269:240–242. doi: 10.1126/science.269.5221.240. [DOI] [PubMed] [Google Scholar]
- 36.MacCrimmon HR, Wren CD, Gots BL. Can J Fish Aquat Sci. 1983;40:114–120. [Google Scholar]
- 37.Harden JW, Neff JC, Sandberg DV, Turetsky MR, Ottmar R, Gleixner G, Fries TL, Manies KL. Global Biogeochem Cycles. 2004;18:GB3014. [Google Scholar]
- 38.Amirbahman A, Ruck PL, Fernandez IJ, Haines TA, Kahl JS. Water Air Soil Pollution. 2004;152:313–331. [Google Scholar]
- 39.Mast MA, Campbell DH, Krabbenhoft DP, Taylor HE. Water Air Soil Pollution. 2005;164:21–42. [Google Scholar]
- 40.Mailman M, Bodaly RA. Environ Pollution. 2005;138:161–166. doi: 10.1016/j.envpol.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 41.Matilainen T, Verta M, Korhonen H, Uusi-Rauva A, Niemi M. Water Air Soil Pollution. 2001;125:105–119. [Google Scholar]
- 42.Krabbenhoft DP, Olson ML, Dewild JF, Clow DW, Striegl RG, Dornblaser MM, VanMetre P. Water Air Soil Pollution Focus. 2002;2:233–249. [Google Scholar]
- 43.Garcia E, Carignan R. Can J Fish Aquat Sci. 1999;56:339–345. [Google Scholar]
- 44.Garcia E, Carignan R. Can J Fish Aquat Sci. 2000;57:129–135. [Google Scholar]
- 45.US Environmental Protection Agency. Fact Sheet Update: National Listing of Fish and Wildlife Consumption Advisories. Washington, DC: Environ Protection Agency; 1998. EPA Publ No 823-F-98-009. [Google Scholar]
- 46.Forestry Department, Food and Agriculture Organization of the United Nations. Global Forest Resources Assessment 2005 Progress Towards Sustainable Forest Management. Rome: Forestry Department, Food and Agriculture Organization of the United Nations; 2005. pp. 60–65. FAO Forestry Paper No 147. [Google Scholar]
- 47.St Louis VL, Rudd JWM, Kelly CA, Beaty KG, Flett RG, Roulet NT. Environ Sci Technol. 1996;30:2719–2729. [Google Scholar]
- 48.US Environmental Protection Agency. Draft Method 1631: Mercury in Water by Oxidation, Purge and Trap, and Cold Vapour Atomic Fluorescence Spectrometry. Washington, DC: Environ Protection Agency; 1996. EPA Publ No 821-R-96-012. [Google Scholar]
- 49.Olson ML, Cleckner LB, Hurley JP, Krabbenhoft DP, Heelan TW. Fresen J Anal Chem. 1997;358:392–396. [Google Scholar]
- 50.Horvat M, Liang L, Bloom NS. Anal Chim Acta. 1993;282:153–168. [Google Scholar]
- 51.Zar JH. Biostatistical Analysis. 4th Ed. Vol. 332. Upper Saddle River, NJ: Prentice–Hall; 1999. pp. 360–368. [Google Scholar]